Abstract
Background
COVID-19 patients are extensively treated with antibiotics despite few bacterial complications. We aimed to study antibiotic use in hospitalized COVID-19 patients compared to influenza patients in two consecutive years. Furthermore, we investigated changes in antibiotic use from the first to second pandemic wave.
Methods
This prospective study included both patients from two referral hospitals in Bergen, Norway, admitted with influenza (n = 215) during the 2018/2019 epidemic and with COVID-19 (n = 82) during spring/summer 2020, and national data on registered Norwegian COVID-19 hospital admissions from March 2020 to January 2021 (n = 2300). Patient characteristics were compared, and logistic regression analysis was used to identify risk factors for antibiotic use.
Results
National and local COVID-19 patients received significantly less antibiotics (53% and 49%) than influenza patients (69%, p < .001). Early antibiotics contributed to >90% of antibiotic prescriptions in the two local hospitals, and >70% of prescriptions nationally. When adjusted for age, comorbidities, symptom duration, chest X-ray infiltrates and oxygen treatment, local COVID-19 patients still had significantly lower odds of antibiotic prescription than influenza patients (aOR 0.21, 95%CI 0.09–0.50). At the national level, we observed a significant reduction in antibiotic prescription rates in the second pandemic wave compared to the first (aOR 0.35, 95% CI 0.29–0.43).
Conclusion
Fewer COVID-19 patients received antibiotics compared to influenza patients admitted to the two local hospitals one year earlier. The antibiotic prescription rate was lower during the second pandemic wave, possibly due to increased clinical experience and published evidence refuting the efficacy of antibiotics in treating COVID-19 pneumonia.
Introduction
The World Health Organization (WHO) has declared increasing antibiotic resistance a major threat to global health. Widespread use of broad-spectrum and long antibiotic treatment courses are important driving factors for development of resistance [Citation1]. Community-acquired infections, particularly acute respiratory infections (ARI), are the main indicators for antibiotic prescription in hospitals [Citation2]. Viral pathogens are detected in up to one-third of community-acquired cases of pneumonia (CAP) [Citation3,Citation4], but remains challenging to distinguish from ARI with bacterial or mixed aetiology in the clinic. Consequently, antibiotics are often given empirically to hospitalized patients with ARI, even after detection of a viral pathogen [Citation4,Citation5]. The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), actualizes the risk of antibiotic overuse. Initial published reports on treatment of COVID-19 included excessive antibiotic use, despite evidence of low rates of concurrent bacteraemia (3.8%) and other bacterial complications (6–15% of hospitalized cases) outside of intensive care units (ICU) [Citation6–10]. However, the latest WHO interim guidance recommends antibiotic therapy only in severe COVID-19, or when signs of bacterial infection are present, and antibiotics should be adjusted to local microbiological epidemiology [Citation11]. Prior to the COVID-19 pandemic, influenza accounted for the highest respiratory virus disease burden globally, with up to 650,000 deaths annually despite available vaccines and antiviral drugs [Citation12]. Furthermore, influenza entails a significant risk of concurrent bacterial infections (co-infections), found in 10–35% of hospitalized patients, and secondary bacterial pneumonia (after onset or clearance of the initial viral infection), associated with fatality during the 1918 and 2009 influenza pandemics [Citation13–16]. Co- and secondary bacterial infections require appropriate treatment, but despite awareness of antimicrobial resistance, antibiotic prescription rates increase annually during influenza season [Citation17,Citation18]. There is concern that the COVID-19 pandemic has halted progress in antibiotic stewardship and changed the antibiotic prescription patterns in hospitals. To address this, we initiated a prospective comparative cohort study and hypothesized that, after adjusting for clinical characteristics and severity of illness, hospitalized COVID-19 patients were prescribed more antibiotics, particularly broad-spectrum antibiotics, than influenza patients.
Methods
Study design
In this study, we compared clinical data from hospitalized patients ≥18 years old in Bergen, Norway, admitted with either influenza during the 2018/2019 influenza epidemic or with COVID-19 during March 2020–September 2020.
