Abstract
Background
Coronavirus disease 2019 (COVID-19) ranges from a mild illness to acute respiratory distress syndrome (ARDS), multiorgan dysfunction, and death. Transplant recipients are vulnerable due to comorbidities and immunosuppressants that render them susceptible to infections. The information on COVID-19 in kidney transplant recipients remains limited to small case series.
Methods
A systematic literature search was conducted, and 12 case series totalling 204 kidney transplant recipients with COVID-19 were identified. Data were extracted, pooled and analysed.
Results
Most patients (74%) were men. The most frequent symptoms were fever (76%), cough (64%) and dyspnoea (43%). At admission, over 70% of the patients had abnormal radiological findings. Leukocyte counts were in the lower normal range. C-reactive protein, ferritin, and D-dimer were consistently increased. Treatments included lowering immunosuppression, hydroxychloroquine, antivirals, tocilizumab and intravenous immunoglobulins. Thirty-one percent of the patients were admitted to intensive care units (ICUs), and 16% required intubation. The overall mortality was 21.2%. Patients who died were significantly older than those who survived (61 ± 12 vs. 51 ± 15, p < .01). Logistic regression revealed that the odds for death increased by 4.3% for each additional year of age (odds ratio [OR] 1.043, 95% confidence interval [CI] 1.005–1.083, p value = .0265).
Conclusions
No substantial conclusions could be drawn on the efficacy of any particular treatment. More rigorous patient stratification is needed when analysing and reporting data to facilitate future meta-analyses.
Keywords:
Introduction
Coronavirus disease 2019 (COVID-19) is a clinical syndrome caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [Citation1]. The infection ranges from mild disease to severe, acute respiratory distress syndrome (ARDS) characterized by a hyperinflammatory syndrome, multiorgan dysfunction, and death [Citation2,Citation3]. The COVID-19 outbreak emerged in China in December 2019, spread rapidly over the globe, is an immense public health challenge, and was declared a pandemic by the World Health Organization (WHO) on 11 March 2020. The number of confirmed COVID-19 cases worldwide reported to the WHO as of 4 June 2020, exceeded 6,4 million, with almost 400,000 deaths [Citation4].
Given the short time elapsed since the start of the outbreak as well as the resulting immense medical, social and economic strain, the information on COVID-19 is both limited and rapidly evolving. Several risk factors predicting unfavourable outcomes have been identified. These include old age, diabetes, hypertension, chronic kidney disease, coronary heart disease and chronic lung disease [Citation5]. In addition, accumulating clinical, laboratory and pathology data suggest that an aberrant host immune response and the inflammatory cytokine storm play a key role for progression and mortality in COVID-19 patients [Citation3,Citation6]. Several ongoing trials are exploring various immunomodulating and immunosuppressive strategies to ameliorate the dysregulation of the immune response [Citation7,Citation8].
Kidney transplant recipients frequently have one or several risk factors, and, therefore, run a high risk for unfavourable outcome. Two major risk factors, i.e. hypertension and cardiovascular diseases, are particularly frequent in this patient group. In addition, these patients take combinations of immunosuppressive drugs, including calcineurin inhibitors, antimetabolites and steroids, which alter both the innate and adaptive immunity and render them more susceptible to bacterial and viral infections. On the other hand, the immunosuppressive medication may theoretically modulate and reduce the inflammatory response following SARS-CoV-2 infection.
Despite widespread concern about the potential for high prevalence and severity of COVID-19 among renal transplant recipients, aside from a few small case series, a more extensive analysis of this population is lacking [Citation9–20]. Whereas the mortality of COVID-19 in the general population is unclear and under intense scrutiny and debate [Citation21,Citation22], the most extensive analysis to date from a mixed transplant population indicates an early mortality of 24% for inpatients [Citation23]. In contrast, a recent study of 8910 inpatients with COVID-19 in the general population and similar median age, showed a mortality rate of 5.8% [Citation8].
