Abstract
Background
In health care workers SARS-CoV-2 has been shown to be an occupational health risk, often associated with transmission between health care workers. Yet, insufficient information on transmission dynamics has been presented to elucidate the precise risk factors for contracting SARS-CoV-2 in this group.
Methods
In this cross-sectional study, we investigated association between questionnaire answers on potential exposure situations and SARS-CoV-2-positivity. Health care workers with and without COVID-19-patient contact at nine units at Skåne University Hospitals in Malmö and Lund, Sweden and university employees from Lund University, Sweden were enrolled. To limit impact of health care worker to health care worker transmission, units with known outbreaks were excluded. A SARS-CoV-2-positive case was defined by a previous positive PCR or anti-SARS-CoV-2 IgG in the ZetaGene COVID-19 Antibody Test.
Results
SARS-CoV-2-positivity was detected in 11/51 (22%) health care workers in COVID-19-units, 10/220 (5%) in non-COVID-19-units and 11/192 (6%) University employees (p = .001, Fischer’s exact). In health care workers, SARS-CoV-2-positivity was associated with work in a designated COVID-19-unit (OR 5.7 (95CI 2.1–16)) and caring for COVID-19-patients during the majority of shifts (OR 5.4 (95CI 2.0–15)). In all participants, SARS-CoV-2-positivity was associated with a confirmed COVID-19 case (OR 10 (95CI 2.0–45)) in the household.
Conclusion
Our study confirmed previous findings of elevated risk of acquiring SARS-CoV-2 in health care workers in COVID-19-units, despite exclusion of units with known outbreaks. Interestingly, health care workers in non-COVID-19-units had similar risk as University employees. Further measures to improve the safety of health care workers might be needed.
Previous findings of elevated risk of contracting SARS-CoV-2 in health care workers with COVID-19 patient contact was confirmed, despite exclusion of wards with known SARS-CoV-2 outbreaks.
Further measures to improve the safety of health care workers might be needed.
KEY POINTS
Introduction
Following the early global spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) an increased risk among health care workers (HCWs) to develop coronavirus disease 19 (COVID-19) was reported [Citation1–4]. This increased risk has subsequently been confirmed [Citation5–8]. Additionally, a nationwide Scottish study showed a tripled risk for hospital admission due to COVID-19 in HCWs with patient contact as well as a doubled risk for their household members [Citation9]. Furthermore, several reports have shown a high psychosocial burden in HCWs during the COVID-19-pandemic [Citation10,Citation11].
While knowledge is increasing on the occupational health risks involved in the care of COVID-19-patients, the reasons for the reported increased risks are multifactorial [Citation12]. Transmission from and between asymptomatic patients and HCWs; workplace congestion; lack of or inadequate use of appropriate personal protective equipment (PPE) are all factors that have been associated with increased risk of transmission [Citation13]. Likely, the transmission risk further increases in aerosol generating procedures (AGPs) and during prolonged exposure, especially in poorly ventilated areas [Citation12,Citation14–16]. Yet, better understanding of the risks and the effects of interventions are needed in both healthcare settings and the general population [Citation12].
In this study we examined SARS-CoV-2-positivity rates in HCWs in COVID-19 and non-COVID-19-units and investigated associations between positivity and specified potential exposure situations. We also enrolled University employees from the same region and examined their SARS-CoV-2-positivity rate as well as investigated specified societal exposure situations and their association to positivity.
Methods
Study design, setting and participants
In this cross-sectional study all participants were recruited by e-mail invitations between 8th September and 10th November 2020. Participating HCWs were enrolled from two designated COVID-19-units (Infectious Disease units) and five non-COVID-19-units (Internal Medicine units) units at the Skåne University Hospital in Malmö and Lund, Skåne Region, Sweden. None of the units had known previously reported HCW to HCW SARS-CoV-2 transmission or identified nosocomial outbreaks in infection control investigations. In addition, University employees were recruited from the Biomedical Centre and Department of Economics at Lund University, Lund, Skåne Region, Sweden.
All participants completed a self-administered questionnaire on exposure risks and baseline characteristics. Venipuncture blood samples were collected and analysed with a rapid antibody test for SARS-CoV-IgG. Questionnaires included questions on potential exposure risks dating from February 2020, when the first case of SARS-CoV-2 was diagnosed in the Skåne Region, Sweden.