Patients were prospectively included from two academic referral hospitals in Bergen with emergency care services, Haukeland University Hospital (HUH) and Haraldsplass Deaconess Hospital (HDH). To investigate differences between local and national antibiotic prescription patterns, as well as changes in COVID-19 treatment during consecutive pandemic waves, we included national data on COVID-19 patients hospitalized between March 2020 and January 2021 from the Norwegian Intensive Care and Pandemic Registry (NIPaR) as a separate, national comparison. Similar surveillance on national influenza admissions do not exist. The NIPaR included the vast majority of hospital admissions due to COVID-19 since the first case on February 26, 2020. Registration became compulsory from March 30, 2020, and most admissions prior to this date were included retrospectively. We defined the second pandemic wave as the period from July 2020 to January 2021. According to viral aetiology and geographic location, we assigned patients to one of three cohorts; local influenza or COVID-19 cohorts – admitted to HUH or HDH – and the national COVID-19 cohort, the latter with data limited to age, gender, comorbidities, antibiotic use, in-hospital complications, length-of-stay (LOS) and 30-days mortality.
Data collection and patient consent statement
Patients recruited from HUS and HDH, or by next-of-kin when necessary, provided written informed consent (the KVIKKFLU study, #2018/1772; COVID-19 study #118664) [Citation19]. NIPaR is based on the right for reservation, as a result active consent was waived for this group of patients.
The study was approved by the Western Norway Ethics committee (#118664) and conducted according to the principles of good clinical practice (GCP) and the Declaration of Helsinki.
Diagnostic assay
The diagnosis of influenza was confirmed by either a commercially available nucleic acid amplification test (Abbott™ ID NOW Influenza A and B 2 (Abbott Park, IL), Cepheid GeneXpert® II (Sunnyvale, CA) with Xpert Xpress Flu/RSV and Xpert Flu test kit, Eplex Respiratory pathogen panel from GenMark Dx®) or an in-house reverse transcription-polymerase chain reaction (RT-PCR). Both hospitals used a common in-house RT-PCR test to confirm the diagnosis of COVID-19.
Statistical analysis
Patient characteristics were compared using chi-square statistics and Fisher’s exact test. The significance of differences in median and interquartile range for continuous variables was assessed using the Mann–Whitney U test. As antibiotic stewardship aims to shift prescription practices from resistance driving broad-spectrum towards narrow-spectrum antibiotics, the frequency of broad- and narrow-spectrum antibiotic prescriptions in the two diagnostic groups were compared. We classified second- and third-generation cephalosporins, piperacillin-tazobactam, macrolides, quinolones and carbapenems as broad-spectrum, and phenoxy methyl- and benzyl-penicillins, aminopenicillins, and aminoglycosides as narrow-spectrum antibiotics. Odds ratios (ORs) between dichotomous categorical variables were calculated using binomial logistic regression. Factors with a significance level <0.05 in bivariable analysis were included as covariates in the multiple logistic regression analysis of factors associated with antibiotic prescription in local patients (age, diagnosis, symptom duration, comorbidities, oxygen treatment and chest X-ray infiltrates). Age was assessed as a continuous and categorical variable in the exploratory bivariable analysis, but as a continuous variable in the multiple logistic regression analysis. When adjusted analysis included national COVID-19 patients, covariates were limited to diagnosis, age, comorbidities and chest X-ray infiltrates, due to lack of data on symptom duration and oxygen treatment in this cohort. Microbiological data on co-infections were assessed but found insufficient for inclusion in statistical analysis.
Statistical analyses were performed in IBM SPSS statistics version 26 (SPSS, Inc., Chicago, IL) and Prism version 8.1.2 (GraphPad Software Inc., La Jolla, CA).
Results
Overall, 215 patients were included in the influenza cohort, and 82 patients in the local COVID-19 cohort. National data on COVID-19 patients from NIPaR was screened (n = 2331), and hospital admissions of adult patients (≥18 years old) were included in the subsequent data analysis (n = 2300), representing 2177 individual patients as shown in . The distribution of gender, age- and comorbidities was comparable in local and national COVID-19 patients (). Among national COVID-19 patients, there was a significantly higher proportion of male patients than in the local influenza cohort (59% versus 51%, p = .015). Fewer COVID-19 patients than influenza patients had comorbidities, temperature above 37.5° and respiratory symptoms upon admission (, Supplementary Table 1). Influenza patients were older than local COVID-19 patients (65 years versus 57 years, p = .048), but not significantly older than national patients (median age 61 years, p = 0.083, ). COVID-19 patients were significantly more obese (body mass index >30) than influenza patients (33% versus 18%, x2 n = 1296, p < .001). Smoking was significantly more prevalent in influenza patients (16%) than in local and national COVID-19 patients, 6% (p = .027) and 3% (p < .001), respectively. Local patients reported symptom duration upon admission. Influenza patients were symptomatic for 3 days before admission, compared to 7 days in local COVID-19 patients (p < .001, ). Chest X-ray infiltrates were more common in COVID-19 patients (73% locally and 67% nationally) than in influenza patients (35%, both p < .001). COVID-19 patients had higher 30- day mortality rate than influenza patients (7% nationally and 4% locally versus 2%, p = .002 and p = .399), and longer hospital stays, with a median length-of-stay of 5 days compared to 2 days, p < .001 ().