Case series do not allow in-depth analyses and firm conclusions due to inherent limitations, such as small sample size and centre bias. However, many reports provide detailed and valuable information on individual patients. Herein, we attempted to overcome the inherent restrictions of these limited, single-centre observations by identifying and pooling both individual and aggregated data from the available case-series. We aimed to provide a multicentre account based on a larger patient group that may hopefully overcome some type 1 and 2 errors and offer a more reliable insight into the statistics and risk factors of COVID-19 in kidney transplant patients.
Materials and methods
A systematic review was conducted using the PRISMA guidelines. We performed a literature search of the MEDLINE/PubMed and Excerpta Medica dataBASE (EMBASE) using the search terms: (‘COVID-19’[All Fields] OR ‘COVID-2019’[All Fields] OR ‘severe acute respiratory syndrome coronavirus 2’[Supplementary Concept] OR ‘severe acute respiratory syndrome coronavirus 2’[All Fields] OR ‘2019-nCoV’[All Fields] OR ‘SARS-CoV-2’[All Fields] OR ‘2019nCoV’[All Fields] OR ((‘Wuhan’[All Fields] AND (‘coronavirus’[MeSH Terms] OR ‘transplants’[All Fields] OR ‘transplant’[All Fields] OR ‘transplantation’[MeSH Terms] OR ‘transplantation’[All Fields]). All publications fitting these criteria published between 1 January 2020 and 4 June 2020 were retrieved. Two researchers (JB and MO) independently reviewed the titles and abstracts of the articles, and all studies in which kidney transplantations were reported were identified. We excluded reports with less than five cases and reports in which data from kidney transplant recipients were presented in aggregated form along with data from other types of organ transplant recipients. After omitting redundant studies, the full texts of the studies were assessed against the eligibility criteria. We collected information on patient age, gender, comorbidities, clinical presentation, baseline immunosuppression and patient management during COVID-19. Whenever possible, individual participant data (IPD) were retrieved. Aggregate data concerning only kidney transplant recipients were also used.
Quantitative data are shown as mean ± standard deviation (SD) or as median (range) while qualitative variables are expressed as absolute and relative frequencies. Continuous variables were compared using the Mann–Whitney U test.
Logistic regression with a step-wise selection of factors to include in the model (the threshold for inclusion of factor was p < .3 and for keeping variables p < .35) was performed for the outcome of death, and the composite endpoints death or intubation with mechanical ventilation, and death, mechanical ventilation or intensive care unit (ICU) admission. In order to avoid losing patients for whom information was lacking, a separate level termed ‘unknown’ was introduced for categorical data that were missing. The resulting models included age (indicating higher odds for event with increasing age) regardless of endpoint. For death, age was the only variable included in the model. For death or mechanical ventilation, and for death, mechanical ventilation or ICU admittance, we included a variable indicating whether the patient was within the first year since transplantation. SAS version 9.4 (SAS Insitute, Cary, NC) was used for all statistical analyses.
Results
Literature search results
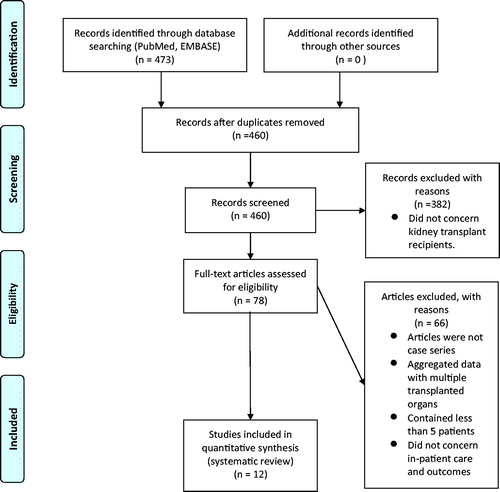
We found a total of 473 articles amongst which 13 duplicates were identified and removed (). The remaining 460 articles were screened by title and abstract, resulting in identification of 78 articles related to kidney transplantation. The remaining 382 items were removed. Among the 78 articles concerning kidney transplantation, we found 24 publications describing the clinical course of COVID-19 in kidney transplant recipients. Of these, only 12 included five or more patients. Twelve smaller studies (i.e. case reports) were removed due to concern about publication bias. The remaining 12 publications showed no signs of duplicate patients or data. This resulted in the identification of 204 kidney transplanted patients with PCR-confirmed Covid-19.
