Abstract
Background
Information about the contagiousness of new SARS-CoV-2 variants, including the alpha lineage, and how they spread in various locations is essential. Country-specific estimates are needed because local interventions influence transmissibility.
Methods
We analysed contact tracing data from Oslo municipality, reported from January through February 2021, when the alpha lineage became predominant in Norway and estimated the relative transmissibility of the alpha lineage with the use of Poisson regression.
Results
Within households, we found an increase in the secondary attack rate by 60% (95% CI 20–114%) among cases infected with the alpha lineage compared to other variants; including all close contacts, the relative increase in the secondary attack rate was 24% (95% CI −6%−43%). There was a significantly higher risk of infecting household members in index cases aged 40–59 years who were infected with the alpha lineage; we found no association between transmission and household size. Overall, including all close contacts, we found that the reproduction number among cases with the alpha lineage was increased by 24% (95% CI 0%−52%), corresponding to an absolute increase of 0.19, compared to the group of index cases infected with other variants.
Conclusion
Our study suggests that households are the primary locations for rapid transmission of the new lineage alpha.
Introduction
New, more transmissible variants of the SARS-CoV-2 pose a significant challenge to the response against the ongoing COVID-19 pandemic. The alpha lineage that emerged in the United Kingdom (UK) in September 2020 [Citation1] and subsequently spread to other countries is reported to be more transmissible than the previously circulating lineages [Citation2,Citation3]. There is also evidence of higher risk of hospitalization for the alpha variant [Citation4,Citation5]. Though our study is not the first to estimate the relative transmissibility of the alpha variant, local estimates are imperative due to differences in infection control measures, population behaviour, and variations in circulating variants. Contact tracing data contain important information that can be used to estimate secondary attack rates (SAR) and reproduction numbers during an emerging epidemic [Citation6].
Using data from the contact tracing database PasInfo in Oslo Municipality, Norway, we compared the number of secondary cases among close contacts of persons infected with the alpha lineage with those infected with other circulating lineages to estimate the increased transmissibility of the lineage alpha.
During the study period in early 2021, testing upon arrival at the Norwegian border was obligatory, and all direct flights from the United Kingdom were cancelled for several weeks. As COVID-19 infections rose in Oslo, measures to reduce contacts in the population were implemented. When the first non-imported cases of the alpha lineage were detected on January 23, all non-essential shops closed, and teleworking was obligatory when possible. Some of these restrictions were eased on February 16 [Citation7].
Materials and methods
Data
The contact tracing data contain information on primary cases that tested positive in early 2021, from January 4 until February 28. In most cases, PCR tests were used to confirm infection with SARS-CoV-2 and screen for the alpha variant; however, antigen rapid tests was used for 13 individuals included in the sample. Until 20 January 2021, sequencing of samples initially categorised as alpha were performed using a mixture of Whole-Genome Sequencing (WGS; IIllumina) and Sanger sequencing. After 20 January, diagnostic tests were based on the Illumina sequencing platform exclusively.
Primary cases were eligible for inclusion if the virus lineage test result was based on WGS or Sanger sequencing, and they were recorded as infected outside their household. For each primary case, the record contains age, number of close contacts, and number of SARS-CoV-2 positive close contacts within and outside the household. Close contacts are defined as persons who had direct physical or close contact (≥15 min and <2 m) within 48 h of symptom onset of the case or time of test for asymptomatic cases.
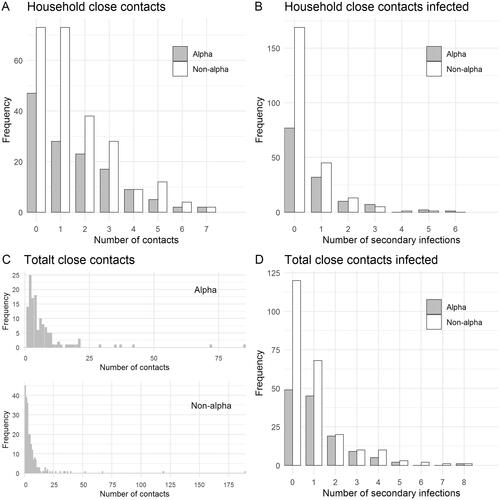
Figure 1. Frequency distributions of the number of close contacts and secondary infections of the 415 index cases. (A) household close contacts; (B) secondary infections within households; (C) total close contacts; (D) total secondary infections. Index cases with zero close contacts were included in the estimation of reproduction numbers.