Figure 1. Study design. Local influenza and COVID-19 patients were included from Haukeland University Hospital and Haraldsplass Deaconess Hospital during the 2018/2019 influenza season and spring/summer of 2020. The national cohort included COVID-19 patient data from the Norwegian Intensive Care and Pandemic Registry. Inclusion criteria were age > = 18 years, and a diagnosis of either influenza in 2018/2019 or COVID-19 in 2020/2021.
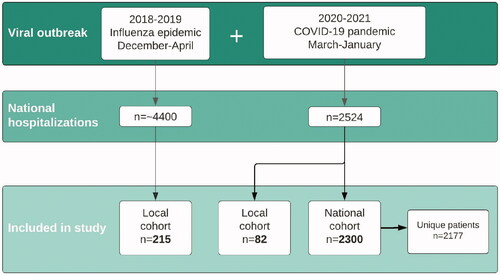
Table 1. Clinical characteristics and outcomes of COVID-19 and influenza patients.
Complete data on antibiotic prescription were available for all local patients and 95% nationally. Influenza patients received antibiotics (69%) significantly more often than both local and national COVID-19 patients (49% and 53% of patients respectively, p = .001 and p < .001). Antibiotics initiated within 24 h accounted for 90% of the prescriptions in local COVID-19 patients and 96% in influenza patients. In the national COVID-19 cohort, 72% of the antibiotics were given within the first 24 h of admission. Overall, COVID-19 patients nationally received broad-spectrum antibiotics more frequently than local influenza patients (36% versus 25%, p = .002) and less frequently narrow-spectrum antibiotics (28% versus 60%, p < .001). In local COVID-19 patients, the use of broad-spectrum antibiotics (23%) was similar to that of influenza patients (p = .728) and narrow-spectrum antibiotics (37%) similar to that of national COVID-19 patients (p = .446).
Among national COVID-19 patients receiving antibiotics, the most commonly prescribed were penicillins and second- and third-generation cephalosporins. Penicillins were prescribed to 51% of national COVID-19 patients who received antibiotics, compared to 75% of local COVID-19 and 86% of influenza patients. Cephalosporins were prescribed to 49% of national COVID-19 patients receiving antibiotics, but only to 33% of local COVID-19 and 22% of influenza patients. Internationally, azithromycin gained attention due to a possible effect on COVID-19, as it was shown to possess antiviral properties against multiple viral agents in vitro and anti-inflammatory effects in vivo [Citation20,Citation21]. In our study, only 3% of national patients received treatment with macrolides, mainly in the beginning of the pandemic. To study whether increased knowledge of the clinical picture of COVID-19 influenced the choice of antibiotic treatment, we divided patients into two groups, corresponding to the first (spring 2020) and the second wave (autumn 2020) of the pandemic in Norway ( and ). The adjusted ORs of antibiotic prescription in the two pandemic waves compared to influenza are presented in (crude OR in Supplementary Figure 1), demonstrating higher odds of broad-spectrum antibiotic prescription in the first pandemic wave than in influenza, but higher odds of overall and narrow-spectrum antibiotic prescriptions in influenza patients. In the second wave, use of broad-spectrum antibiotics was reduced by 20% (), and comparable to prescription rates in influenza patients (aOR 0.96, 95% CI 0.64–1.43). The adjusted ORs of antibiotic prescription in local versus national COVID-19 patients during the first pandemic wave are shown in , demonstrating higher odds of the use of overall and broad-spectrum antibiotics nationally. Furthermore, national COVID-19 patients received significantly less antibiotics during the second pandemic wave than during the first (42% compared to 65% respectively, aOR 0.35, 95% CI 0.29–0.43, ). The reduction was due to reduced rates of early antibiotic prescriptions (from 49% to 27%, p < .001, ). Length-of-stay was significantly shorter during the second wave, with a median of 4.6 days versus 5.8 days in the first wave from admission to discharge (p < .001).