Patients
A total of 204 patients were reported in the 12 case series. The number of patients in the case series varied between five and 36 (). Information on 87 patients was available as individual patient data (IPD) and formed the basis of our statistical analysis. Both IPD and aggregated data were used for descriptive statistics.
Table 1. The reports included in the analysis.
The mean age of the patient groups in the 12 reports analysed herein ranged between 49.6 and 61 years. The mean age of the entire patient group was 53 ± 15 years, whereas the median age was 54 years (range 21–80). In all the reports the majority were men who represented over 70% of the cases (). The absolute majority of the kidney grafts came from deceased donors, and only 9% of the transplants were performed in the year preceding the COVID-19 infection.
Table 2. Clinical characteristics, findings at presentation, treatment and outcome of kidney transplant recipients with COVID-19.
More than 80% of the subjects had a diagnosis of hypertension, whereas diabetes was present in about half of the subjects. The frequency of smoking and lung diseases was low.
Non-survivors had significantly higher median Charlson comorbidity index (3, range 0–6) compared to survivors (2, range 0–7) (p = .02).
Clinical presentation and findings at admission
The most common presenting symptom was fever, which was reported in over 70% of the cases, followed by cough (66%) and dyspnoea (43%) (). A combination of at least two of these major symptoms was universal. Digestive symptoms (diarrhoea) and myalgia were present in 22% of the cases. Malaise, chills and fatigue were also reported. The time period between symptom onset and diagnosis varied greatly, from two days to up to four weeks. Still, admission to hospital generally occurred within two weeks from the initial symptoms.
Over 75% of the cases displayed abnormal radiological findings at admission including ground-glass opacities or focal, nodular consolidations and frequently bilateral. Despite lower diagnostic yield, bed-side, plain x-ray examinations were favoured both at admission and during hospitalization while computed tomography examinations were deliberately limited due to the risk of intra-hospital transmission of COVID-19 during patient transfers.
Laboratory data at the time of diagnosis revealed a similar pattern in the studies analysed. In most reports, white blood cell count was in the lower normal range, and lactate dehydrogenase was marginally increased. An increase in C-reactive protein, ferritin and D-dimer were reported in all the articles in which this information was presented. Most patients displayed impaired renal function (increased creatinine) at admission and about one third developed signs of acute kidney injury.
Treatment
Most patients (86%) in these case series were hospitalized, but at least 28 patients were managed on an outpatient basis. The absolute majority of the patients required supportive therapy, ranging from oxygen supplementation to mechanical ventilation.
Due to the lack of consensus and evidence concerning antiviral therapies against SARS-CoV-2, management protocols varied significantly between centres especially in the earliest stages of the pandemic. Several expert opinion guidelines were developed jointly by different organ transplant groups and made available in late March 2020, possibly leading to a more consistent approach.
Immunosuppression was lowered in two-thirds of the patients. The first change in immunosuppression in the majority of patients was the reduction or complete cessation of antimetabolites (). In addition, the dose of tacrolimus was reduced to a target through level around 5 ng/mL. In patients receiving antivirals (ritonavir/lopinavir), calcineurin inhibitors were discontinued due to pharmacologic interactions.
A majority of the patients received hydroxychloroquine in various doses (i.e. 200 or 400 mg b.i.d), while caution was exercised in individuals with pre-existing QT prolongation or at risk for QT prolongation. Azithromycin was used initially but was not used in more recent cases.