Statistical analyses
We used Poisson regression with the number of secondary cases as the response to estimate the SAR and the relative risk, including age-adjusted estimates. To obtain the lineage-specific SAR, we fitted separate intercept-only models without covariates to the alpha lineage data and the other lineages data. The relative risk was estimated using an indicator variable for the cases infected with the alpha lineage. We included an offset to account for the number of close contacts in all models; hence the SAR is the probability of secondary infection per close contact. Age group was added to the models using sum-to-zero constraints to preserve the interpretation of the intercept and of the relative risk parameters. We used the same Poisson regression approach to estimate reproduction numbers but without adjusting for the total number of contacts. The analyses were performed using the R software, version 4.0.2.
Results
After exclusion of 45 cases with incomplete information, the contact tracing data included 415 index cases and their 2718 reported close contacts, of which 368 tested positive (). In total, 5818 positive cases were registered in Oslo in the same period. shows the demographic composition of the sequenced primary cases. One hundred and forty-six of these were infected with the alpha lineage, while 269 were infected with other lineages. For 251 of the cases (85 alpha lineage, 166 other lineages) at least one close contact within her/his household was recorded and included in the analysis of household transmission.
Table 1. The number of primary cases and their average number of close contacts and secondary infections by age and lineage.
Among those infected with the alpha lineage, the average number of close contacts was 6.83 (standard deviation (SD): 10.9), and the average number of secondary infections was 1.01 (SD: 1.3), see . Within the household, the average number of close contacts was 1.45 (SD: 1.7), and the average number of secondary infections was 0.61 (SD: 1.1).
In the group infected with the other variants, the average number of close contacts was 6.4 (SD: 15.3), and the average number of secondary infections was 0.82 (SD: 1.3). Within the household, the average number of close contacts was 1.36 (SD: 1.6), and the average number of secondary infections was 0.36 (SD: 0.8). The total number of close contacts of index cases in the age group < 19 years is higher likely due to cohorting and school class testing. The number of contacts is overdispersed, in accordance with previous findings from Ireland [Citation8].
shows the estimated SAR for primary cases with the alpha lineage and other lineages, in addition to the ratio between them. Within households, the estimated SAR was 0.27 for the non-alpha lineages, and 0.42 for the alpha lineage. The alpha lineage was estimated to be 60% (20%−114%) more transmissible within households than the other circulating variants. Among index cases infected with the alpha lineage, individuals aged 40–59 years had a significantly higher risk of infecting household members. Household size was not found associated with higher SAR. Independently of the location, the estimated SAR was 0.13 for the non-alpha lineages, while it was 0.15 for the alpha lineage. The alpha lineage was estimated to be 16% (-6%−43%) more infectious than the other circulating variants. Adjusting for the age of the primary cases did not substantially change the estimates.
Table 2. The estimated secondary attack rates (SAR) for the alpha lineage and for other lineages, with the relative risk between them, and univariate analyses of the impact of age of primary case and household size on SAR within households for the alpha lineageand other lineages.
The results of the reproductive numbers for the alpha lineage and other lineages are shown in . The reproduction number for the alpha lineage is estimated to be 24% greater than for the other lineages, or 0.19 difference in absolute numbers.
Table 3. Estimated reproduction numbers for the alpha lineage and for other lineages, as well as the relative ratio between them.
Discussion
We estimate a 60% higher transmissibility of lineage alpha within households. To our knowledge, this is the first estimate of the relative transmissibility of the alpha variant in household settings. Our estimate is compatible with general results from the United Kingdom that report higher transmissibility in the range 43–82% [Citation2] and 40–80% [Citation9], respectively. Our findings are also in accordance with results from Denmark, which report 36% higher transmissibility for the alpha variant than other locally circulating variants [Citation3]. Note that our 95% confidence interval is wide (20–114%).
When we also consider contacts outside households, we find lower relative transmissibility. There are two potential explanations for this. There could be bias in the data when considering all close contacts. By restricting our analysis to contacts within the household, we control for potential selection bias of contacts introduced by the contact tracing, as contacts within households are more likely to be complete in any case and thus comparable across variants. Another possibility is that there is very little difference in the transmissibility for contacts outside households because of stringent and efficient distancing interventions. Transmission in the home setting has been shown to be more important during strict interventions [Citation10]. The fact that the estimates are not affected when we control for age means that the susceptibility by age group is similar for the alpha variant and the other circulating lineages. This finding is in line with studies from other countries, for example, from UK showing that alpha is associated with increased transmissibility in all age groups [Citation11].
We estimated a household attack rate of 27% for non-alpha lineages, which is lower than 45%, reported in a Norwegian prospective household study enrolling cases between February and April 2020 [Citation12]. As testing was not easily available during the early phase of the pandemic, delay in testing may have increased the exposure time for household members, thereby contributing to the high transmission observed in this study. Still, both estimates are high compared to 17%, found in a systematic review of studies of the spread of SARS-CoV-2 within households; however, documenting high heterogeneity between studies [Citation13].