Figure 2. Monthly COVID-19 hospital admissions and antibiotic prescriptions from February 2020 to January 2021. Upper part: National COVID-19 hospital admissions per month (green line). Admissions peaked during spring and autumn of 2020 corresponding to the first and second pandemic wave (divided by the vertical dotted line). Lower part: Proportion of admitted patients receiving antibiotics any time during admission (pink line) and within 24 h of admission (purple line).
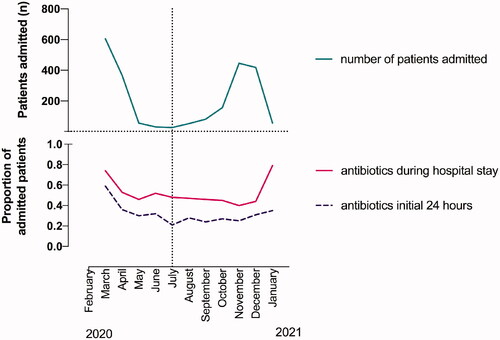
Figure 3. Adjusted odds ratios for antibiotic prescription. Adjusted odds ratios (aOR) for antibiotic prescription in (a) COVID-19 patients compared to influenza patients, (b) local COVID-19 patients compared to national COVID-19 patients in the first pandemic wave and (c) national COVID-19 patients in the second compared to first pandemic wave. Odds were adjusted for chest X-ray infiltrates, age and comorbidities.
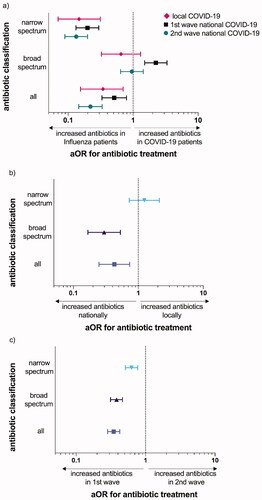
Table 2. Clinical characteristics of COVID-19 patients during the first and second pandemic wave.
Local cohorts of influenza and COVID-19 patients were combined in the analysis of association between diagnosis and antibiotic use in the two referral hospitals. In the bivariable and multivariable analysis, a significant association between influenza and antibiotic prescription was found (). Other factors associated with antibiotic use in multivariable analysis of all local patients were chest X-ray infiltrates and oxygen treatment. In addition, the bivariable analysis showed significantly higher odds of antibiotic prescription with increasing age, shorter symptom duration, and underlying comorbidities (in particular cardiovascular disease, hypertension, and immunosuppression).
Table 3. Factors associated with antibiotic prescription.
Discussion
We were surprised, that contrary to our hypothesis, when adjusted for important differences in patient populations, antibiotic prescription rates in hospitalized COVID-19 patients were lower than in influenza patients in the same two referral hospitals.
Our study provides detailed findings and comparison of antibiotic prescription practices during the COVID-19 pandemic and 2018/2019 influenza epidemic, contributing to the growing evidence of differences in clinical management, and patient outcomes of the two viral diseases [Citation22,Citation23].
In the spring of 2020, reports from European countries, such as Italy, depicted a healthcare system collapsing in the encounter with the pandemic virus SARS-CoV-2. The fear of the novel virus affected decision-making at many levels in society and may have impacted on antibiotic use. Since the previous influenza epidemic, national guidelines on antibiotic prescription remained unchanged through the first year of the pandemic, and did not include consideration of infection markers [Citation24]. In influenza, co- and secondary bacterial infections require appropriate treatment, as they aggravate disease outcome [Citation22,Citation23]. COVID-19 has proved to be more lethal than seasonal influenza [Citation22,Citation25], possibly encouraging high initial antibiotic use.
Since the start of the COVID-19 pandemic, knowledge of the prevalence of bacterial coinfections, and experimental treatment options have rapidly advanced [Citation6,Citation26–28]. The development of co- and secondary infections appears to be rare in COVID-19 [Citation29,Citation30]. At time, reports on antibiotic prescription trends over time are scarce [Citation31,Citation32]. We observed a significant reduction in antibiotic prescriptions in clinically comparable patients from the first to second wave of the COVID-19 pandemic, indicating that the reduction in antibiotic prescriptions was due to fundamental changes in prescribing practices rather than changes in patient populations.
These findings are encouraging and show that important change in prescribing patterns is possible, especially with rapidly evolving knowledge during a pandemic.
