Abstract
Background: There is a lack of evidence on treatment of urinary tract infections (UTIs) in male patients in a primary care setting, and whether narrow-spectrum antibiotics are safe and effective.
Objectives: To explore antibiotic switch rates after treatment with UTI antibiotics in men over the last 11 years.
Material: We analysed data from the Norwegian Prescription Database (NorPD). Men ≥16 years receiving cefalexin, ciprofloxacin, cotrimoxazole, nitrofurantoin, ofloxacin, pivmecillinam or trimethoprim during the period 2008–2018 were included. Antibiotic switch was defined as being prescribed a different antibiotic drug appropriate for UTI within 14 days after initial treatment. We calculated rates of antibiotic switch and corresponding odds ratios for each antibiotic drug.
Results: Seven hundred twenty-six thousand and ninety-six (726,096) prescriptions to 429,807 men were defined as possible UTI episodes. Fluoroquinolones, pivmecillinam and cotrimoxazole were most frequently prescribed. Forty-nine thousand five hundred and thirty-one (49,531) (6.8%) of the treatments resulted in antibiotic switch. Compared to cotrimoxazole, the risk of antibiotic switch was higher for pivmecillinam (OR: 2.46; 95% CI, 2.39–2.53) and trimethoprim (OR: 2.12; 95% CI, 2.04–2.20), and lower for fluoroquinolones (OR: 0.40; 95% CI, 0.39–0.42) and cefalexin (OR: 0.28; 95% CI, 0.26–0.30). Treatment duration of ≥7 days and age of ≥50 years were associated with an increased risk of antibiotic switch.
Conclusion: Fluoroquinolones and cefalexin were associated with lower antibiotic switch rates than the recommended UTI antibiotics (pivmecillinam, nitrofurantoin and trimethoprim). However, the rates of antibiotic switch following treatment of male patients with first-line empirical UTI antibiotics are relatively low, indicating that the current guidelines are safe.
Introduction
Antibiotic resistance in common organisms is an increasing global concern, and the need to reduce over-use of antibiotics is evident [Citation1]. In Norway, approximately 84% of all antibiotics for human treatment are prescribed in primary health care [Citation2]. Urinary tract infections (UTIs) are among the most common infections treated with antibiotics in general practice in Norway, estimated to account for almost 25% of all antibiotic prescriptions in general practice [Citation2,Citation3]. Approximately, 20% of all UTIs occur in men [Citation4,Citation5]. The incidence of UTI in the community is estimated to 0.9–2.4 cases per 1000 men below the age of 55 years, increasing with age to levels at or above 7.7 per 1000 men of 85 years and older [Citation4,Citation6,Citation7].
Traditionally, UTIs are categorised as uncomplicated or complicated infections [Citation8]. It is common practice to treat all UTIs in male patients as complicated infections, and most international guidelines recommend fluoroquinolones or trimethoprim-sulfamethoxazole (cotrimoxazole) for 7–14 days [Citation9,Citation10]. Randomised trials have shown that 14 days of ciprofloxacin is favourable to both 7 days and 28 days in male patients with a febrile UTI [Citation11,Citation12]. However, there are on-going discussions regarding the safety and efficacy of treating acute cystitis in male patients as uncomplicated UTIs [Citation13]. Some newer studies suggest that men with acute cystitis do not need treatment for more than 5–7 days [Citation14–16], and others argue that nitrofurantoin or pivmecillinam are appropriate as empirical treatment options [Citation17,Citation18].
In Norway, the current treatment guidelines recommend treating acute cystitis in male patients empirically with nitrofurantoin, pivmecillinam or trimethoprim for 5–7 days, whereas at-risk patients or men with suspected pyelonephritis or prostatitis should be treated with cotrimoxazole or ciprofloxacin for 7–14 days [Citation19]. The lack of evidence calls for studies investigating how to effectively treat male patients with acute cystitis in general practice.
The aim of this study was to explore the rates of antibiotic switch as a proxy for treatment failure in men receiving antibiotic treatment appropriate for UTI in the last 11 years, and to investigate whether the rate of antibiotic switch varied between different antibiotic treatments. A secondary aim was to describe the prescription patterns in the study period.