Hospital setting
During the period February – September 2020, Skåne had low SARS-CoV-2 transmission compared to other densely populated areas in Sweden and at the finalisation of the study in November 2020, the second wave in Skåne and Sweden was on the rise. The PPE recommendations when caring for a patient with suspected or confirmed COVID-19 during this period, included surgical masks (IIR), reusable and manually disinfected face shields, disposable aprons and optional gloves. Use of respirators (N95, FFP2/FFP3) were recommended in what was considered potentially aerosol generating procedures (e.g. intubation, manual ventilation, non-invasive ventilation, high-flow nasal cannula and airway suctioning). On September 30th 2020 recommendations were changed and the use of surgical masks in situations with less than 1 m between the patient and HCWs were implemented. Masking of HCWs beyond patient contact was not implemented. During the period of the study the recommended PPE was continuously available at the hospital.
Sampling and SARS-CoV-2 testing
Blood was drawn in serum tubes and allowed to coagulate for one hour at room temperature or at 8 °C over night followed by centrifugation at 2000 g for 10 min. Qualitative detection of SARS-CoV-2 IgG was performed using ZetaGene COVID-19 Antibody Test IgM/IgG (ZetaGene Ltd., Sweden; www.zetagene.com) according to the manufacturer’s instructions [Citation17]. In brief, 10 μl of serum was dispensed into the test sample well, followed by the addition of 100 μl of diluent buffer provided in the kit. The presence of SARS-CoV-2 IgG or IgM was visualised between 15–20 min.
Variables
SARS-CoV-2-infection was defined as positive IgG using the ZetaGene COVID-19 Antibody Test IgM/IgG [Citation17] or a previous or current positive reverse-transcriptase polymerase chain reaction (PCR)-result, independent of IgG-status. If participants were IgM-positive, a PCR-test was performed to confirm or refute the diagnosis. If negative, IgM-positivity alone did not fulfil the case definition.
For all HCWs, baseline characteristics included age, gender, comorbidities (previously healthy, cardiovascular disease, pulmonary disease, immunodeficiency including medically induced and diabetes mellitus), smoking status, profession (nurse assistant, nurse, physician, other without patient contact (e.g. administrative personnel, service workers) or other with patient contact (e.g. physiotherapists, dietitians)). The participants were asked whether they had used appropriate PPE during COVID-19-patient care always, almost always, most of the time or sometimes. If they had no direct contact with COVID-19-patients, this question was blanked and excluded from analysis. The participants who answered most of the time or sometimes were defined as not adherent to hygiene recommendations. To confirm that units with outbreaks had been excluded, participants were asked if nosocomial transmission had occurred on the ward they had worked at. Potential exposure since February 2020 was investigated with yes/no questions on workplace congestion, travel outside living area within Sweden (Skåne) or international travel, daily commuting by public transport to work, confirmed or suspected case of COVID-19 in the household and whether they had worked alongside a colleague who was tested positive for SARS-CoV-2 during a shift. Then, the HCWs were asked about the proportion of days they had worked with COVID-19-patients. This was categorised as; every day, a majority of the days, half of the days, rarely or never. HCWs who worked half of the days or more with COVID-19-patients were considered highly exposed. Questions on participation in two specific situations hereafter defined as situation with increased exposure were investigated. Situations included potentially aerosol generating procedures (e.g. intubation and airway handling including suctioning) where respirator use was recommended and bedside surveillance for >3 h with a COVID-19-patient during a shift, when respirator use was not recommended.
For non-HCW-participants recruited at the University, the same baseline characteristics were investigated. Potential exposure since February 2020 was investigated with yes/no questions on workplace congestion, travel outside living area (Skåne) or Sweden, daily commuting by public transport to work and confirmed or suspected case of COVID-19 in the household.
Statistical analysis
Continuous variables were described with median and interquartile ratio. Binary variables were described as counts and percentages. Baseline characteristics and SARS-CoV-2-positivity were presented descriptively in HCWs in COVID-19 and non-COVID-19-units and University employees. Potential risk factors and association to SARS-CoV-2-positivity were compared using the Fischer’s exact test with p-values provided and through exact unadjusted and adjusted logistic regression with odds ratio (OR) with 95% confidence intervals (95CI) provided.
Due to the relatively low SARS-CoV-2-seroprevalence in the Skåne Region we expected outcomes to be few, yet sufficient to adjust OR using one additional covariate in logistic regression analyses. Our primary analysis was designed as a logistic regression investigating association of SARS-CoV-2-positivity with potential risk factors solely in HCWs in COVID-19- and non-COVID-19-units, thus excluding University employees. We expected several risk factors in COVID-19-units to be collinear, i.e. HCWs in COVID-19-units are likely to be exposed to several risks. Therefore, a priori, we chose to adjust logistic regressions by adding the covariate exposure to a situation with increased exposure to evaluate to what extent these defined situations affected overall risk.
