Abstract
Background
Staphylococcus aureus bacteraemia (SAB) is recognized as an infection that is difficult to treat and with high risk of device related infection. Extraction/explantation of cardiac implantable electronic devices (CIED) is recommended in SAB patients but studies evaluating long-term prognosis are scarce.
Materials and methods
In this retrospective cohort study, 626 consecutive SAB patients were identified in routine diagnostics (November 2014–October 2016). Patient characteristic, infective endocarditis (IE) incidence and mortality were compared for patients with and without CIED.
Results
SAB patients with CIED (n = 33) compared to non-CIED patients (n = 593) were older (83 versus 70 years, p = .0001), had a higher 30-day mortality (12/33, 36% versus 119/593, 20%, p = .044) and higher incidence of IE (9/33, 27% versus 41/593, 7%, p = .0006). One-year mortality was 19/33 (58%) among the SAB CIED patients. Echocardiography was performed in all nine patients with CIED-IE but only in 14/24 (58%) of the 24 SAB CIED patients that were considered not having IE. However, if patients with very early mortality were excluded, echocardiography was performed in 14/17 (82%) of SAB CIED-non-IE patients. CIED extraction/explantation during intravenous antibiotic treatment was only performed in three patients with SAB CIED-IE and in one non-IE patient. One year post treatment initiation, 14 out of 33 SAB CIED patients were alive of whom only one had CIED extraction/explantation performed as part of treatment.
Conclusion
Staphylococcus aureus bacteraemia in CIED patients is associated with poor prognosis but in a subgroup of patients survival beyond one year was seen despite retainment of the electronic device.
Introduction
Staphylococcus aureus bacteraemia (SAB) is recognized as an infection that is difficult to treat and associated with severe complications and high mortality [Citation1]. Large variations are seen between different studies in respect of patient characteristics, management and outcome [Citation2,Citation3]. SAB carries a high risk of foreign body engagement subsequent to haematogenous bacterial seeding of biofilms and removal of infected devices is a cornerstone of SAB management. Extraction/explantation of cardiac implantable electronic devices (CIED) in patients who experience a blood stream infection with Staphylococcus aureus is recommended even if objective signs of device involvement (pocket infection, lead vegetations) are not present according to European consensus statement, and for patients with persistent bacteraemia in HRS recommendations [Citation4,Citation5]. The benefits of these recommendations are well recognized but prospective randomized studies on CIED removal in SAB patients are lacking as are studies evaluating long-term prognosis in these patients. Strategies to identify patients in whom CIED removal may not be necessary remains to be established.
Prospective antibiotic treatment studies are rare in SAB but a randomized, double-blind, placebo-controlled trial comparing standard intravenous treatment to standard intravenous treatment in combination with rifampicin failed to show improved outcome in the combination treatment arm group [Citation6]. In a post-hoc analysis of a multinational cohort of SAB patients, combination antibiotic treatment was not linked to better outcome except for a possible association to lower mortality and fewer SAB-related late complications in the subgroup of patients with implanted foreign bodies/devices [Citation7].
The aim of this study was to explore long-term outcome of CIED patients with SAB in an unselected population of consecutive patients. A secondary aim was to correlate patient outcome to device extraction/explantation and intravenous antibiotic treatment duration.
Materials and methods
This is a retrospective cohort study which included consecutive patients with at least one positive blood culture with Staphylococcus aureus (SAB patient) identified from the Laboratory Database at the Department of Clinical Microbiology, Sahlgrenska University Hospital, Gothenburg, Sweden, during a 2 year period (November 2014–October 2016). Each patient was included only once and individuals with multiple positive blood cultures or relapse of SAB within the study period were not re-included. Other microbiological tests such as pocket and electrode cultures were not recorded. Electronic medical records for all patients were reviewed and variables entered into a database. Demographical data, mode of acquisition (community/nosocomial), comorbidities and 30-day mortality were collected in all SAB patients. In patients with CIED and SAB additional data were collected and registered regarding type of device, time since implantation and latest device manipulation as well as echocardiography execution and findings, duration of intravenous antibiotic therapy and 1-year mortality. Nosocomial acquisition was defined as first positive blood culture collected >48 h after admission or in case of readmission ≤5 days from previous discharge.
For basic data, SAB patients with CIED were compared to SAB patients without CIED. Further, CIED patients with an established diagnosis of infective endocarditis (SAB CIED-IE) were compared to CIED patients without infective endocarditis (SAB CIED-non-IE) including antibiotic treatment duration and extraction/explantation of CIED. Infective endocarditis was considered present when this diagnose was stated in the electronical medical record, also considering electrode vegetation as major criteria.