Materials and methods
Ethics
The Regional Committee for Medical and Health Research Ethics granted a dispensation from professional secrecy requirements (ref 2018/2496/REK sør-øst C). Data protection was approved by the Norwegian centre for Research Data (NSD number: 796958). The study protocol was approved by the Norwegian Prescription Database (ref 19/10717). The study was conducted in accordance with the Declaration of Helsinki, as well as national and institutional standards.
Setting and design
We analysed data from the Norwegian Prescription Database (NorPD), a national electronic database with a complete listing of all prescription drugs dispensed by pharmacies in Norway since 2004 [Citation20]. In Norway, all antibiotics are available by prescription only, and the database contains information on drugs dispensed to patients in ambulatory care, that is, general practice, out-of-hours services and outpatient treatment in specialist health services. The NorPD covers the entire Norwegian population (5,328,212 inhabitants per 1 January 2019). Each prescription record contains a unique anonymised person-identifier, age, sex, municipality, full account of the dispensed product, the dispensed quantity and the date of purchase. The NorPD contains diagnosis codes (ICD-10 or ICPC2) only for prescriptions given through the general reimbursement programme for chronic conditions (The Blue Prescription Programme). The database does not contain information on signs, symptoms or diagnosis for patients receiving standard non-reimbursed prescriptions.
From the NorPD database, we extracted all prescriptions of cefalexin, ciprofloxacin, cotrimoxazole, fosfomycin, nitrofurantoin, ofloxacin, pivmecillinam, sulfadiazine and trimethoprim to male patients in the period January 2008 to December 2018. We decided not to include amoxicillin in our study as it is not recommended for UTI treatment in the national guidelines, and it is more frequently used to treat respiratory tract infections [Citation19]. Patients were eligible for inclusion if they were male and ≥16 years old when the prescription was dispensed. Patients treated with fosfomycin or sulfadiazine were excluded, as the drugs are infrequently used in Norway. The DDDs used in the dataset were according to the ATC/DDD index of 2019 [Citation21]. For population statistics, we used Norwegian population data from Statistics Norway (SSB) [Citation22].
Identification of UTI episodes
For each individual, we identified a UTI episode as a prescription of a UTI antibiotic, defined as cefalexin, cotrimoxazole, fluoroquinolones (ciprofloxacin or ofloxacin), nitrofurantoin, pivmecillinam or trimethoprim. To be defined as a UTI episode, prescriptions had to contain a sufficient quantity and dosage, with cut-offs at minimum two DDD and maximum 14 DDD. We did not include prescriptions if there were more than one antibiotic prescription on the same day. As a measure to identify “new” infections and to avoid prophylactic treatment, we only included prescriptions if the patient had at least 90 days without treatment with the relevant antibiotics prior to the start of UTI treatment. The prescription had to be attained at least 90 days after the start of the study period (i.e. 30 March 2008 at the earliest), and before 28 days prior to the end of the study period (i.e. 3 December 2018 at the latest).
Outcome
Antibiotic switch was defined as a prescription of a different UTI antibiotic than the initial treatment within the first 14 days after start of treatment. Each follow-up period started at the time of initial prescription.
Statistical analysis
Patient characteristics are described with mean (standard deviation, SD) or median (interquartile range, IQR) for numerical variables and with numbers (percentage) for categorical variables. Binary responses relating to antibiotic switch were collected at different time points for each patient. This rendered the standard logistic regression model inappropriate. Therefore, we used a mixed effect binary logistic regression model to account for the variability between the patients. The modelling process was executed in two steps. First, unadjusted models were fitted to the data. Secondly, based on the results from step 1, all the variables with p < .05 were used to fit an adjusted model. We obtained an estimate of intra-cluster correlation (ICC), which explained the amount of variability in the response variable that was due to differences between the patients. All analyses were conducted using commercially available software (STATA, version 16.0; StataCorp LLC) and the significance level was set at α = .05.