Our secondary analysis included all participants (both HCWs and University employees). Here, non-hospital specific risk factors for COVID-19 were investigated for association with SARS-CoV-2-positivity. First, unadjusted exact logistic regression was performed. Then, based on previous data on increased risk for SARS-CoV-2 in HCWs [Citation9], a priori, HCW-status was chosen as the added covariate in adjusted regression analysis. Here, HCW-status was designed as a dummy variable, where both HCWs in COVID-19 and non-COVID-19-units were labelled as 1.
To further evaluate increased exposure situations, a post-hoc analysis was performed where the associations with SARS-CoV-2-positivity were investigated for AGPs and bedside surveillance >3 h independently. In adjusted analysis of these associations, working in a COVID-19-unit was used as an added covariate in the adjusted logistic regression.
To avoid confounding effects of major SARS-CoV-2-outbreaks and to evaluate the risk of contracting SARS-CoV-2 outside of an outbreak situation, units with known major outbreaks were not included in the study. The nosocomial outbreak situation was defined in the following way. If a case of COVID-19 was diagnosed in either a HCW or an admitted patient (>2 days after admission) contact tracing was initiated to investigate if a nosocomial outbreak could have occurred. If a second case was identified in the same unit during one incubation period (2-14 days), this was considered a possible nosocomial outbreak. Also, since PCR was rarely available for the general public due to testing shortages in the Skåne Region during March-May 2020 and possibly not similarly available for all HCWs, a sensitivity analysis was performed for SARS-CoV-2-positivity rates and associations with exposure situations using solely IgG-positivity as a marker for SARS-CoV-2-positivity.
Statistical significance was defined as p < .05. In the case of missing data, complete case analysis was performed. Statistical analyses were performed using STATA (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). Figures were made using GraphPad Prism (version 9.0.0 for Mac, GraphPad Software, San Diego, California USA).
Results
Participants
In total, 463/864 (54%) invited employees accepted to participate in the study. Among HCWs at COVID-19-units participation rates were 51/119 (43%), at non-COVID-19-units 220/314 (70%) and among non-HCW employees at the University 192/460 (42%).
Baseline characteristics
Baseline characteristics in HCW at COVID-19 and non-COVID-19 units and University employees are presented with descriptive statistics in . In short, age was similar in groups, whereas females were overrepresented in HCWs. Comorbidities shared similar distribution between the three groups and the majority of participants reported to be previously healthy. HCWs in COVID-19-units had a higher proportion of smokers.
Table 1. Baseline characteristics and SARS-CoV-2-positivity in University employees and health care workers (HCWs) in COVID-19- and non-COVID-19-units.
Distributions of occupations in HCWs at COVID-19 and non-COVID-19-units were comparable and are shown in . In brief, a majority worked as assistant nurses or nurses although COVID-19-units had a small overrepresentation of assistant nurses. Physicians, other HCWs with or without patient contact were few. Poor compliance to hygiene recommendations or nosocomial transmission at their ward was rarely reported from HCWs in COVID-19 and non-COVID-19-units ().
Outcomes
SARS-CoV-2-positivity was found in 11/51 (22%) of HCWs in COVID-19-units, in 10/220 (5%) in non-COVID-19-units and in 11/192 (6%) of University employees (p = .001, Fischer’s exact) ().
In the primary analysis, we examined unadjusted associations between SARS-CoV-2-positivity and potential risk factors among HCWs in COVID-19- and non-COVID-19-units. Unadjusted analysis showed that working in a COVID-19-unit (OR 5.7 (95CI 2.1–16)), with COVID-19-patients during the majority of the days since February 2020 (OR 5.4 (95CI 2.0–15)) and reporting to have worked in a situation with increased exposure (OR 3.2 (95CI 1.1–8.7)) were associated with SARS-CoV-2-positivity (, ). Reporting to have worked alongside a colleague who was tested positive for SARS-CoV-2 during a shift was not significantly associated with SARS-CoV-2-positivity. When the possible risk factor situation with increased exposure was added in adjusted analysis of above stated risks, it did not affect ORs from unadjusted analyses ().
