Abstract
Background
Virtually all living organisms, including microbes and humans, depend on iron to survive and grow. During an infection, the plasma level of iron and several iron-related proteins change substantially. We hypothesized that iron and iron-related proteins could predict short- and long-term outcomes in community-acquired pneumonia.
Methods
Blood samples from a prospective cohort of 267 in-patients with community-acquired pneumonia were analysed for hepcidin, ferritin, iron, transferrin, transferrin saturation, and soluble transferrin receptor at admission and 6-weeks post-discharge. Adverse short-term outcome was defined as admission to intensive care unit or death within 30 days, and long-term outcome was assessed as 5-year overall mortality. Logistic regression, Kaplan Meier survival curves, and Cox regression models with cut-offs at median for the potential biomarkers were used for statistical evaluation.
Results
Low admission levels of hepcidin predicted 5-year overall mortality, independently of age, sex, comorbid conditions, and anaemia. Low levels of ferritin at admission as well as low levels of iron and transferrin saturation and high levels of soluble transferrin receptor at the 6-week follow-up were predictors of 5-year overall mortality in univariable, but not in multivariable analyses. Neither of these potential biomarkers predicted adverse short-term outcomes.
Conclusions
In hospitalized patients with community-acquired pneumonia, low levels of hepcidin at admission predicted 5-year overall mortality, but not short-term adverse outcome.
Keywords:
Introduction
Lower respiratory tract infections remain the leading infectious cause of death worldwide [Citation1]. In community-acquired pneumonia, the disease course ranges from mild to fatal. To date, the only widely accepted tools for prediction of short-term clinical course are combined scores such as CURB-65 (Confusion, Urea, Respiratory rate, Blood pressure, Age ≥65 years) [Citation2] and PSI (Pneumonia Severity Index) [Citation3]. Despite limitations of these scoring systems, no biological biomarker has yet proven superior [Citation4]. In particular, specific assessment tools for evaluating long-term prognosis after pneumonia hospitalizations are not established. Biomarkers related to the iron-regulatory system have been proposed as potential predictors of patient outcome in sepsis and critically ill patients [Citation5–8]. In patients with Covid-19, high levels of serum ferritin and hepcidin have mainly been associated with poor short-term outcome [Citation9–11]. Biomarkers that can improve prediction of the clinical course in community-acquired pneumonia patients are needed to guide assessment of disease severity at hospital admission, and to identify patients in need of intensified treatment and surveillance, both in the short- and long-term perspective. Moreover, iron-related biomarkers may potentially provide insight into the pathogenesis of these infections.
Iron is required for vital microbial and human functions, and restricting iron access to microbes is a possible strategy to combat invading microorganisms [Citation12]. Inflammatory cytokines such as interleukin-6 affect the regulation of iron-related proteins. The iron-regulatory hormone hepcidin is increased in plasma during infections, in parallel with a decrease of iron and an increase of the iron-storage protein ferritin [Citation13]. Compromized iron availability for erythropoiesis contributes to the development of anaemia of inflammation [Citation14]. Other short- and long-term clinical consequences of iron restriction are to a lesser degree explored.
We hypothesized that short- and long-term outcomes in community-acquired pneumonia relate to iron regulation reflected by plasma levels of iron and iron-related proteins, with particular focus on hepcidin.
Materials and methods
Study population and design
Over a 3-year period (1 January 2008–31 January 2011), adults (≥18 years) admitted to Drammen Hospital (Vestre Viken Hospital Trust, Norway) with suspected community-acquired pneumonia were recruited to a prospective cohort study (NCT01563315) as previously described [Citation15].
Based on the following criteria, community-acquired pneumonia was diagnosed within the first 48 h of admission: 1) identification of a new pulmonary infiltrate on chest x-ray, 2) rectal temperature ≥38.0 °C, and 3) at least one of the following: productive or non-productive cough, dyspnoea, respiratory chest pain, crackles, or reduced respiratory sounds. Exclusion criteria were x-ray-findings of other non-infectious pulmonary diseases or discharge from hospital within two weeks prior to admission. The 6-week follow-up included blood sampling and a renewed chest x-ray.