Results
Total antibiotic prescriptions
During the 11-year study period, 476,423 men received in total 1,515,878 prescriptions of the relevant antibiotics. Overall mean age at prescription date was 63.4 years. Mean age varied between the different antibiotics, and was higher for nitrofurantoin, trimethoprim and pivmecillinam, and lower for cefalexin or fluoroquinolones (). Men <65 years received 67.7% of the cefalexin and 55.0% of the fluoroquinolone prescriptions but only 29.8% of the nitrofurantoin, 33.5% of the trimethoprim and 36.2% of the pivmecillinam prescriptions.
Table 1. Total UTI antibiotic prescriptions to men ≥16 years in Norway 2008–2018.
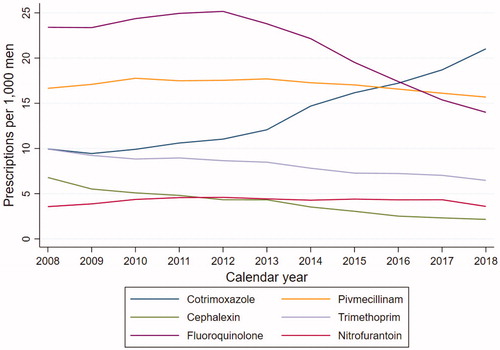
During the study period, the total number of prescriptions of UTI antibiotics decreased from 70.3 prescriptions per 1000 male inhabitants in 2008 to 62.9 prescriptions per 1000 male inhabitants in 2018. The frequency of fluoroquinolone prescriptions decreased from 23.4 to 14.0 prescriptions per 1000 men from 2008 to 2018, while usage of cotrimoxazole increased from 10.0 to 21.0 prescriptions per 1000 men (). Pivmecillinam, nitrofurantoin and cefalexin usage marginally decreased. In 2018, cotrimoxazole, pivmecillinam and fluoroquinolones were the most frequently prescribed UTI antibiotics.
UTI episodes
Seven hundred twenty-six thousand and ninety-six (726,096) prescriptions in a total of 429,807 men fulfilled the criteria to be defined as UTI episodes. The mean age of men with a UTI episode was 61.3 years. The most frequently prescribed antibiotics for treatment of the UTI episodes were the fluoroquinolones ciprofloxacin and ofloxacin (225,439 prescriptions) and pivmecillinam (206,814 prescriptions). The UTI prescriptions had a median duration of 10 DDD [5.6–10]. Regardless of antibiotic type, the median DDD did not differ between UTI episodes and the overall prescriptions described in .
Antibiotic switch
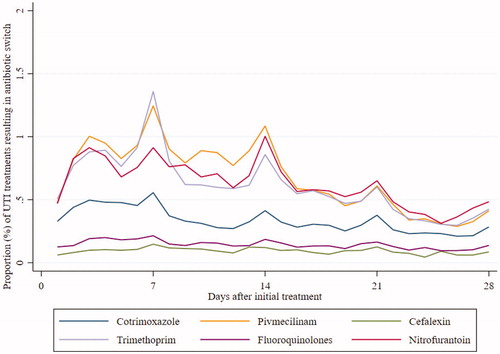
Ninety-two thousand five hundred and sixty four (92,564; 12.8%) of the initial prescriptions were followed by a new prescription of a UTI antibiotic within the first 14 days. Of these, 49,531 (6.8%) prescriptions were switches to another type of antibiotic than the initial treatment, while 43,033 (6.3%) were re-prescriptions of the same antibiotic as the initial treatment. When we prolonged the follow-up period to 28 days, we found a switch rate of 10.9% and rate of repeat prescriptions at 19.2%. Table S1 displays which antibiotics were prescribed as repeat prescriptions. The rates of antibiotic switch increased slightly from 6.5% in 2008 to 7.1% in 2018. The antibiotic switch rate was highest between day 3 and day 14 after initial treatment (). Thereafter, the rates decreased steadily with exceptions on days 21 and 28. The antibiotic switch rates were highest for pivmecillinam and trimethoprim, 12.5% and 10.8%, respectively, and lowest for fluoroquinolones and cefalexin, 2.3% and 1.5%, respectively.