Further therapeutic considerations included the addition of antivirals (most frequently lopinavir/ritonavir) or steroids in bolus or high dose, IVIG infusion and tocilizumab to counteract the deleterious cytokine activity.
Outcome
Thirty-four percent of the kidney transplant recipients with COVID-19 were admitted to an ICU, and 19.7% required mechanical ventilation. Forty-three out of the 204 patients (21%) died. Patients who died were significantly older than those who survived (61 ± 12 years vs. 51 ± 15 years, p < .01). Among the patients requiring intubation, 72.7% died.
Sixty-three patients were discharged, and 97 patients were still hospitalized when the reports were submitted for publication ().
Old age was strongly related to both death (OR 1.043, 95% CI 1.005–1.083, p = .0265) and the two combined outcomes death or intubation (OR 1.035, 95% CI 1.001–1.071, p = .0436) and death, intubation and ICU admittance (OR 1.039, 95% CI 1.005–1.073, p = .0241).
Graft outcomes were generally poorly described, and only one rejection episode was clearly reported. Biopsies were avoided in spite of the combination of lowered immunosuppression and evidence of renal dysfunction. Thirteen patients required continuous renal replacement therapy upon admission.
Discussion
This systematic review is the most extensive analysis to date on COVID-19 in kidney transplant recipients by summarizing information from twelve case series coming from nine centres in six different countries. Although the clinical presentation appeared similar to that of the general population, the mortality rate was 21.2% in this particular patient group. This key finding is similar to the observations in a mixed population of transplant recipients from New York City, showing 24% mortality among inpatients in the largest study published so far [Citation23]. For comparison, the largest study of the general population presented to date reported 1023 deaths among 44,672 confirmed cases from Wuhan (without stratification for disease severity), resulting in an overall mortality of 2.3% [Citation22]. Although high mortality rates have been observed in several risk groups of hospitalized patients [Citation24,Citation25], one of the highest reported rates of unfavourable outcomes of COVID-19 appears to occur in transplant recipients. Considering that almost half of the patients included in the case-series reviewed were still hospitalized and thus counted as alive at the time when the reports were submitted for publication, any additional deaths that may have occurred would further increase the mortality. This may also imply that the real odds ratio (OR) for death and death or intubation could turn out to be higher than the results presented herein which were calculated using the published data. The very high mortality of transplanted patients requiring mechanical ventilation appears similar to that observed in non-transplanted patients [Citation22].
Advanced patient age has been repeatedly shown to be a risk factor for unfavourable outcome following SARS-CoV-2 infection [Citation5,Citation22,Citation25]. Our results are the first to show that among kidney transplant recipients with COVID-19 mortality was significantly higher in older patients than in younger ones. In addition, the analysis revealed that nonsurvivors had more comorbidities and a significantly higher CCI. This is in line with previous reports showing a higher risk of death of sepsis patients with a CCI higher than 2 [Citation26].
The reduction of immunosuppression was one of the most frequent interventions in this patient group. Withdrawal of antimetabolites and transition to corticosteroid monotherapy were cornerstones of patient management. This approach is similar in other circumstances when the recipient’s immune system is allowed to recover to fight a viral infection, but this may also increase the risk of acute rejection [Citation27]. The increase in corticosteroids may have provided additional benefit for outcome in renal transplant recipients. This is supported by the recent RECOVERY trial, in which treatment with dexamethasone lead to improved survival in patients from the general population with a severe COVID-19 infection [Citation28].
Although the prevalence of renal dysfunction was high, and the patients were on low or no immunosuppression, no graft biopsies were performed. Whereas only one acute rejection episode was reported, one has to take into account the short follow-up time in all studies as acute rejection may take some time to develop. Performing a biopsy in a patient infected with COVID-19 is associated with a potential SARS-CoV-2 exposure in the health care facility and the risk-benefit ratio must be considered under these circumstances [Citation29]. Several patients developed supratherapeutic tacrolimus levels contributing to nephrotoxicity due to pharmacologic interactions and required continuous renal replacement therapy. While this may be due to other factors as well, it is difficult to rule out that some patients may have developed an acute rejection. Rejection should be kept in mind as a cause of renal dysfunction together with infection-related kidney involvement seen in about one-third of the hospitalized, non-transplanted COVID-19 patients [Citation30,Citation31]. As SARS-CoV-2 binds to ACE2 protein which is abundantly expressed on the proximal tubule and in podocytes, it is conceivable that the virus, apart from systemic inflammation, may directly affect the kidneys.