All participants signed an informed consent form. The study was approved by the Regional Committee for Medical and Health Research Ethics in South-Eastern Norway (reference number S-06266a) and a waiver of consent was obtained from the committee to link patient data to death certificates (2012/467 A).
Data collection and definitions
Clinical information was extracted from paper case records forms and medical records. An experienced study radiologist blinded to the clinical data re-evaluated all chest x-rays. Microbial aetiology was established through an extensive investigation as previously described [Citation15]. In comparative analysis of microbial aetiology, single or multiple bacterial (typical and/or atypical) and viral infections were analysed separately. The clinical scoring tool CURB-65 was used to assess severity of illness; i.e. low-risk CURB-65 score ≤2 and high-risk CURB-65 score ≥3. Anaemia was subclassified according to recommendations from the World Health Organization as mild (haemoglobin (Hb) 11.0-11.9 g/dL in women and 11.0-12.9 g/dL in men), moderate (Hb 8.0-10.9 g/dL), and severe anaemia (Hb <8 g/dL) [Citation16].
Sample collection
Blood samples were collected within 48 h of admission and at the 6-week follow-up. Serum clotted for at least 30 min, followed by refrigerated centrifugation at 2000 g for 12 min within 60 min from sampling (both serum and EDTA plasma). Aliquots were stored at −80 °C and analyses were performed in batches for the iron-related biomarkers of interest.
Biomarker analyses
All samples were included in the analyses, given sufficient material. Plasma hepcidin analyses were performed by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS), a method that selectively measures hepcidin-25, the biological active isoform. The method has been validated for clinical applications according to Eurachem guidelines [Citation17] and as part of work in the Working Group on Clinical Quantitative Mass Spectrometry Proteomics of the International Federation of Clinical Chemistry (IFCC). Samples used for hepcidin analyses had been through one freeze-thaw cycle prior to analysis. All samples were handled in the same way, and serum hepcidin differences over four freeze-thaw cycles have been reported to be of minor importance [Citation18]. Analyses of serum ferritin, iron, and transferrin were performed on Architect ci16200 (Abbott Diagnostics, Abbott Park, IL, USA). Transferrin saturation (%) was calculated by the ratio of serum iron to the total iron-binding capacity of serum transferrin (iron/(transferrin x 25,1)). Serum soluble transferrin receptor was analysed on BN ProSpec (Siemens Healthineers, Marburg, Germany). Measurements of pO2 and sO2 were obtained from analysis on the blood gas analyser ABL 725 (Radiometer, Copenhagen, Denmark). CRP and haemoglobin were measured by routine methods. Detection of HFE gene variants (Cys282Tyr and His63Asp) were performed by PCR amplification and melt curve analysis on Lightcycler 480 (Roche Diagnostics, Oslo, Norway) using in vitro diagnostics kit from TIB MOLBIOL (Berlin, Germany). Results of the iron-related biomarkers, CRP and IL-6 in this cohort are previously reported [Citation19,Citation20].
Clinical outcome measures
Short-term adverse outcome was evaluated by a combination of intensive care unit admissions and 30-day mortality. Long-term outcome was evaluated as 5-year overall survival from discharge, excluding mortality within 30-days of admission.
Statistical analyses
Categorical data are presented as n (%). Categorical groups were compared by Pearson Chi-Square test or by McNemar’s Chi-Square test for paired observations. Normally distributed continuous variables are presented as mean (SD) and skewed data as median (interquartile range; IQR). The Mann-Whitney U test was used for comparison of independent continuous variables. The Spearman Rho correlation coefficient was used to assess correlations between continuous variables. The potential biomarkers were dichotomized at median for all prognostic evaluations and referred to as low and high groups. Short-term adverse outcome was evaluated by logistic regression. 5-year overall survival was displayed by Kaplan-Meier curves. Differences in survival rates for patients in the low- and high-value groups were assessed by the log-rank test. Cox regression analyses were performed uni- and multivariable. Age, sex, and a combined comorbidity parameter of prospectively registered conditions (graded as 0, 1, 2, or ≥3 comorbid conditions) were included as covariates in the multivariable model. The included comorbid conditions are listed in (see footnote for all single comorbid conditions) and further elaborated in a previous publication [Citation21]. A 2-sided significance level of 0.05 was considered statistically significant in all analyses. Analyses were performed using SPSS (IBM, version 26, Chicago, IL, USA) and GraphPad Prism (GraphPad Software, version 9.0.1, San Diego, California, USA).