Figure 1. Odds ratios for SARS-CoV-2-positivity in health care workers (HCW) (n = 192) by potential risk factors for exposure. An increased exposure situation was defined as a potentially aerosol generating procedure (e.g. intubation and airway handling including suctioning) or bedside surveillance for >3 h with a COVID-19-patient during a shift.
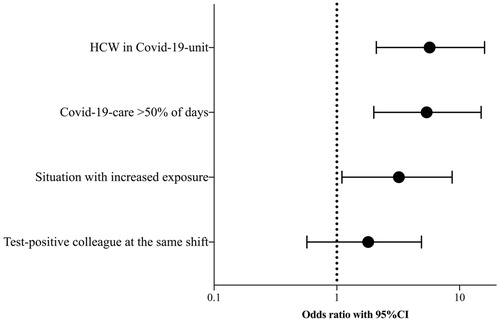
Table 2. Associations and odds ratios (OR) for SARS-CoV-2-positivity for hospital-related risk factors among health care workers (n = 271).
Situations with increased exposure were reported as a composite, but a post-hoc analysis of each increased exposure situation was also performed. Here, only bedside surveillance for >3 h was associated to SARS-CoV-2-positivity. In adjusted analysis we added working at a COVID-19-unit as a covariate, whereafter significance of this association disappeared (Supplementary Table 1).
Despite the high prevalence of SARS-CoV-2-positivity in HCWs in COVID-19-units, there was no significant difference in SARS-CoV-2 positivity when all HCWs were compared to University employees (OR 1.4 (0.62–3.3)).
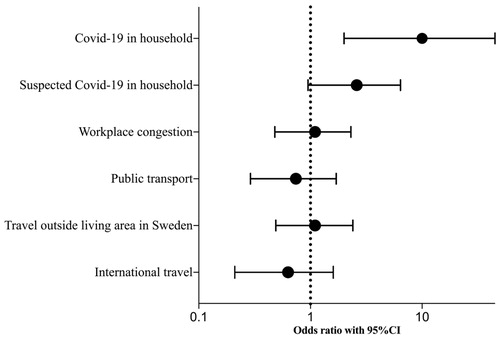
In the secondary analysis, we examined associations between SARS-CoV-2-positivity and societal and non-hospital-based potential exposure situations in all HCWs and University employees. A confirmed or suspected COVID-19 case in the same household was significantly associated with SARS-CoV-2-positivity (p < .01 and p = .04 respectively), whereas workplace congestion, commuting by public transport to work and travel outside of regional living area or internationally did not have a significant association with SARS-CoV-2-positivity ().
Table 3. Associations and odds ratios (OR) for SARS-CoV-2-positivity for society-related risk factors among 463 participants (271 health care workers and 192 University employees).
Unadjusted OR for SARS-CoV-2-positivity showed significant associations for sharing household with a confirmed case of COVID-19 (OR 10 (95CI 2.0–45)), and had the tendency of being significant for a suspected case (OR 2.6 (95CI 0.95–6.4)) (, ). In adjusted analyses, to account for the previously described increased risk among HCWs compared to non-HCWs, HCW-status was added as a covariate but did not affect ORs from unadjusted analyses ().
Figure 2. Odds ratios for SARS-CoV-2-positivity in all participants (n = 463, health care workers and University employees) by potential risk factors for exposure.
We performed a sensitivity analysis using only SARS-CoV-2-IgG seropositivity thus excluding PCR-results to account for possible confounding by availability of PCR-testing. In this sensitivity analysis described associations remained unaltered.
Discussion
In this cross-sectional study the SARS-CoV-2-positivity rate was higher among HCWs working in COVID-19-units. Interestingly, HCWs working in non-COVID-19-units and University employees had similar prevalence of SARS-CoV-2-positivity. Among HCWs, the risks associated with SARS-CoV-2-positivity included working in a designated COVID-19-unit, caring for COVID-19-patients during a majority of the days since February 2020 or reporting to be involved in an increased exposure situation (AGP or bedside surveillance >3 h). However, when the risk seen in HCWs working in COVID-19-units were adjusted for increased exposure situations, risks remained unaltered suggesting collinearity. In all participants, the presence of a confirmed case of COVID-19 in the household was identified as the most significant risk factor, with a caveat for the directional uncertainty of transmission for this risk factor.