We find it concerning that almost 70% of influenza patients received antibiotics, and that early antibiotics accounted for 96% of prescriptions, despite rapid influenza testing in the Emergency Department, short median symptom duration of 3 days and established knowledge of influenza pathology [Citation33,Citation34]. Prescription rates in our study were lower than or comparable to several international studies [Citation35–38]. A recent study documented higher rates of 30-day respiratory disease readmission in influenza patients only treated with antivirals as compared to both antivirals and antibiotics, although the absolute differences in risk were low [Citation39]. In COVID-19 patients early antibiotic prescriptions were significantly reduced from the first to second pandemic wave (from 49% to 27%, proportionally 76% and 65% of all prescriptions). In comparison, a study from the US reported a wide range (27–84%) of early empirical antibiotic use in COVID-19 patients in 32 hospitals [Citation32]. High rates of empirical antibiotic treatment indicate the presence of unnecessary prescribing, potentially both resistance-driving and harmful at patient level, thus an important target for antimicrobial stewardship. In our experience, SARS-CoV-2 test turn-around-times has improved since the beginning of the pandemic outbreak, possibly affecting antibiotic prescribing patterns. Simultaneously, the superior local rapid influenza test turn-around-times is not reflected in lower empiric antibiotic prescriptions.
We found a higher prevalence of respiratory symptoms in local influenza patients than in local COVID-19 patients, in line with results of a recent meta-analysis [Citation40]. The presence of respiratory symptoms and clinical findings has previously been associated with antibiotic prescribing in respiratory tract infections [Citation41]. However, our study was not designed to examine such an association.
Broad-spectrum antibiotics was used more prevalently in COVID-19 patients than in influenza patients. The most common co-infecting pathogens in influenza are Streptococcus pneumonia, Staphylococcus aureus, and Haemophilus influenzae [Citation34,Citation42], most often treatable with narrow-spectrum antibiotics in Norway. Accumulated data demonstrates low prevalence of community-onset bacterial co-infection in COVID-19 patients, however, numerous different co-pathogens have been detected internationally [Citation32,Citation43]. These findings might encourage the use of broad-spectrum antibiotics in COVID-19 patients with suspected bacterial co-infection.
Currently, Norway has no national registry of antibiotic treatment of hospitalized influenza patients, and, to our knowledge, our current cohorts are the most comprehensive in the country, including both regional influenza patients and all COVID-19 hospitalizations in Norway until January 2021. In a national survey of antibiotic stewardship, one of the two participating hospitals, -HDH-, ranked top in the country in adhering to narrow-spectrum antibiotic use when appropriate, while HUS was among those using most broad-spectrum antibiotics. Both hospitals are more restrictive than the country as a whole concerning antibiotic treatment of COVID-19. In Norway, there is low prevalence of multi-resistant bacteria compared to most other countries. Hence, some of our findings on selection and prescription of antibiotics may only be generalizable to countries with similar microbial resistance patterns. Another limitation is that we lacked data on microbiological findings in most patients and therefore could not evaluate the appropriateness of the antibiotic prescription in each case. Furthermore, our study focussed solely on the proportionate use of antibiotics, and not on treatment duration. The core elements of antibiotic stewardship, particularly in patients with COVID-19, such as reassessment, de-escalation and early termination, should be investigated in future studies.
The 30-day mortality reported in our study was exceptionally low compared to other studies [Citation30,Citation44,Citation45]. This could be influenced by a tendency to treat elderly and frail nursing home residents with COVID-19 outside hospital, where the majority of deaths during the early phase of the pandemic occurred [Citation46].
We believe it is important to analyze present antibiotic prescribing patterns in the context of previous practices. Our study forms a valuable backdrop for reflection on decisive factors for antibiotic prescription in viral lung infections. A preprinted study of hospitalized influenza patients in Norway between 2014-2018 reported of unchanged antibiotic use in the study period [Citation47], whereas in hospitalized COVID-19 patients, we observed rapid changes in antibiotic prescription rates during 2020. Improved rapid diagnostic tools, and targeted stewardship measures to reduce discrepancies between the true prevalence of bacterial co-infection and antibiotic use in viral respiratory infections is urgently needed, as antibiotic resistance may well be our next pandemic threat.
Short summary
Hospitalized COVID-19 patients received less antibiotics than influenza patients. The majority of antibiotic prescriptions were early and empirical in both COVID-19 (>70%) and influenza (>90%). Significant reduction of COVID-19 antibiotic prescriptions was observed from the first to second pandemic wave.