Figure 2. Proportion (%) of prescriptions of UTI antibiotic resulting in antibiotic switch by days after initial treatment.
Comparing treatment for 2-9 DDD vs. 10-14 DDD revealed an increased switch rate for shorter courses of trimethoprim (11.0% vs. 8.8%) and nitrofurantoin (11.1% vs. 6.4%), and a decreased switch rate for shorter courses of pivmecillinam and fluoroquinolones (Table S2).
presents the results of the multilevel logistic regression model on antibiotic switch during the first 14 days after initial treatment. Pivmecillinam and trimethoprim yielded significantly higher ORs compared to treatment with cotrimoxazole, whereas cefalexin and fluoroquinolones yielded significantly lower ORs. Higher age and duration of therapy of seven DDD or more was associated with higher odds for antibiotic switch. The year of prescription did not significantly influence the odds for antibiotic switch. From the adjusted model, we obtained an ICC estimate of 0.119, meaning that 11.9% of the variability in antibiotic switch was due to differences between patients.
Table 2. Multivariate logistic analysis showing factors associated with antibiotic switch within 14 days after initial prescription.
Discussion
Strengths and limitations
The Norwegian Prescription database covers the entire population of Norway, and contains every prescription dispensed at Norwegian pharmacies during the study period. As all antibiotic drugs in Norway are available by prescription only, our data represents almost all outpatient treatments for all males in Norway during the study period. However, this study was retrospective and patients were not randomised to receive particular treatments. It is possible therefore, that the choice of antibiotic drug and the duration of treatment reflect symptom severity and specific patient risk factors rather than the arbitrary preferences of the individual physician.
The NorPD-database does not link prescription data to patient journal records. Nor does it provide information about symptom or diagnosis codes such as ICD or ICPC of non-reimbursed prescriptions. Hence, we are not able to distinguish between acute cystitis and febrile UTI. In reality, a prescription of our selected antibiotics did not necessarily represent a bacterial urinary tract infection. About half of the prescriptions defined as UTI episodes were dispensed to men <65 years. UTIs mainly occur in men older than 65 years [Citation4,Citation23]. We found a higher mean age for antibiotics exclusively used to treat UTIs (pivmecillinam, trimethoprim and nitrofurantoin), and lower mean age for antibiotics used to treat both UTIs and other infections (fluoroquinolones and cefalexin). This might imply that a substantial part of the younger patients did not actually have a UTI but were treated for other infections.
We did not have information on patient hospitalisation or death. This could potentially lead to an over-estimation of effectiveness of treatments that potentially resulted in complications such as pyelonephritis, urosepsis or death, and not just the need for a new prescription as an outpatient. Drugs given during hospitalisation or at nursing homes are not usually registered in the database. Therefore, the elderly patients included in the study are likely among the healthiest among their age. This, and the fact that the patients could end up in a hospital or in a nursing homes following unsuccessful treatment, might explain the relatively low rate of repeat prescriptions for men aged 85 years and older, especially when compared to men in the age group 50–64 and 65–84 years.
Antibiotic use in the study period
The most prescribed antibiotics in the 11-year study period were fluoroquinolones and pivmecillinam. From 2012, there has been a steady decline of the use of fluoroquinolones and an increased use of cotrimoxazole. The reduction in overall antibiotic use in general, and fluoroquinolones in particular, is also seen in other countries [Citation24], probably caused by the European and Norwegian stewardship programmes and guidelines, and a wanted shift from fluoroquinolones as empiric treatment in primary care due to increasing resistance and warnings about adverse effects [Citation25,Citation26]. Overall antibiotic sales in Norway have decreased by 24% since 2012 [Citation2]. In the last 10 years, the resistance rates in urine cultured Escherichia coli have remained relatively stable with a slight increase for most antibiotics. For trimethoprim and cotrimoxazole, the resistance rates has been stable at approximately 20–25%. The rates for pivmecillinam has been at approximately 4–6%, whereas the rates have increased for ciprofloxacin from 4% to 9% [Citation2].