The data are subject to several limitations. We cannot distinguish between the case infecting the contact or vice versa. There can be undetected cases: close contacts who are not registered, infected contacts that escape detection, and infected contacts who have not yet developed the infection (censored). Some non-positive contacts could be immune due to a previous infection. However, we do not expect these problems to occur more often for particular lineages than others. Hence, our relative estimates should be less affected by these sources of bias.
Besides, there are potential sources of bias in the contact tracing data. Even though the definition of close contact and contact tracing instructions were the same for the different lineages, there were likely differences in how the tracing was performed in practice, with an increased focus on tracing and testing contacts of cases infected with the alpha variant. Due to the increasing circulation of the alpha lineage in Oslo, the TICTQ (Test-Isolation-Contact Tracing-Quarantine) intervention was strengthened. In addition to the general requirement of quarantine for all close contacts of confirmed SARSCov-2 cases, since 5 February, all close contacts were tested at the beginning and end of their quarantine. In addition, household members of close contacts of cases confirmed with lineage alpha infection were asked to quarantine until the contact was confirmed negative. Since March, quarantining the household members of close contacts was extended to include all close contacts regardless of the variant. The expanded TICTQ targeting lineage alpha cases may have introduced bias in the number of contacts registered outside the home, depending on lineage, thereby affecting our relative SAR estimate based on the total number of contacts. However, it should not affect the relative household SAR, as household members are known and followed up regardless.
Our study suggests that households are major locations for rapid transmission of the lineage alpha. Therefore, lowering the risk of spread within the families is pivotal to controlling the COVID-19 pandemic with new and more transmissible lineages, given the Norwegian infection control policy with expansive household quarantining. However, it is strenuous to avoid transmission in households and more difficult under crowded conditions, potentially introducing a socioeconomic gradient [Citation14]. The Norwegian government is now proposing a more extensive use of quarantine hotels related to inbound travel. This intervention may also be encouraged for domestic cases with more transmissible virus variants, particularly in situations of unvaccinated household members in the risk groups.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Rambaut A, Loman N, Pybus O, et al. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the uk defined by a novel set of spike mutations. Technical report, Virological.org, 2020. Available at: https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563.
- Davies NG, Abbott S, Barnard RC, et al. Estimated transmissibility and severity of novel SARS-CoV-2 variant of concern 202012/01 in England. MedRxiv. 2021;2020–2012.
- SSI-DK. Vurdering af smitsomhed af nye varianter. Technical report, Statens Serum Institut, January 2021. Available at: https://covid19.ssi.dk/-/media/cdn/files/vurdering-af-smitsomhed-af-nye-varianter_210121.pdf?la=da.
- Bager P, Wohlfahrt J, Fonager J, et al. Increased Risk of Hospitalisation Associated with Infection with SARS-CoV-2 Lineage B.1.1.7 in Denmark. Preprints with The Lancet, 2021. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3792894.
- Challen R, Brooks-Pollock E, Read JM, et al. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372:n579. doi:
- Soetens L, Klinkenberg D, Swaan C, et al. Real-time estimation of epidemiologic parameters from contact tracing data during an emerging infectious disease outbreak. Epidemiology. 2018;29(2):230–236.
- The Government of Norway. Tidslinje: myndighetenes håndtering av koronasituasjonen, 2021. [Online; accessed 10-March-2021]. Available at: https://www.regjeringen.no/no/tema/Koronasituasjonen/tidslinje-koronaviruset/id2692402/.
- McAloon CG, Wall P, Butler F, et al. Numbers of close contacts of individuals infected with SARS-CoV-2 and their association with government intervention strategies. medRxiv. 2021.
- Volz E, Mishra S, Chand M, et al. Transmission of SARS-CoV-2 Lineage B. 1.1. 7 in England: Insights from linking epidemiological and genetic data. medRxiv. 2021.
- Curmei M, Ilyas A, Evans O, et al. Estimating household transmission of SARS-CoV-2. medRxiv. 2020.
- Public Health England. Investigation of novel sars-cov-2 variant, variant of concern 202012/01 technical briefing 3. Technical report, December 2020. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/959360/Variant_of_Concern_VOC_202012_01_Technical_Briefing_3.pdf.
- Kuwelker K, Zhou F, Blomberg B, et al. High attack rates of SARS-CoV-2 infection through household-transmission: a prospective study. medRxiv- 2020.
- Madewell ZJ, Yang Y, Jr Longini IM, et al. Household transmission of SARS-CoV-2: a systematic review and Meta-analysis. JAMA Netw Open. 2020;3(12):e2031756.
- Forland F, Aavitsland P. Should high household attack rates change public health polices? The Lancet Regional Health - Europe. 2021;3:100031.