Table 1. Baseline characteristics of 267 patients hospitalized with community-acquired pneumonia.
Results
Out of 320 adult community-acquired pneumonia patients screened for participation, 267 were included, as previously detailed [Citation15]. Baseline characteristics are shown in . Fifty-one patients (19%) experienced an adverse short-term outcome of intensive care unit admission (n = 48, 18%) and/or death within 30 days from admission (n = 10, 3.7%). One patient was lost to follow-up and was censored post-discharge, leaving 256 certain short-term survivors for inclusion in 5-year overall survival analyses, of which 67 patients (26%) died within 5 years. Median survival time for the patients who died during the 5-year follow-up was 26 months (IQR 7-40 months). Due to certain missing biomarker values and short-term deaths, the number of patients (events) included in Cox regression analyses were at the lowest 221 (55) at admission and 223 (50) at the 6-week follow-up.
Short-term outcome
None of the iron-related biomarkers were associated with short-term adverse outcome (Supplementary Table). However, at admission, 158 patients (59%) were anaemic according to the definition of the World Health Organization, which was reduced to 59 patients (26%) at the 6-week follow-up (p < 0.001). Notably, compared with no anaemia, moderate or severe anaemia were significantly associated with an adverse short-term outcome whereas mild anaemia showed no significant association. Finally, patients carrying an HFE variant did not have an increased risk of an adverse short-term outcome compared to those with the wild-type genotype (OR 0.77, 95% CI 0.38-1.56, p = 0.47).
Long-term outcome
Patients with high hepcidin and ferritin levels at admission had significantly improved 5-year overall survival compared to patients with low values (). The difference was most prominent for hepcidin, in which 86% of the patients in the high hepcidin group were alive at 5 years, compared to 63% in the low group (p < 0.001). However, at the 6-week follow-up, hepcidin and ferritin levels were not associated with 5-year survival (Supplementary Figure).
Figure 1. Kaplan–Meier curves of 5-year overall survival by biomarker values at hospital admission for community-acquired pneumonia, excluding deaths within 30 days from admission. Biomarker cut-offs at median (hepcidin 88 ng/mL, ferritin 386 μg/L, iron 2.9 μmol/L, transferrin 1.65 g/L, transferrin saturation 7.15%, soluble transferrin receptor 1.02 mg/L).
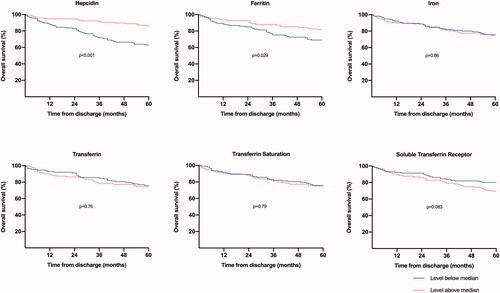
Similarly, univariable cox regression analyses show that low hepcidin and ferritin values at admission were associated with a significantly higher risk of death within 5 years, but only hepcidin remained significant in the multivariable model (HR 0.46, 95% CI 0.26-0.84, p = 0.011) (), which adjusted for age, sex, and an overall count of comorbid conditions (listed in ). The same pattern was seen if the comorbidity variable was defined as dichotomous, graded as 0, 1, or ≥2 comorbidities, or included as a continuous variable (data not shown). At the 6-week follow-up, but not at admission, low levels of iron and transferrin saturation, high levels of soluble transferrin receptor as well as anaemia, were associated with higher risk of death within 5 years, but only anaemia remained significant in the multivariable analysis.
Table 2. 5-Year overall mortality by Cox regression analysis.
