Abstract
Background
Nosocomial outbreaks of coronavirus disease 2019 (COVID-19) can have devastating consequences from both a resource cost and patient healthcare perspective. Relying on reverse transcription–polymerase chain reaction (RT-PCR) for identifying infected individuals may result in missed cases. Screening for antibodies after an outbreak can help to find missed cases and better illuminate routes of transmission.
Methods
In this study, we present the results of a serological screening of the healthcare workers (HCWs) on a ward for infectious diseases in Sweden with a point-of-care antibody test 8 weeks after an outbreak of COVID-19. In all, 107/123 (87%) of HCWs who were tested with RT-PCR in the outbreak investigation participated in this study on seroprevalence. Participants were also asked to fill out a questionnaire entailing epidemiological data. The cohort was stratified by RT-PCR result and the resulting groups were compared to each other.
Results
Six (8%) HCWs who were tested RT-PCR negative during the outbreak investigation had developed specific IgG antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). These HCWs had all worked shifts with colleagues who later were tested RT-PCR positive during the outbreak.
Conclusions
Our results indicate that a serological follow-up screening after an outbreak may be used as a complement to virus detection in an outbreak situation. However, immunoglobulin (Ig) G-detection should also be performed at the start of an outbreak, to facilitate interpretation of the results.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has abruptly and severely shaken the foundation of healthcare around the world. Coronavirus disease 2019 (COVID-19) presents with only mild symptoms in a majority of patients, but the risk for a more severe course with potential fatality is high if the infection manifests in the elderly or those with pre-existing comorbidity [Citation1]. Therefore, it is of utmost importance that precautions are taken to limit transmission to vulnerable individuals such as hospitalized patients. While interindividual spread among healthcare workers (HCWs) is problematic in itself, nosocomial transmission to patients can have dire consequences [Citation2].
Nosocomial outbreaks of COVID-19 have been reported in a variety of healthcare settings across the globe [Citation3–6]. The strategy for handling outbreaks varies, but usually involves some combination of screening for COVID-19 with reverse transcription–polymerase chain reaction (RT-PCR) based methods, gathering epidemiological and descriptive data of potentially exposed patients and employees, and implementing stricter protective measures. The potential issue with relying on RT-PCR to screen for COVID-19 infection is that the testing is dependent on timing. The sensitivity for RT-PCR is highest if performed in the immediate period following symptom development. The sensitivity then drops and the risk of a negative test result increases with time after symptom onset [Citation7]. Moreover, the symptoms of COVID-19 may be mild or absent and therefore makes it difficult to sample at the optimal time.
Antibody tests can be used as a complement to RT-PCR. By proving the presence of SARS-CoV-2 specific immunoglobulin (Ig) G and/or IgM antibodies, patients who have undergone infection can be identified. Most patients have developed antibodies two weeks after the onset of symptoms [Citation8]. From an epidemiological point of view, investigating the prevalence of antibodies is a robust method less reliant on timing when compared to RT-PCR that requires the presence of viral RNA. This could be applied for use in outbreak investigations, such as the one described below.
This study aimed to investigate if serological screening of HCWs eight weeks after an outbreak in a hospital ward could add additional information about the number of persons affected by SARS-CoV-2.
Methods
An invitation to participate was sent out via mail to all HCWs who had been tested with RT-PCR for SARS-CoV-2 during an outbreak at Skåne University Hospital in May–June 2020. Lists of affected HCWs were obtained from the respective heads of department. During a period of 3 weeks in August 2020, venous blood samples were drawn after informed consent had been procured. The participants were also asked to fill out a questionnaire entailing epidemiological factors such as comorbidity, present risk factors, self-reported exposure, preventive measures taken and symptom manifestations during the outbreak. A detailed analysis of symptoms will be presented elsewhere. Out of 123 HCWs who were tested during the outbreak, 107 participated in this study on seroprevalence. The presence of antibodies was tested using the ZetaGene COVID-19 rapid IgM/IgG test (ZetaGene Ltd., Malmö, Sweden) according to the accompanied user manual [Citation9].
Statistical analysis was performed using IBM SPSS® Statistics version26 (SPSS Inc., Chicago, IL). For the bivariate variables, the chi-squared and the Fisher’s exact tests were used. For larger than two by two contingency tables, the Fisher–Freeman–Halton test was used instead. For the continuous variables, the independent samples t-test was used. p Values <.05 were considered statistically significant.
The Swedish national ethics committee granted approval for this study (2020-03168).