In accordance with previous studies [Citation1–9], we confirm the reported increased risk of acquiring SARS-CoV-2 in HCWs in COVID-19-units. However, in our study, units with known HCW to HCW SARS-CoV-2-transmission or nosocomial outbreaks were excluded which adds a unique feature allowing the interpretation that the reported risk persists also outside of the outbreak situation in COVID-19-care. Interestingly, in HCWs in non-COVID-19-units and University employees we found similar and relatively low rates of SARS-CoV-2-positivity. While an increased risk among all HCWs have previously been reported [Citation6] it is probable that risk in HCWs in non-COVID-19-units to a larger extent originates from HCW to HCW-transmission whereas HCWs in COVID-19-units face more multifaceted risks. In this study, HCWs in COVID-19-units remained at increased risk despite reported adherence to guidelines and availability of PPE throughout the pandemic at the study hospitals, highlighting the need to better understand transmission dynamics to guide and improve interventions. With the exception of the AGPs, regional hygiene and PPE guidelines were designed for droplet and contact transmission. Hypothetically, transmission accounting for the increase in risk reported can then occur when guidelines are not followed (e.g. non-compliance to PPE and hygiene recommendations including physical distancing), in situations when precautionary measures (PPE) were not recommended (e.g. HCW to HCW-transmission) or if guidelines are not sufficient (e.g. aerosol transmission [Citation14–16,Citation18]. While acknowledging a potential bias in evaluating adherence to hygiene recommendations through questionnaire answers, adherence was reported as good. In addition, since outbreak units were excluded we believe that HCW to HCW-transmission accounts for a small part of the risk reported. Yet, undetected and rare HCW to HCW-transmission cannot be fully excluded. Then, the potential of aerosol transmission outside of AGPs remains to be discussed. When examining participation in situations with increased exposure, defined as participation in an AGP (where respirator use was recommended) and bedside surveillance for >3 h (where respirator use was not recommended), we found an association with SARS-CoV-2-positivity (). In a post-hoc analysis of these situations, solely bedside surveillance was significantly associated to positivity (Supplementary Table 1). These situations are interesting since increased or prolonged exposure here occur which possibly increases the risk for potential aerosol transmission. However, when unadjusted analyses were adjusted for work at a COVID-19-unit, association with positivity were no longer significant. Thus, while the described increased exposure situations (i.e. bedside surveillance or AGP) cannot be excluded as potential risk factors, our data does not support that the specified situations were major drivers of transmission in this study. Finally, while identifying a confirmed or suspected household case of COVID-19 as a risk factor in all participants, our study did not identify previously proposed exposure situations such as public transport and congestion [Citation13,Citation19] as significant risk factors (), possibly due to being underpowered. Unsurprisingly, having a household contact with COVID-19 was the strongest risk factor identified. This is in line with previous findings of both increased rates of SARS-CoV-2-positivity and increased risk for hospitalisation in household contacts of HCWs [Citation9]. In this situation, naturally, exposure can be both increased and prolonged independent of protective measures applied after diagnosis, since pre- and asymptomatic transmission is known to occur [Citation13].
This study has several limitations. Inclusion bias is probable since we actively chose not to include units with known major nosocomial SARS-CoV-2-outbreaks and had a relatively high non-participation rate among HCWs in COVID-19-units and University employees. In addition, HCWs in COVID-19-units were relatively few. Should units with known outbreaks have been included, it is possible that risk in all HCWs would have been more pronounced in comparison to University employees. Since inclusion of units and employees were neither randomised nor population-based, generalisability is decreased and reliable seroprevalence comparisons cannot be drawn.
Furthermore, analysis of risk factors in HCWs in COVID-19-units suffered from collinearity, where increased exposure situations were identified in participants who, naturally, regularly worked in COVID-19-units and subsequently were subject to several different types of risk. Thus, conclusions from this study are necessarily limited to investigating associations since causal inference between specific situation and SARS-CoV-2-positivity cannot be proven with our study design. Moreover, analyses were performed using IgG-results from a rapid antibody test [Citation17] or PCR and while false positives and negatives in both tests cannot be excluded, uniform antibody test protocols were used on the entire cohort. Nevertheless, availability of PCR-testing was reserved for HCWs and patients requiring admission during the first months and thus, was not available for all cohort participants. To address potential misclassification bias, a sensitivity analysis was performed where PCR-testing was excluded which found that results were robust. Finally, it is also possible that participants could have been IgG-positive previously, but that titres had decreased [Citation20] when included in the study which would underestimate SARS-CoV-2-positivity rates throughout the cohort.