Supplemental Material
Download MS Word (11.9 KB)Supplemental Material
Download TIFF Image (369.1 KB)Supplemental Material
Download MS Word (15.8 KB)Acknowledgements
The authors thank Dr Taule, Dr Bjørneklett, Dr Reimers, Dr Markussen, Dr Steihaug, and all staff in The Emergency Care Clinic, providing support and assisting with the recruitment of patients during very busy times. The authors also thank J. Østensjø, J. Hustveit-Brunell, M. Sævik, H. Søyland, and all staff affiliated with Bergen COVID-19 Research Group for help in recruiting patients and handling data. Finally, a grateful thank you to all patients participating in this study.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Holmes AH, Moore LS, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387(10014):176–187.
- Fridkin S, Baggs J, Fagan R, Centers for Disease Control and Prevention (CDC), et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194–200.
- Holter JC, Muller F, Bjorang O, et al. Etiology of community-acquired pneumonia and diagnostic yields of microbiological methods: a 3-year prospective study in Norway. BMC Infect Dis. 2015;15(1):64.
- Clark TW, Medina M-J, Batham S, et al. Adults hospitalised with acute respiratory illness rarely have detectable bacteria in the absence of COPD or pneumonia; viral infection predominates in a large prospective UK sample. J Infect. 2014;69(5):507–515.
- Falsey AR, Becker KL, Swinburne AJ, et al. Bacterial complications of respiratory tract viral illness: a comprehensive evaluation. J Infect Dis. 2013;208(3):432–441.
- Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–1629.
- Feng Y, Ling Y, Bai T, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–1388.
- Zheng F, Tang W, Li H, et al. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24(6):3404–3410.
- Contou D, Claudinon A, Pajot O, et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann Intensive Care. 2020;10(1):119.
- Sepulveda J, Westblade LF, Whittier S, et al. Bacteremia and blood culture utilization during COVID-19 surge in New York city. J Clin Microbiol. 2020;58(8):e00875–20.
- World Health Organization. COVID-19 Clinical Managment: living guidance. [last updated 2021 Jan 25, cited 2021 Apr 4]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1.
- Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–1300.
- Klein EY, Monteforte B, Gupta A, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016;10(5):394–403.
- Bartley PS, Deshpande A, Yu PC, et al. Bacterial coinfection in influenza pneumonia: rates, pathogens, and outcomes. Infect Control Hosp Epidemiol. 2021:1–6. DOI:https://doi.org/10.1017/ice.2021.96
- Gill JR, Sheng ZM, Ely SF, et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza a/H1N1 viral infections. Arch Pathol Lab Med. 2010;134(2):235–243.
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198(7):962–970.
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Netw Open. 2018;1(2):e180243.
- Durkin MJ, Jafarzadeh SR, Hsueh K, et al. Outpatient antibiotic prescription trends in the United States: a national cohort study. Infect Control Hosp Epidemiol. 2018;39(5):584–589.
- Fjelltveit EB, Cox R, Østensjø J, et al. Point-of-care influenza testing impacts clinical decision, patient flow and length of stay in hospitalized adults. J Infect Dis. 2020;jiaa690. DOI:https://doi.org/10.1093/infdis/jiaa690
- Menzel M, Akbarshahi H, Bjermer L, et al. Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients. Sci Rep. 2016;6:28698.
- Lee N, Wong CK, Chan MCW, et al. Anti-inflammatory effects of adjunctive macrolide treatment in adults hospitalized with influenza: a randomized controlled trial. Antiviral Res. 2017;144:48–56.
- Piroth L, Cottenet J, Mariet AS, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9(3):251–259.
- Auvinen R, Nohynek H, Syrjanen R, et al. Comparison of the clinical characteristics and outcomes of hospitalized adult COVID-19 and influenza patients – a prospective observational study. Infect Dis (Lond). 2021;53(2):111–121.
- Helsedirektoratet. Nasjonale faglige retningslinjer for bruk av antibiotika bør følges også under covid-19-pandemien 2020. [last updated 2020 Apr 3, cited 2021 Apr 8]. Available from: https://www.helsedirektoratet.no/veiledere/koronavirus/vaksiner-smittevernutstyr-og-legemidler/legemidler-og-medisinsk-utstyr/nasjonale-faglige-retningslinjer-for-bruk-av-antibiotika-bor-folges-ogsa-under-covid-19-pandemien.