Chi-square test was used to compare proportions and for categorical variables Mann–Whitney U test or t-test were used as appropriate. 30-day mortality was calculated with log-rank test (Mantel-Cox). p value <.05 was considered significant. Statistical analyses were performed with Graphpad Prism 7 (Graphpad Software, Inc., San Diego, CA). The study was approved by the Regional Ethical Review Authority (EPN 1126-16).
Results
Staphylococcus aureus bacteraemia was identified in 626 patients of whom 33 (5%) had a concomitant CIED (). All electronic devices except two were pacemakers, the remaining being implantable cardioverter defibrillators. SAB patients with CIED were older (83 versus 70 years, p = .0001) and had a higher 30-day mortality (12/33, 36% versus 119/593, 20%, p = .044) compared to non-CIED patients. The number of infective endocarditis was 9/33 (27%) among CIED patients compared to 41/593 (7%) in non-CIED patients (p = .0006) but echocardiography was not performed in all patients and inappropriate or underdiagnosis of IE is possible in both groups (). One patient with CIED IE had a concomitant prosthetic valve endocarditis. Nosocomial SAB acquisition did not differ significantly between patients with and without presence of CIED (8/33, 24%, versus 206/593, 35%, p = .26) nor between CIED patients with and without infective endocarditis (3/9, 33%, versus 5/24, 21%, p = 1.00). Five patients (<1%) had methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia, none of these being in the CIED group.
Figure 1. Flowchart of patients with Staphylococcus aureus bacteraemia included in the study. SAB: Staphylococcus aureus bacteraemia; CIED: cardiac implantable electronic device; IE: infective endocarditis.
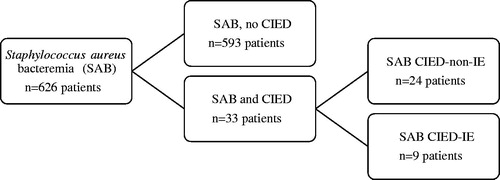
Table 1. Basic characteristics of Staphylococcus aureus bacteraemia patients with and without cardiac implantable electronic devices.
Of the 33 SAB patients with CIED, nine were diagnosed with infective endocarditis (SAB CIED-IE) while 24 patients did not meet the criteria for endocarditis (SAB CIED-non-IE) (). Vegetations were found in six of the SAB CIED-IE patients (lead and/or valve), the remaining being classified as infective endocarditis by presence of minor criteria. There was no age difference between SAB CIED-IE patients and SAB CIED-non-IE patients and no significant difference regarding number of device years or time since last device manipulation was detected. Echocardiography was performed in all nine patients diagnosed as SAB CIED-IE (transoesophageal echocardiography in 6/9) but only in 14/24 (58%) of the patients that were considered as SAB CIED-non-IE. If patients with very early mortality (≤2 days of SAB blood culture collection) or on palliative care were excluded, echocardiography was performed in 14/17 (82%) of SAB CIED-non-IE patients. In SAB CIED-IE, the first echocardiographic exam was performed median day four after index blood culture, in SAB CIED-non-IE on day six.
Table 2. Comparison of patients with Staphylococcus aureus bacteraemia and cardiac implantable electronic device with and without infective endocarditis.
Mortality within 30 days of SAB was 36% (12/33) in the SAB CIED patients and a majority (58%, 19/33) were dead within one year regardless of initially being diagnosed with endocarditis or not. Among the 24 SAB CIED-non-IE patients, six died within two days of blood culture collection and one died in hospice on day four. Hence, the 17 SAB CIED-non-IE patients surviving beyond the very early phase had a 30-day mortality of 2/17 (12%) and a 1-year mortality, 6/17 (35%).
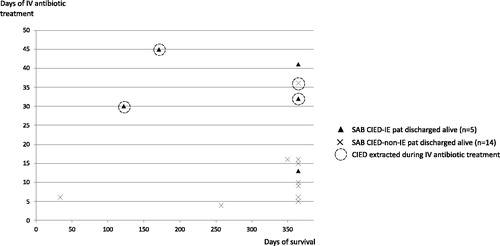
CIED extraction/explantation during intravenous antibiotic treatment was performed in three patients with SAB CIED-IE and in one of the non-IE patients (median day 19). The median (IQR) duration of intravenous antibiotic treatment in the 19 patients that were discharged alive was 31 (19–41) days in SAB CIED-IE patients and 10 (6–16) days in SAB CIED-non-IE. One year post-treatment initiation, 14 out of 33 SAB-CIED patients were alive of whom only one had CIED extraction/explantation performed as part of initial treatment (). Hence, 13 patients survived beyond one year despite that CIED extraction/explantation was not performed and intravenous antibiotic treatment was median 10 days in this subgroup of non-extracted survivors.