Due to a small overrepresentation of assistant nurses and nurses enrolled in COVID-19-units, this could affect positivity rates to increase, since a previous Swedish report have suggested these HCWs to be at a higher risk [Citation6]. Finally, although our aim was to study units without outbreak, we cannot fully exclude silent HCW to HCW transmission. Infection control investigations were always performed after newly diagnosed COVID-19 in HCWs, but larger screening programs to ensure lack of transmission were not performed before May 2020.
In conclusion, our data collectively suggest and confirm the previously reported increased risk for SARS-CoV-2-positivity in HCWs working with COVID-19-patients. Further, we highlight that this increased risk persists outside of apparent HCW to HCW transmission and unit outbreaks and imply that the risk at non-COVID-19-units is lower and similar to that of University employees in our study setting. While our study aimed to investigate risks associated with AGPs and prolonged exposure to COVID-19-patients during bedside surveillance, we are unable to draw firm conclusions on these situational exposure risks. Thus, current knowledge on risks associated with AGPs, aerosol transmission as well as how to efficiently avoid HCW to HCW transmission is insufficient. To improve understanding of transmission dynamics and decrease the continuously reported [Citation5–9] risk of infection or hospitalisation among HCWs, larger and more specific studies are needed to better guide infection control interventions in hospitals.
Ethical approval
This study was performed in accordance with the ethical standards of the Helsinki Declaration, written informed patient consent after oral and written information was obtained from all participants and was approved by the National Ethics Review Board Sweden (number 2020/03168).
Supplemental Material
Download MS Word (12.6 KB)Acknowledgements
The authors thank Alejanda Maria Castilla for expert help with blood sampling and logistics, Associate Professor Andreas Inghammar for important support, unit managers for important help in recruiting participants and ZetaGene Ltd. (Sweden) for donation of rapid antibody tests.
Disclosure statement
DN and MR reports grants from Swedish Government Funds for Clinical Research (ALF), during the conduct of the study. DN, JN, AH, CJF and MR reports no conflicts of interest. YDM is the founder of and has an equity interest in ZetaGene Ltd. (Sweden).
Additional information
Funding
References
- Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395(10231):1225–1228.
- Nguyen LH, Drew DA, Graham MS, COronavirus Pandemic Epidemiology Consortium, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475–e483.
- Chou R, Dana T, Buckley DI, et al. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med. 2020;173(2):120–136.
- Zhang Z, Liu S, Xiang M, et al. Protecting healthcare personnel from 2019-nCoV infection risks: lessons and suggestions. Front Med. 2020;14(2):229–231.
- Iversen K, Bundgaard H, Hasselbalch RB, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20(12):1401–1408.
- Rudberg A-S, Havervall S, Månberg A, et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11(1):5064.
- Eyre DW, Lumley SF, O'Donnell D, et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. eLife. 2020;9:e60675.
- Wilkins JT, Gray EL, Wallia A, et al. Seroprevalence and correlates of SARS-CoV-2 antibodies in healthcare workers in Chicago. Open Forum Infectious Diseases. 2020;8(1):ofaa582.
- Shah ASV, Wood R, Gribben C, et al. Risk of hospital admission with coronavirus disease 2019 in healthcare workers and their households: nationwide linkage cohort study. BMJ. 2020;371:m3582.
- Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901–907.
- Liu Q, Luo D, Haase JE, et al. The experiences of health-care providers during the COVID-19 crisis in China: a qualitative study. Lancet Glob Health. 2020;8(6):e790–e798.
- Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–1987.
- Cevik M, Marcus JL, Buckee C, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Transmission Dynamics Should Inform Policy. Clinical Infectious Diseases. 2020:ciaa1442.
- Tran K, Cimon K, Severn M, et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4):e35797.
- Wilson N, Corbett S, Tovey E. Airborne transmission of COVID-19. BMJ. 2020;370:m3206.
- Morawska L, Milton DK. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2020;71(9):2311–2313.
- De Marinis Y, Sunnerhagen T, Bompada P, et al. Serology assessment of antibody response to SARS-CoV-2 in patients with COVID-19 by rapid IgM/IgG antibody test. Infect Ecol Epidemiol. 2020;10(1):1821513.
- Wilson NM, Norton A, Young FP, et al. Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers: a narrative review. Anaesthesia. 2020;75(8):1086–1095.
- Kissler SM, Kishore N, Prabhu M, et al. Reductions in commuting mobility correlate with geographic differences in SARS-CoV-2 prevalence in New York City. Nat Commun. 2020;11(1):4674.
- Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383(11):1085–1087.