Antibiotic switch vs. treatment failure
Antibiotic treatment failure may be due to multiple factors, for example, antibiotic resistance, faulty diagnosis by the physician and/or poor compliance. As a consequence of our definition of antibiotic switch, prescriptions given as a result of laboratory findings and treatment for new infections with other organisms have been included in our analyses. As we did not have access to the diagnosis for each prescription, we wanted to reduce the risk of including recurrent or new infections by choosing 14 days as our cut-off of follow-up. Previous comparable studies have used 28–31 days as their limit [Citation27–30]. In a clinical setting, however, we consider 14 days to be sufficient for the patient to get a new doctor’s appointment and receive additional treatment if needed. We also differentiated between repeat prescriptions and antibiotic switches, as a prolonged treatment with the same antibiotic did not answer the question of adequate initial treatment in terms of antibiotic choice. It could, however, implicate a too short course of treatment, and suggest a need for longer treatment duration.
During the 11-year study period, the switch rate was 6.8% within the first 14 days after initial antibiotic treatment. As previously seen in a Norwegian study using data from NorPD [Citation31], our data show peaks in repeat prescription every seven days ( and Figure S1). Most Norwegian pharmacies are closed on Sundays; thus, every seventh day cannot be a Sunday if the initial treatment was dispensed on a weekday. Also, arranging a follow-up consultation in one or two weeks is common practice in Norwegian primary care.
Our findings are fairly similar to previous studies of treatment failure rates of empirical antibiotic treatment in general practice [Citation32,Citation33]. In a previous study examining UTI and treatment failure, Montelin et al. [Citation33] investigated men treated for UTI with pivmecillinam, nitrofurantoin or trimethoprim, and reported a 30% rate of re-consultation and/or re-prescription within 91 days after initial treatment, with day 14 being the median day of re-prescription. Tandan et al. [Citation29] found that re-consultation rates for men treated with antibiotics for UTIs were 18% (31 of 177 patients) within 30 days after initial treatment. Our findings suggest that the rate of antibiotic switch (11% within 28 days) and repeat prescriptions (19% within 28 days) was relatively low during the study period, and comparable to the switch and repeat prescription rates for UTI in female patients at 8–16% within 4 weeks [Citation27,Citation28].
Treatment duration and antibiotic type
As more studies are suggesting shorter optimal antibiotic treatment duration [Citation14,Citation15], our data also suggest no obvious benefit of treatment for more than 7 days. While treatment duration of 7 days or less was associated with higher crude OR for antibiotic switch compared to 8–14 days, the adjusted OR was lower. As current guidelines recommend 5–7 days of treatment for acute cystitis and 7–10 days of treatment for febrile/complicated UTI, one might expect that shorter courses might represent treatment for acute cystitis, while longer courses might represent treatment for febrile UTI. The switch rate of pivmecillinam, which may not reach therapeutic concentrations in the kidneys or the prostate [Citation34], was indeed slightly higher in the 10–14 DDD treatment (12.9%) compared with 2–9 DDD (11.6%). However, for trimethoprim and nitrofurantoin, which are not recommended for febrile UTI treatment, switch rates were considerably lower in 10–14 DDD treatments compared with 2–9 DDD treatments. Hence, the number of DDD does not seem to distinguish between acute cystitis and febrile UTI in our material.
The current Scandinavian guidelines rely heavily on the concept that narrow spectrum antibiotics targeted to a specific infecting organism are less likely to induce resistance than more broad-spectrum antibiotics, such as fluoroquinolones, and should be preferred when possible to reduce the increase in antimicrobial resistance [Citation35]. Even though the narrow-spectrum antibiotics nitrofurantoin, pivmecillinam and trimethoprim yielded higher odds for antibiotic switch than the more broad-spectrum alternatives cotrimoxazole and fluoroquinolones, we find that the rates are satisfactory low, and comparable to treatment of female UTI. The need for future randomised controlled studies investigating both duration of treatment and efficacy of narrow-spectrum antibiotic drugs for treatment of acute cystitis in male patients is evident.