No significant conclusions could be drawn from this analysis concerning the efficacy of any particular therapeutic intervention. In addition to lowering immunosuppression, most of the patients received hydroxychloroquine. Emerging evidence indicates a lack of benefit for hydroxychloroquine in the management of COVID-19 [Citation28,Citation32,Citation33]. While hydroxychloroquine is not strictly immunosuppressive, it has complex immunomodulatory effects. By affecting both the innate and the adaptive immune system, it ultimately alters both T-cell and B-cell responses [Citation34]. As some centres adopted hydroxychloroquine-free protocols already in the early stage of the COVID-19 pandemic [Citation35] it could have been interesting to comparatively assess the impact of hydroxychloroquine on the outcome of transplant patients. However, data from Solidarity, a multinational clinical trial organized by the WHO to compare untested treatments for severe COVID-19 and the recently announced results from the UK’s Recovery trial [Citation28,Citation36] indicate that hydroxychloroquine does not improve mortality of hospitalized COVID-19 patients, when compared with standard of care. Hence, such an analysis is no longer mandated. Similarly, the addition of tocilizumab, a recombinant humanized anti-interleukin (IL)-6 receptor monoclonal antibody did not show any benefit in this series, possibly because it was given to sicker patients with higher IL-6 levels (data not shown).
The majority of the patients reviewed herein were hospitalized, which may have introduced a selection bias towards sicker patients as it is thought that mild or moderate disease is more likely to be managed on an outpatient basis [Citation15,Citation29]. Whereas some reports have not explicitly described patient disease severity, it is apparent that many patients had very severe disease. As COVID-19 outcome is closely related to disease severity, the dismal results reported in many of these series may have been due to a selection bias towards cases with more advanced disease.
This analysis bears the numerous limitations of its constituent reports. Although the absolute majority of the patients were hospitalized, thus bringing some homogeneity to the cohort, the severity of the COVID-19 disease remained mostly unaccounted for. One report found that the SOAR (systolic blood pressure, oxygenation, age and respiratory rate) pneumonia score was lower in survivors, which was the only variable among almost fifty various parameters differing significantly between surviving and non-surviving renal transplant recipients [Citation18]. Any studies comparing therapies and outcomes in this or other patient groups should include COVID-19 severity in their reports. Additionally, the patient cohorts were heterogeneous with respect to ethnicity, comorbidities and concurrent medications. Another significant limitation is the very short time-frame covered by all the studies and the high proportion of COVID-19 patients still hospitalized. This limitation is present in studies of both the general population and the transplant recipients and leaves several questions related to the long term outcomes unanswered. The possibility of re-infection and other key epidemiological outcome measures (e.g. case fatality ratio) remain unclear.
In conclusion, this analysis indicates that transplanted patients with COVID-19 share several clinical characteristics with the general population, such as a higher proportion of men and advanced age risk as risk factors. The main finding, however, is the very high mortality in hospitalized kidney transplant recipients with COVID-19, which increases with age.
| Abbreviations | ||
| AR | = | acute rejection |
| ARDS | = | acute respiratory distress syndrome |
| CI | = | confidence interval |
| CCI | = | Charlson comorbidity index |
| COVID-19 | = | Coronavirus disease 2019 |
| ICU | = | intensive care unit |
| IPD | = | individual patient data |
| IL | = | interleukin |
| SARS-CoV-2 | = | severe acute respiratory syndrome coronavirus 2 |
| WHO | = | World Health Organization |
Acknowledgements
This study is dedicated to the memory of all healthcare personnel who lost their lives while caring for COVID-19 patients worldwide.