Results
Outbreak investigation
During the month of May in 2020, an outbreak of COVID-19 occurred at a ward for infectious diseases at Skåne University Hospital in Lund, Sweden. This ward was formed in March 2020 specifically for the purpose of admitting patients with suspected COVID-19. The initial case responsible for the outbreak was not possible to define. A HCW was the first to be identified, and two patients soon followed. The two patients presented with only vague symptoms and no isolation measures were instituted since they initially had been tested negative for SARS-CoV-2. Suspicion of COVID-19 transmission was raised when HCWs who had cared for the patients developed COVID-19-related symptoms. The unit for infection prevention and control (IPC) was involved and a rigorous investigation to track down and control the potential outbreak was initiated. All patients and all HCWs were monitored for COVID-19 symptoms daily, physical distancing and more stringent cleaning routines were adopted. All HCWs were screened by RT-PCR regardless of symptoms. The use of facemasks was enforced in all HCW-patient interactions. All HCWs were updated frequently on the outbreak and the strategy of halting the outbreak. The aforementioned suspected patients were re-tested and confirmed positive.
In total, seven patients and 42 of 123 HCWs were tested RT-PCR positive for COVID-19 during the outbreak. All HCWs included in this study were on average screened with RT-PCR for SARS-CoV-2 on two separate occasions at an interval of two weeks. Thirteen positive HCWs were tested negative during the first round of screening, but were tested positive at a later date. At the beginning of June 2020, the outbreak was deemed over as no new cases were identified.
Main analysis
Cohort inclusion and main outcomes of the study are presented in . General cohort characteristics are shown in . Of the 107 included HCWs, 35 (33%) were tested positive for SARS-CoV-2 by RT-PCR during the outbreak investigation. Thirty-one (89%) of these were positive for specific IgG antibodies. Of the RT-PCR negative HCWs (n= 72), six (8%) were tested positive for IgG antibodies. The RT-PCR negative, IgG positive HCWs (n= 6) were compared to (a) the RT-PCR negative, IgG negative HCWs (n= 66) and (b) to the RT-PCR positive HCWs (n= 35). The results of these comparisons are shown in . The RT-PCR negative, IgG positive HCWs had shared shifts with colleagues who later were tested positive for SARS-CoV-2 to a significantly higher degree than their RT-PCR negative, IgG negative counterparts. One (17%) of six HCWs who were RT-PCR negative and IgG positive was screened with RT-PCR once during the outbreak, four (67%) were screened twice (mean interval of 14 d between tests) and one was screened three times. Of the RT-PCR negative, IgG positive HCWs, four experienced COVID-19-related symptoms. Two of these four individuals experienced mild symptoms such as rhinorrhoea and throat pain while two experienced more severe symptoms such as fever and anosmia.
Figure 1. Flow chart displaying the included cohort participants who were tested by RT-PCR for SARS-CoV-2 during an outbreak investigation in a hospital ward for infectious diseases in Lund, Sweden during the months of May and June, 2020.
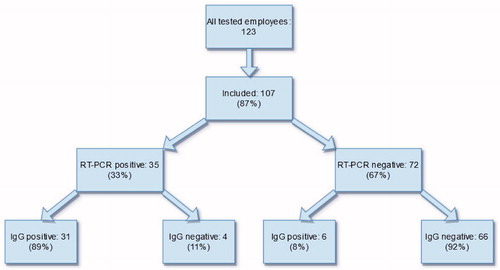
Table 1. Collected data describing the cohort as a whole.
Table 2. Comparison between the RT-PCR negative, IgG positive healthcare workers, the RT-PCR negative, IgG negative and the RT-PCR positive healthcare workers.
Subanalysis
A subanalysis was conducted comparing the features of the RT-PCR positive HCWs and the RT-PCR negative HCWs (). The two groups did not differ significantly when looking at general demographics. However, in regards to profession, assistant nurses were significantly overrepresented in the RT-PCR positive group (p< .003), whereas physicians were overrepresented in the RT-PCR negative group (p< .001). In the group with a positive RT-PCR result, a significantly higher proportion believed that one or more of their family members had contracted COVID-19, when compared to the RT-PCR negative group (p= .001).
Table 3. Comparison of the included healthcare workers when stratifying by RT-PCR result.
Discussion
Considering that COVID-19 may manifest asymptomatically [Citation10], exclusively using RT-PCR for screening can make it difficult to accurately evaluate the extent of an outbreak.