Conclusion
This study gives doctors in primary care some observational evidence of the effectiveness of empirical antibiotic treatment of UTIs in men. Our findings suggest that regardless of the antibiotic prescribed, about 12.8% of the patients will get a second prescription, and 6.8% will get a different antibiotic than initially prescribed within 14 days. Although pivmecillinam, trimethoprim and nitrofurantoin were associated with higher risk of antibiotic switch than fluoroquinolones, cefalexin and cotrimoxazole, we did not find evidence to suggest that these drugs are inappropriate as first-line empirical treatment options for UTI treatment in male patients. As general practitioners should want to avoid excessive use of fluoroquinolones, the current Norwegian guidelines seem appropriate for empirical treatment of UTIs in male patients. There is, however, a great need for clinical trials on acute cystitis in men, exploring the effectiveness and safety of different antibiotics and treatment durations.
Supplemental Material
Download MS Word (115 KB)Acknowledgements
We would like to thank Ibrahimu Mdala for his help with the statistical analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- World Health Organization. Antimicrobial resistance: global report on surveillance. Geneva: World Health Organization; 2014.
- NORM/NORM-VET 2018. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Tromsø/Oslo: NORM/NORM-VET; 2019. ISSN:15002-2307 (print)/1890-9965 (electronic).
- Straand J, Rokstad KS, Sandvik H. Prescribing systemic antibiotics in general practice. A report from the Møre & Romsdal prescription study. Scand J Prim Health Care. 1998;16(2):121–127.
- Griebling TL. Urologic diseases in America project: trends in resource use for urinary tract infections in men. J Urol. 2005;173(4):1288–1294.
- Lipsky BA. Urinary tract infections in men. Epidemiology, pathophysiology, diagnosis, and treatment. Ann Intern Med. 1989;110(2):138–150.
- Caljouw MAA, den Elzen WPJ, Cools HJM, et al. Predictive factors of urinary tract infections among the oldest old in the general population. A population-based prospective follow-up study. BMC Med. 2011;9(1):57–57.
- Ahmed H, Farewell D, Jones HM, et al. Incidence and antibiotic prescribing for clinically diagnosed urinary tract infection in older adults in UK primary care, 2004–2014. PLoS One. 2018;13(1):e0190521.
- Gupta K, Grigoryan L, Trautner B. Urinary tract infection. Ann Intern Med. 2017;167(7):ITC49–ITC64.
- Naber KG, Bergman B, Bishop MC, et al. EAU guidelines for the management of urinary and male genital tract infections. Urinary Tract Infection (UTI) Working Group of the Health Care Office (HCO) of the European Association of Urology (EAU). Eur Urol. 2001;40(5):576–588.
- Wagenlehner FH, Fünfstück R, et al. Epidemiology, diagnostics, therapy and management of uncomplicated bacterial community acquired urinary tract infections in adults S3 Guideline Urinary Tract Infections Urologenportal. 2010. AWMF-Register-Nr. 043/044.
- Ulleryd P, Sandberg T. Ciprofloxacin for 2 or 4 weeks in the treatment of febrile urinary tract infection in men: a randomized trial with a 1 year follow-up. Scand J Infect Dis. 2003;35(1):34–39.
- van Nieuwkoop C, van der Starre WE, Stalenhoef JE, et al. Treatment duration of febrile urinary tract infection: a pragmatic randomized, double-blind, placebo-controlled non-inferiority trial in men and women. BMC Med. 2017;15(1):70.
- Trautner BW. New perspectives on urinary tract infection in men comment on “urinary tract infection in male veterans: treatment patterns and outcomes” and on “preoperative urine cultures at a veterans affairs medical center. JAMA Intern Med. 2013;173(1):68–70.
- Germanos GJ, Trautner BW, Zoorob RJ, et al. No clinical benefit to treating male urinary tract infection longer than seven days: an outpatient database study. Open Forum Infect Dis. 2019;6(6):ofz216.
- Drekonja DM, Rector TS, Cutting A, et al. Urinary tract infection in male veterans: treatment patterns and outcomes. JAMA Intern Med. 2013;173(1):62–68.