Disclosure statement
There are no conflicts of interest to be reported.
References
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513.
- Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000.e3
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
- Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200604-covid-19-sitrep-136.pdf?sfvrsn=fd36550b_2
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395(10229):1054–1062.
- Lin L, Lu L, Cao W, et al. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727–732.
- Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7):102568.
- Mehta PM, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034.
- Abrishami A, Samavat S, Behnam B, et al. Clinical course, imaging features, and outcomes of COVID-19 in kidney transplant recipients. Eur Urol. 2020. DOI:10.1016/j.eururo.2020.04.064
- Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477.
- Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97:1083–1088.
- Banerjee D, Popoola J, Shah S, et al. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020; 97:1076–1082.
- Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20(7):1849–1858.
- Columbia University Kidney Transplant Program. Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol. 2020;31:1150–1156.
- Husain SA, Dube G, Morris H, et al. Early outcomes of outpatient management of kidney transplant recipients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020. DOI:10.2215/CJN.05170420
- Montagud-Marrahi E, Cofan F, Torregrosa JV, et al. Preliminary data on outcomes of SARS-CoV-2 infection in a Spanish single centre cohort of kidney recipients. Am J Transplant. 2020. DOI:10.1111/ajt.15970
- Nair V, Jandovitz N, Hirsch JS, et al. COVID-19 in kidney transplant recipients. Am J Transplant. 2020;20(7):1819–1825.
- Trujillo H, Caravaca-Fontán F, Sevillano Á, et al. SARS-CoV-2 infection in hospitalized patients with kidney disease. Kidney Int Rep. 2020;5:905–909.
- Zhang H, Chen Y, Yuan Q, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020;77:742–747.
- Zhu L, Gong N, Liu B, et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77:748–754.
- Richardson S, Hirsch JS, Narasimhan M, Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):e206775.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239.
- Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808.
- Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091.
- Hou W, Zhang W, Jin R, et al. Risk factors for disease progression in hospitalized patients with COVID-19: a retrospective cohort study. Infect Dis (Lond). 2020;52(7):498–505.
- Oltean S, Ţǎţulescu D, Bondor C, et al. Charlson’s weighted index of comorbidities is useful in assessing the risk of death in septic patients. J Crit Care. 2012;27(4):370–375.
- Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-guidelines of the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9):e13512.
- Effect of dexamethasone in hospitalized patients with COVID-19—preliminary report. 22 Jun 2020. Available from: https://www.medrxiv.org/content/10.1101/2020.06.22.20137273v1.full.pdf
- Gleeson SE, Formica RN, Marin EP. Outpatient management of the kidney transplant recipient during the SARS-CoV-2 virus pandemic. Clin J Am Soc Nephrol. 2020;15:892–895.
- Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218.
- Batlle D, Soler MJ, Sparks MA, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31(7):1380–1383.
- Chowdhury MS, Rathod J, Gernsheimer J. A Rapid Systematic Review of Clinical Trials Utilizing Chloroquine and Hydroxychloroquine as a Treatment for COVID-19. Acad Emerg Med. 2020;27(6):493–504.
- Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849.
- Meyerowitz EA, Vannier AGL, Friesen MGN, et al. Rethinking the role of hydroxychloroquine in the treatment of COVID-19. FASEB J. 2020;34(5):6027–6037.
- Hoek RAS, Manintveld OC, Betjes MGH, et al. Covid-19 in solid organ transplant recipients: a single center experience. Transpl Int. 2020. DOI:10.1111/tri.13662
- World Health Organization. Targeted update: safety and efficacy of hydroxychloroquine or chloroquine for treatment of COVID-19. Available from: https://www.who.int/publications/m/item/targeted-update-safety-and-efficacy-of-hydroxychloroquine-or-chloroquine-for-treatment-of-covid-19