Subgroup analyses
Significant correlations were found between admission values of hepcidin and CRP (rho 0.45, p < 0.001) and interleukin-6 (rho 0.55, p < 0.001). In further subgroup analyses, the predictive value of hepcidin at admission only proved significant for bacterial aetiology (HR 0.19, 95% CI 0.06-0.56, p = 0.003) and not for viral aetiology (HR 0.47, 95% CI 0.12-1.76, p = 0.26). The number of events in each microbial group was limited (bacteria: n = 18, viruses: n = 11), which prevented separate analyses of atypical and typical bacterial infections. The proportions of patients classified with anaemia in the low and high hepcidin group were similar (62% versus 55%, p = 0.31), and the predictive value of hepcidin remained unchanged upon adjustment for anaemia (HR 0.33, 95% CI 0.18-0.58, p < 0.001). In the low hepcidin group, 55% of the patients had soluble transferrin receptor levels above median compared to 41% in the high hepcidin group (p = 0.028), but adding soluble transferrin receptor as a covariate did not affect the predictive value of hepcidin (HR 0.29, 95% CI 0.15-0.54, p < 0.001). pO2 did not significantly differ between the two hepcidin groups (low hepcidin group: median 8.36 (IQR 2.18), high hepcidin group: median 8.41 (IQR 1.78), p = 0.30), nor did sO2 (low hepcidin group: median 92.7 (IQR 6.2), high hepcidin group: median 94.0 (IQR 4.5), p = 0.12). Long-term overall survival did not significantly differ between patients carrying HFE gene variants and wild-type genotypes (HR 1.50, 95% CI 0.83-2.69, p = 0.18). In the low hepcidin group, 71% of patients had at least one comorbid condition, compared with 46% in the high hepcidin group (p = 0.001).
Discussion
In this cohort study of hospitalized community-acquired pneumonia patients, low hepcidin levels at admission were independently associated with 5-year mortality. Because inflammation cause high levels of hepcidin, it is intriguing that an association between high levels of this biomarker and superior long-term prognosis was revealed.
We have previously described a substantial increase of both hepcidin and ferritin in the acute-phase of community-acquired pneumonia and showed that high hepcidin and ferritin levels in particular were associated with atypical bacterial aetiology [Citation19]. In the current study, we found no association between hepcidin, ferritin or any of the other iron-related biomarkers and short-term outcomes. Moderate to severe anaemia, however, was significantly related to adverse short-term outcomes.
Anaemia of inflammation seems to involve hepcidin-mediated iron-restricted erythropoiesis, iron- and hepcidin independent suppression of erythropoiesis, and reduced erythrocyte lifespan [Citation14]. The finding of anaemia in three of five patients at admission of acute-phase community-acquired pneumonia is interesting and higher than in previous reports, however, these studies have defined anaemia at lower haemoglobin values, which impairs comparability [Citation22,Citation23]. Similar to our results, development of anaemia (Hb ≤ 10 g/dL) in acute-phase community-acquired pneumonia has been found independently associated with 90-day mortality [Citation22]. Six weeks after the pneumonia, the proportion of anaemic patients was significantly reduced and, as previously reported, the levels of hepcidin and ferritin were substantially lower compared with admission levels [Citation19]. Thus, the impact of community-acquired pneumonia on levels of hepcidin, ferritin, and haemoglobin seems to be temporary for a majority of these hospitalized community-acquired pneumonia patients.
Our major finding in the present study was the strong association between low levels of hepcidin in the acute phase of community-acquired pneumonia and adverse long-term outcomes. Although a common perception is that biomarkers reflecting enhanced inflammation are associated with worse short-term outcomes, a previous long-term observation study of community-acquired pneumonia patients has shown the opposite [Citation24]. Moreover, the net balance of hepcidin is a result of the relative impact of numerous factors. Inflammation is potentially a strong inducer of hepcidin, but in severe iron deficiency, hepcidin levels may be low despite the presence of inflammation [Citation25]. Thus, iron deficiency and its original cause, rather than the hepcidin level itself, may affect long-term prognosis. Nevertheless, we found no significant difference in the prevalence of anaemia between the low- and high-level hepcidin groups. Hypoxia may also reduce plasma hepcidin levels [Citation26], but measurements of pO2 and sO2 did not support hypoxia as a considerable factor in our study. Other factors such as pre-existing liver disease, kidney disease, or genetic disorders could similarly affect the net hepcidin production [Citation25] as well as independently impact long-term prognosis. Although we did not have the prerequisites to specifically account for all potential influencing factors, overall comorbidity was significantly more frequent in the low hepcidin group. However, our observation remained significant upon adjustment for both age, sex, an overall count of comorbid conditions, and anaemia.