- Mospan GA, Wargo KA. 5-Day versus 10-day course of fluoroquinolones in outpatient males with a urinary tract infection (UTI). J Am Board Fam Med. 2016;29(6):654–662.
- Boel JB, Jansåker F, Hertz FB, et al. Treatment duration of pivmecillinam in men, non-pregnant and pregnant women for community-acquired urinary tract infections caused by Escherichia coli: a retrospective Danish cohort study. J Antimicrob Chemother. 2019;74(9):2767–2773.
- Ingalsbe ML, Wojciechowski AL, Smith KA, et al. Effectiveness and safety of nitrofurantoin in outpatient male veterans. Ther Adv Urol. 2015;7(4):186–193.
- Helsedirektoratet. Anitbiotikabruk i primaerhelsetjenesten. Nasjonal faglig retningslinje for antibiotikabruk i primaerhelsetjenesten. 2012.
- The Norwegian Institute of Public Health. The Norwegian Prescription Database 2019. Vol 2019. Available from: http://www.norpd.no.
- WHO Collaborating Centre for Drug Statistics Methology, Guidelines for ATC classification and DDD assignment 2019. Oslo, Norway, 2018.
- Statistics Norway 2019. Population, by sex, age, contents and year. Available from: http://www.ssb.no/en/statbank/table/07459.
- Rowe TA, Juthani-Mehta M. Diagnosis and management of urinary tract infection in older adults. Infect Dis Clin North Am. 2014;28(1):75–89.
- Appaneal HJ, Caffrey AR, Lopes VV, et al. National trends in the treatment of urinary tract infections among Veterans’ Affairs Community Living Center residents. Infect Control Hosp Epidemiol. 2019;40(10):1087–1093.
- Aypak C, Altunsoy A, Düzgün N. Empiric antibiotic therapy in acute uncomplicated urinary tract infections and fluoroquinolone resistance: a prospective observational study. Ann Clin Microbiol Antimicrob. 2009;8(1):27.
- Tandan M, Cormican M, Vellinga A. Adverse events of fluoroquinolones vs. other antimicrobials prescribed in primary care: a systematic review and meta-analysis of randomized controlled trials. Int J Antimicrob Agents. 2018;52(5):529–540.
- Bjerrum L, Dessau RB, Hallas J. Treatment failures after antibiotic therapy of uncomplicated urinary tract infections. A prescription database study. Scand J Prim Health Care. 2002;20(2):97–101.
- Lawrenson RA, Logie JW. Antibiotic failure in the treatment of urinary tract infections in young women. J Antimicrob Chemother. 2001;48(6):895–901.
- Tandan M, Duane S, Cormican M, et al. Reconsultation and antimicrobial treatment of urinary tract infection in male and female patients in general practice. Antibiotics (Basel, Switzerland). 2016;5(3):31.
- Goettsch WG, Janknegt R, Herings R. Increased treatment failure after 3-days’ courses of nitrofurantoin and trimethoprim for urinary tract infections in women: a population-based retrospective cohort study using the PHARMO database. Br J Clin Pharmacol. 2004;58(2):184–189.
- Blandhol M, Tysland T, Blix HS, et al. Antibiotic switch during treatment with antibiotics against respiratory tract infections in ambulatory care in Norway. Infect Dis (London, England). 2017;49(11–12):854–858.
- Currie CJ, Berni E, Jenkins-Jones S, et al. Antibiotic treatment failure in four common infections in UK primary care 1991–2012: longitudinal analysis. Br Med J. 2014;349(3):g5493–g5493.
- Montelin H, Forsman K-J, Tängdén T. Retrospective evaluation of nitrofurantoin and pivmecillinam for the treatment of lower urinary tract infections in men. PloS One. 2019;14(1):e0211098.
- Ulleryd P. Febrile urinary tract infection in men. Int J Antimicrob Agents. 2003;22:89–93.
- Peterson LR. Squeezing the antibiotic balloon: the impact of antimicrobial classes on emerging resistance. Clin Microbiol Infect. 2005;11(Suppl 5):4–16.