Figure 2. Relation between days of intravenous antibiotic treatment and post-discharge survival in CIED patients with Staphylococcus aureus bacteraemia with (n = 5) or without infective endocarditis (n = 14). IV: intravenous.
Empirical antibiotic treatment (data not shown) was cefotaxime in 20 out of 33 SAB-CIED patients, later switched to cloxacillin in a majority (13 patients) of cases. Other empirical treatment choices were piperacillin/tazobactam (7 patients), cloxacillin (4 patients), and meropenem (1 patient). One patient died before antibiotic was started. Combination treatment, with the exception of sporadic days, was not used in any patient.
Discussion
This work demonstrates that Staphylococcus aureus bacteraemia in CIED patients is associated with poor prognosis. One-third of patients died in hospital and less than 50% of SAB CIED patients survived beyond one year, irrespective of initial infective endocarditis diagnosis or not. Mortality in the SAB CIED group of patients was higher compared to mortality among patients not having a CIED. However, SAB CIED patients were also significantly older compared to the general SAB population, and age has consistently been shown to correlate to in-hospital as well as long-term mortality [Citation3,Citation8,Citation9]. A higher age among CIED patients with SAB is not unexpected since prevalence of cardiac electronic devices correlate strongly to age [Citation10] but age-correlated SAB incidence in CIED patients has to our knowledge not been reported elsewhere. A similar age span for patients with CIED infections was reported by Tan et al. [Citation11], including both systemic and pocket infections in that study, while other studies report a lower median age in patients with CIED infections [Citation12]. Identification of SAB patients from consecutive positive blood cultures found in routine laboratory diagnostics probably captures a different population compared to studies including patients on the basis of suspected/possible CIED infection.
The discouraging mortality rates in our SAB study population are in line with other recent studies [Citation8,Citation13]. Despite older age in SAB-CIED patients compared to the SAB population as a whole, a subgroup with better prognosis could be identified among patients surviving the first few days of SAB onset and not having CIED endocarditis. In fact, infection-related death due to SAB after 30 days was rare in a prospective Norwegian cohort study and the authors stated that follow up beyond 30 days unlikely added any significant information with respect to infection-related mortality. The aspect of device related infection was not specifically addressed [Citation9]. Delay from onset of symptoms to SAB diagnosis is often considered a risk factor for device seeding and poor prognosis. This delay was not registered in our study but there was no difference in the proportion of community-onset and nosocomial SAB between CIED patients diagnosed with infective endocarditis or not.
Echocardiography was performed in all patients with a verified CIED IE but only in 58% of patients without IE diagnosis, hence, an underdiagnosis of IE in that group is possible. Additionally, interpretation of a sterile thrombus or “strand” on a lead as a vegetation is a well-documented phenomenon [Citation14]. Noteworthy, among the 24 non-IE patients in our material, seven patients died so early that echocardiography was not feasible and among the remaining patients, echocardiography was performed in more than 80% mirroring a real world application of consensus guidelines [Citation15,Citation16] and national recommendations [Citation17]. In a prospective cohort of 2008 SAB patients described by Le Moing et al. [Citation18], 67% of all patients had an echocardiographic examination done. Of all SAB patients in that study population, 10% were CIED carriers compared to 5% of SAB patients in our study, and 20% of the French SAB CIED patients were diagnosed with CIED infective endocarditis comparable to the 27% seen in the present study. The proportion of SAB patients with an intracardiac electronic device developing a CIED infection is reported to be 34–45% in previous studies [Citation19,Citation20], which is a rational for recommendations of device extraction/explanation in patients with SAB also in the absence of echocardiographic verified lead engagement [Citation4,Citation5].
The most interesting finding in our study was that in SAB CIED patients without diagnosed infective endocarditis, discharged alive and surviving beyond one year, this favourable outcome was seen despite that CIED extraction/explantation was not undertaken in 13 of 14 patients. Intravenous antibiotic treatment was median 10 days in this subgroup of non-extracted survivors, which is interesting and raises questions regarding individualized treatment duration. On the other hand, among nine patients diagnosed as CIED-IE not more than three patients had their devices removed and 1-year mortality was as high as 67%.