We speculate that low hepcidin levels may be related to pre-existing conditions and that low hepcidin levels increase the risk of non-transferrin-bound iron and tissue damage, which in turn may affect long-term survival. Because this finding was robust upon adjustment for comorbidity, age, sex, and anaemia, the effect may be independent of an underlying cause of the low hepcidin level. In addition, one may speculate if high hepcidin levels restrict iron availability to bacteria and thereby attenuate long-term harmful effects, as the association between hepcidin levels and long-term outcome was only seen in bacterial infections. However, at present, the reasons for the association between hepcidin levels and long-term prognosis are elusive and need further investigation.
We have not identified comparable studies examining the ability of iron-related biomarkers to predict outcome in community-acquired pneumonia, and prognostic studies of iron-related biomarkers in other infections show divergent results. In sepsis and patients admitted to the intensive care unit, low serum iron [Citation5], high transferrin saturation [Citation6], and high hepcidin and ferritin [Citation7] levels have been linked to unfavourable outcomes, whereas others have reported an association between low hepcidin levels at intensive care unit discharge and increased one-year mortality [Citation8]. Studies of Covid-19 patients have found associations between high levels of ferritin and severe and/or fatal cases [Citation9,Citation10]. Higher admission levels of hepcidin have been reported in non-surviving Covid-19 patients [Citation11], whereas another study found lower hepcidin levels in critically ill patients with Covid-19 compared to healthy controls [Citation27].
The primary aim of this explorative study was to assess relationships between iron-related biomarkers and prognosis. The association between biomarker levels and outcomes may however not be linear. Previous studies have shown J-shaped relationships for some biomarkers, where values in both ends of the scale are unfavorable [Citation28]. Due to the limited number of study participants and events, we did not have the power to make such a differentiation in the current study. In addition, this study did not include a control group, and as a single-center study with defined inclusion and- exclusion criteria, the external validity must be carefully considered. Importantly, this observational study was not designed to prove causal relationships.
Conclusion
In this prospective cohort study of hospitalized community-acquired pneumonia patients, low hepcidin levels at admission predicted 5-year overall mortality independent of age, sex, comorbidity, and anaemia. None of the iron-related parameters predicted short-term outcomes. The reasons for these novel and intriguing findings need further investigation.
Supplemental Material
Download MS Word (157.1 KB)Supplemental Material
Download MS Word (14.9 KB)Acknowledgements
We thank Ulla Helene Enger (Department of Laboratory Medicine, Drammen Hospital, Vestre Viken Hospital Trust, Drammen, Norway) for technical assistance and guidance in the analysis of HFE gene variants.
Disclosure of interest
The authors declare no conflict of interest.
Additional information
Funding
References
- World Health Organization. The top 10 causes of death [published 2020; updated 2020 Dec 09; cited 2021 June 05]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382.
- Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–250.
- Viasus D, Del Rio-Pertuz G, Simonetti AF, et al. Biomarkers for predicting short-term mortality in community-acquired pneumonia: a systematic review and Meta-analysis. J Infect. 2016;72(3):273–282.
- Xia JJ, Wang F, Jiang XN, et al. Serum iron levels are an independent predictor of in-hospital mortality of critically ill patients: a retrospective, single-institution study. J Int Med Res. 2019;47(1):66–75.
- Tacke F, Nuraldeen R, Koch A, et al. Iron parameters determine the prognosis of critically ill patients. Crit Care Med. 2016;44(6):1049–1058.