In the presented work, we screened HCWs after a nosocomial outbreak for SARS-CoV-2 specific antibodies. We were especially interested in investigating the potential discrepancy between RT-PCR test results and the prevalence of antibodies. Out of 72 RT-PCR negative HCWs, six (8%) were tested positive for IgG. This may suggest that these HCWs were missed in the outbreak investigation. It is possible that these HCWs would have been identified during the outbreak if they were tested on more occasions with shorter time spans between tests. Moreover, we cannot exclude the possibility that the HCWs were infected either before or after the outbreak. Unfortunately, blood samples were not collected and stored during the outbreak investigation so we cannot be sure when the seroconversion occurred. This is a major limitation of this study. Interestingly, all RT-PCR negative HCWs who developed IgG antibodies had shared shifts with colleagues who were later tested positive for SARS-CoV-2. This finding gives strength to the hypothesis that these HCWs indeed had been infected with SARS-CoV-2 which, due to mild or absent symptoms and repeated negative test results, remained undetected. The relatively small sample size hampers the power of the analysis, however.
While no detailed data on the background seropositivity rate in Skåne during the study period exists, the seroprevalence on other wards in the same hospital ranged from 3% (non-COVID-19-units) to 18% (other COVID-19-units) two months after this study was performed [Citation11].
There were four (11%) HCWs in the RT-PCR positive group who were tested negative for IgG antibodies. Plausible explanations for this observation may include antibody titres below the detection threshold, and latent or missing seroconversion. However, the point-of-care antibody test utilized for determining the presence of antibodies in this study has been validated in our previous works [Citation9]. The test has an estimated sensitivity of 80% for the detection of IgG in convalescent samples from non-hospitalized patients.
Physicians were overrepresented in the RT-PCR negative group. However, many of the physicians included in this study rotated between wards and were thus not as highly exposed as the other staff. We, therefore, cannot draw any conclusions about a possible decreased risk for physicians to contract SARS-CoV-2, though such findings have been reported in previous studies [Citation12].
With increasing immunity due to vaccination, nosocomial outbreaks as the one described here will hopefully not occur. Antibody tests targeting non-S-protein antigens could, however, still be valuable to screen for possible missed cases of COVID-19 in vaccinated individuals. Moreover, S-protein targeted antibody tests might prove useful to determine optimal timing for renewed vaccination.
In conclusion, this study indicates that cases of COVID-19 may be missed during outbreak investigations if diagnosis is solely based on virus detection by RT-PCR. Repeated testing with short time spans between tests is necessary to minimize the risk of missing asymptomatic individuals. Alternatively, IgG-detection in blood samples drawn 4–6 weeks after an outbreak can be used as a complement to RT-PCR for identifying missed cases of COVID-19. IgG-detection should, however, also be performed in the beginning of an outbreak so that correlation between outbreak and seroconversion may be established. Identifying additional persons affected by an outbreak can help to distinguish flaws in the current routines for limiting spread and allow for improving thereof.
Acknowledgements
The authors would like to thank the department heads Malin Inghammar, Åsa Myrefelt, Nina Sackmann, Patricia Villarroel and Ann-Sofie Bond for their cooperation during this study. We would furthermore also like to thank all of the HCWs who participated in this study on seroprevalence. Finally, we would like to thank Zetagene Ltd. for supplying us with the tests utilized in this study.
Disclosure statement
Yang De Marinis is the founder of, and has an equity interest in Zetagene Ltd. All other authors have no conflicts of interest to declare.
References
- Wu Z, McGoogan JM. Characteristics of and important lessions from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242.
- Rickman HM, Rampling T, Shaw K, et al. Nosocomial transmission of COVID-19: a retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clin Infect Dis. 2020;72:690–693.
- Wang X, Zhou Q, He Y, et al. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur Respir J. 2020;55(6):2000544.
- Van Praet JT, Claeys B, Coene AS, et al. Prevention of nosocomial COVID-19: Another challenge of the pandemic. Infect Control Hosp Epidemiol. 2020;41:1355–1356.
- Yau K, Muller MP, Lin M, et al. COVID-19 outbreak in an urban hemodialysis unit. Am J Kidney Dis. 2020;76:690–695.
- Schwierzeck V, König JC, Kühn J, et al. First reported nosocomial outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a pediatric dialysis unit. Clin Infect Dis. 2020;72:265–270.
- Kucirka LM, Lauer SA, Laeyendecker O, et al. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;173(4):262–267.
- Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848.
- Marinis YD, Sunnerhagen T, Bompada P, et al. Serology assessment of antibody response to SARS-CoV-2 in patients with COVID-19 by rapid IgM/IgG antibody test. Infect Ecol Epidemiol. 2020;10(1):1821513.
- Gao Z, Xu Y, Sun C, et al. A systematic review of asymtomatic infections with COVID-19. J Microbiol Immunol Infect. 2020;54:12–16.
- Nygren D, Norén J, Marinis YD, et al. Association between SARS-CoV-2 and exposure risks in health care workers and university employees – a cross-sectional study. Infect Dis (Lond). 2021;53:460–468.
- Eyre DW, Lumley SF, O’Donnell D, et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. eLife. 2020;9:e60675.