- Brehm TT, van der Meirschen M, Hennigs A, et al. Comparison of clinical characteristics and disease outcome of COVID-19 and seasonal influenza. Sci Rep. 2021;11(1):5803.
- Youngs J, Wyncoll D, Hopkins P, et al. Improving antibiotic stewardship in COVID-19: bacterial co-infection is less common than with influenza. J Infect. 2020;81(3):e55–e57.
- Butler CC, Dorward J, Yu L-M, et al. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;397(10279):1063–1074.
- Abaleke E, Abbas M, Abbasi S, et al. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10274):605–612.
- Karami Z, Knoop BT, Dofferhoff ASM, et al. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: results from a multicentre retrospective cohort study in The Netherlands. Infect Dis (Lond). 2021;53(2):102–110.
- Thelen JM, Buenen AGN, van Apeldoorn M, et al. Community-acquired bacteraemia in COVID-19 in comparison to influenza A and influenza B: a retrospective cohort study. BMC Infect Dis. 2021;21(1):199.
- Brehm TT, Heyer A, Roedl K, et al. Patient characteristics and clinical course of COVID-19 patients treated at a German tertiary center during the first and second waves in the year 2020. J Clin Med. 2021;10(11): 2274.
- Vaughn VM, Gandhi T, Petty LA, et al. Empiric antibacterial therapy and community-onset bacterial co-infection in patients hospitalized with COVID-19: a multi-hospital cohort study. Clin Infect Dis. 2021;72(10):e533–e541.
- Grabowska K, Hogberg L, Penttinen P, et al. Occurrence of invasive pneumococcal disease and number of excess cases due to influenza. BMC Infect Dis. 2006;6:58.
- Weinberger DM, Simonsen L, Jordan R, et al. Impact of the 2009 influenza pandemic on pneumococcal pneumonia hospitalizations in the United States. J Infect Dis. 2012;205(3):458–465.
- Cohen R, Babushkin F, Geller K, et al. Characteristics of hospitalized adult patients with laboratory documented influenza A, B and respiratory syncytial virus – a single center retrospective observational study. PLoS One. 2019;14(3):e0214517.
- Akers IE, Weber R, Sax H, et al. Influence of time to diagnosis of severe influenza on antibiotic use, length of stay, isolation precautions, and mortality: a retrospective study. Influenza Other Respir Viruses. 2017;11(4):337–344.
- Brendish NJ, Malachira AK, Beard KR, et al. Impact of turnaround time on outcome with point-of-care testing for respiratory viruses: a post hoc analysis from a randomised controlled trial. Eur Respir J. 2018;52(2):1800555.
- Verma AA, Hora T, Jung HY, et al. Characteristics and outcomes of hospital admissions for COVID-19 and influenza in the Toronto area. CMAJ. 2021;193(12):E410–E418.
- Sutton SS, Magagnoli J, Cummings T, et al. Association between the use of antibiotics, antivirals, and hospitalizations among patients with laboratory-confirmed influenza. Clin Infect Dis. 2021;72(4):566–573.
- Pormohammad A, Ghorbani S, Khatami A, et al. Comparison of influenza type a and B with COVID-19: a global systematic review and meta-analysis on clinical, laboratory and radiographic findings. Rev Med Virol. 2021;31(3):e2179.
- McKay R, Mah A, Law MR, et al. Systematic review of factors associated with antibiotic prescribing for respiratory tract infections. Antimicrob Agents Chemother. 2016;60(7):4106–4118.
- Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017;8:1041.
- Hughes S, Troise O, Donaldson H, et al. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(10):1395–1399.
- Giacomelli A, Ridolfo AL, Milazzo L, et al. 30-Day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158:104931.
- Suleyman G, Fadel RA, Malette KM, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open. 2020;3(6):e2012270.
- Gulseth HL, Helland E, Johansen KI, et al. Deaths after confirmed SARS-CoV-2 in Norway. Tidsskr nor Laegeforen. 2020;140(18). DOI:https://doi.org/10.4045/tidsskr.20.0693
- Waehre T, Tunheim G, Bodin J, et al. Clinical risk scores as predictors of severe outcome in hospitalized influenza patients: an observational cohort study from Norway 2014–2018. Authorea. 2020. DOI:https://doi.org/10.22541/au.160349168.82323157/v1