- Jiang Y, Jiang FQ, Kong F, et al. Inflammatory anemia-associated parameters are related to 28-day mortality in patients with sepsis admitted to the ICU: a preliminary observational study. Ann Intensive Care. 2019;9(1):67.
- Lasocki S, Lefebvre T, Mayeur C, on behalf of the FROG-ICU study group, et al. Iron deficiency diagnosed using hepcidin on critical care discharge is an independent risk factor for death and poor quality of life at one year: an observational prospective study on 1161 patients. Crit Care. 2018;22(1):314. ).
- Taneri PE, Gomez-Ochoa SA, Llanaj E, et al. Anemia and iron metabolism in COVID-19: a systematic review and Meta-analysis. Eur J Epidemiol. 2020;35(8):763–773.
- Zeng F, Huang Y, Guo Y, et al. Association of inflammatory markers with the severity of COVID-19: a Meta-analysis. Int J Infect Dis. 2020;96:467–474.
- Nai A, Lorè NI, Pagani A, et al. Hepcidin levels predict covid-19 severity and mortality in a cohort of hospitalized italian patients. Am J Hematol. 2021;96(1):E32–e35.
- Ganz T. Iron and infection. Int J Hematol. 2018;107(1):7–15.
- Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science. 2012;338(6108):768–772.
- Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133(1):40–50.
- Holter JC, Muller F, Bjorang O, et al. Etiology of community-acquired pneumonia and diagnostic yields of microbiological methods: a 3-year prospective study in Norway. BMC Infect Dis. 2015;15(1):64.
- WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. World Health Organization. Vitamin and mineral nutrition information system web site [published 2011; cited 2021 May 19]. Available from: http://www.who.int/vmnis/indicators/haemoglobin.pdf..
- Magnusson B, Örnemark U (eds.). Eurachem guide: the fitness for purpose of analytical methods – a laboratory guide to method validation and related topics. 2nd ed. 2014. Available from: http://www.eurachem.org
- Kemna EH, Tjalsma H, Podust VN, et al. Mass spectrometry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications. Clin Chem. 2007;53(4):620–628.
- Oppen K, Ueland T, Siljan WW, et al. Hepcidin and ferritin predict microbial etiology in Community-Acquired pneumonia. Open Forum Infect Dis. 2021;8(4):ofab082.
- Siljan WW, Holter JC, Nymo SH, et al. Cytokine responses, microbial aetiology and short-term outcome in community-acquired pneumonia. Eur J Clin Invest. 2018;48(1):e12865.
- Holter JC, Ueland T, Jenum PA, et al. Risk factors for long-term mortality after hospitalization for Community-Acquired pneumonia: a 5-Year prospective Follow-Up study. PLoS One. 2016;11(2):e0148741.
- Reade MC, Weissfeld L, Angus DC, et al. The prevalence of anemia and its association with 90-day mortality in hospitalized community-acquired pneumonia. BMC Pulm Med. 2010;10(1):15.
- Doshi SM, Rueda AM, Corrales-Medina VF, et al. Anemia and community-acquired pneumococcal pneumonia. Infection. 2011;39(4):379–383.
- Guertler C, Wirz B, Christ-Crain M, et al. Inflammatory responses predict long-term mortality risk in community-acquired pneumonia. Eur Respir J. 2011;37(6):1439–1446.
- Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood. 2016;127(23):2809–2813.
- Sonnweber T, Nachbaur D, Schroll A, et al. Hypoxia induced downregulation of hepcidin is mediated by platelet derived growth factor BB. Gut. 2014;63(12):1951–1959.
- Yağcı S, Serin E, Acicbe Ö, et al. The relationship between serum erythropoietin, hepcidin, and haptoglobin levels with disease severity and other biochemical values in patients with COVID-19. Int J Lab Hematol. 2021;43(S1):142–151.
- Grammer TB, Scharnagl H, Dressel A, et al. Iron metabolism, hepcidin, and mortality (the ludwigshafen risk and cardiovascular health study). Clin Chem. 2019;65(7):849–861.