The present report suffers from several limitations, most importantly being a small cohort not allowing for multivariate regression analysis. We were, therefore, unable to identify independent factors associated with a favourable outcome. An additional limitation is that patient variables of interest, such as septic shock at presentation and time to blood culture negativity, were not recorded and 1-year mortality only extracted for patients with SAB CIED, not the total SAB population. Another limitation is that all patients did not undergo echocardiography and that echocardiographic examinations were not systematically re-evaluated considering that non-infected structures on leads might have been classified as vegetations. Standard SAB guidelines were not fully implemented during the study period (i.e. systematic follow up on positive blood cultures) and casual relation between patient management and outcome cannot be established.
Conclusion
Staphylococcus aureus bacteraemia in CIED patients was associated with poor prognosis but in a subgroup of patients survival beyond one year was seen despite retainment of the electronic device. This finding needs to be further evaluated in larger prospective cohorts.
Disclosure statement
The authors report no conflict of interest.
References
- Thwaites GE, Edgeworth JD, Gkrania-Klotsas E, UK Clinical Infection Research Group, et al. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis. 2011;11(3):208–222.
- Nambiar K, Seifert H, Rieg S, International Staphylococcus aureus collaboration (ISAC) study group (with linked authorship to members in the Acknowledgements) and the ESCMID Study Group for Bloodstream Infections and Sepsis (ESGBIS), et al. Survival following Staphylococcus aureus bloodstream infection: a prospective multinational cohort study assessing the impact of place of care. J Infect. 2018;77(6):516–525.
- Asgeirsson H, Thalme A, Weiland O. Staphylococcus aureus bacteraemia and endocarditis – epidemiology and outcome: a review. Infect Dis (Lond). 2018;50(3):175–192.
- Blomstrom-Lundqvist C, Traykov V, Erba PA, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg. 2019;14:515–549.
- Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14(12):e503–e551.
- Thwaites GE, Scarborough M, Szubert A, United Kingdom Clinical Infection Research Group (UKCIRG), et al. Adjunctive rifampicin for Staphylococcus aureus bacteraemia (ARREST): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2018;391(10121):668–678.
- Rieg S, Joost I, Weiss V, et al. Combination antimicrobial therapy in patients with Staphylococcus aureus bacteremia – a post hoc analysis in 964 prospectively evaluated patients. Clin Microbiol Infect. 2016;8:30370–30376.
- Jacobsson G, Nasic S. Long-term outcome of invasive Staphylococcus aureus infections. Scand J Infect Dis. 2012;44(5):350–354.
- Eskesen AN, Belle MA, Blomfeldt A. Predictors of one-year all-cause mortality and infection-related mortality in patients with Staphylococcus aureus bacteraemia. Infect Dis (Lond). 2018;50(10):743–748.
- Annual Statistical Report 2018 Swedish ICD and Pacemaker Registry: Karolinska Hospital, Department of Cardiology; 2018.
- Tan EM, DeSimone DC, Sohail MR, et al. Outcomes in patients with cardiovascular implantable electronic device infection managed with chronic antibiotic suppression. Clin Infect Dis. 2017;64(11):1516–1521.
- Olsen T, Jorgensen OD, Nielsen JC, et al. Incidence of device-related infection in 97 750 patients: clinical data from the complete Danish device-cohort (1982–2018) . Eur Heart J. 2019;40(23):1862–1869.
- Yahav D, Yassin S, Shaked H, et al. Risk factors for long-term mortality of Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis. 2016;35(5):785–790.
- Downey BC, Juselius WE, Pandian NG, et al. Incidence and significance of pacemaker and implantable cardioverter-defibrillator lead masses discovered during transesophageal echocardiography. Pacing Clin Electrophysiol. 2011;34(6):679–683.
- Baddour LM, Wilson WR, Bayer AS, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435–1486.
- Habib G, Lancellotti P, Antunes MJ, ESC Scientific Document Group, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075–3128.
- Diseases SSoI. Swedish guidelines on the management of infective endocarditis 2016 Swedish Society of Infectious Diseases. [cited 2020 Jul 22]. Available from: https://infektion.net/vardprogram/endokardit/2016
- Le Moing V, Alla F, Doco-Lecompte T, VIRSTA Study Group, et al. Staphylococcus aureus bloodstream infection and endocarditis – a prospective cohort study. PLoS One. 2015;10(5):e0127385.
- Uslan DZ, Dowsley TF, Sohail MR, et al. Cardiovascular implantable electronic device infection in patients with Staphylococcus aureus bacteremia. Pacing Clin Electrophysiol. 2010;33(4):407–413.
- Sohail MR, Palraj BR, Khalid S, et al. Predicting risk of endovascular device infection in patients with Staphylococcus aureus bacteremia (PREDICT-SAB). Circ Arrhythm Electrophysiol. 2015;8(1):137–144.
