Abstract
Background
Seasonal influenza causes substantial numbers of hospitalizations annually. We have characterized the clinical picture and treatment practice in hospitalized adult influenza patients and assessed whether clinical risk scores on admission or influenza type were associated with severe outcomes.
Methods
Clinical characteristics and risk scores on admission (CRB65, CRB, SIRS and quick Sequential Organ Failure Assessment [qSOFA]), treatment and severe outcomes (defined as: stay in intensive care unit (ICU), receiving oxygen supplementation or staying ≥5 days in hospital), were recorded in patients hospitalized with influenza at Oslo University Hospital, Norway, between 2014 and 2018.
Results
Among the 156 included patients, 52.6% had influenza A(H3N2), 32.6% influenza B and 12.8% influenza A(H1N1). Median age was 70 years and 59.6% of patients were ≥65 years. Nine (5.8%) of the patients were treated in ICU, 43.0% received oxygen and 47.4% stayed ≥5 days in hospital. Overall, 34.6% of the patients had a high CRB score on admission which was associated with stay in ICU and oxygen supplementation. Multivariate analyses identified age, and pneumonia (46.8%), but not influenza type, to be associated with severe outcomes. Antiviral treatment was given to 37.2% of the patients, while 77.6% received antibiotics. Only 25.5% of patients with influenza B received antiviral therapy.
Conclusions
The influenza patients were mostly elderly, and few patients were treated in ICU. A high CRB score was associated with severe outcomes with possible implications for patient monitoring. Less than 40% of the patients received antiviral therapy, whereas the majority were treated with antibiotics, indicating potential for optimising treatment strategies.
Introduction
Seasonal influenza causes a substantial number of hospitalizations worldwide with high morbidity and mortality, particularly among the elderly [Citation1,Citation2]. A Norwegian study from 2019 found an average seasonal incidence rate of 48 influenza hospitalizations per 100,000 inhabitants, with increasingly higher hospitalization rates with age, in line with reports from other countries [Citation3–5]. The World Health Organization (WHO) defines that persons above 65 years, pregnant women and persons with chronic medical or immunosuppressive conditions are at greater risk of severe influenza disease or complications [Citation1].
Two subtypes of influenza A (H1N1 and H3N2) and two lineages of influenza B (Yamagata and Victoria) have caused seasonal influenza during the last decade (pre-COVID-19) [Citation1]. Influenza B has been suggested to cause less severe disease than the A subtypes, although findings have been inconsistent [Citation6–8]. Whether various influenza types and subtypes are responsible for different clinical features is not clear, but influenza A(H3N2) is possibly more associated with severe disease in older persons, whereas influenza A(H1N1) and B may affect younger persons to a larger extent [Citation9–11].
Clinical scorings systems are frequently used to evaluate severe infections like community acquired pneumonia and suspected sepsis [Citation12,Citation13]. Scoring systems validated for evaluation of community-acquired pneumonia such as the pneumonia severity index (PSI) and the CURB65 score (a variant of CRB65 where levels of P-urea is also included) have shown variable utility in prediction of outcome in hospitalized influenza patients [Citation14–16]. To our knowledge, there are no established clinical risk scores for assessing disease severity on admission in hospitalized influenza patients.
Pneumonia occurs frequently with influenza disease [Citation17]. Distinguishing between viral pneumonia and pneumonia with bacterial co-infections is challenging. Thus, hospitalized influenza patients often receive antibiotic treatment, even without evidence of bacterial coinfections, possibly leading to unnecessary use of antibiotics [Citation15,Citation17]. In contrast, while international guidelines recommend that antiviral treatment with neuraminidase inhibitors always should be considered in hospitalized patients with proven or suspected influenza, such therapy may be underused [Citation18–21].
In this study, we included adult patients with influenza admitted to Oslo University Hospital, Ullevål, Norway (a large tertiary care hospital) between 2014 and 2018. Our primary aim was to characterize the clinical picture and antimicrobial treatment practice. Second, we explored whether established clinical risk scores (CRB65, CRB, qSOFA and SIRS) on admission were associated with severe outcomes in these patients. Finally, we studied whether age and influenza types/subtypes (from here on called (sub)types) were associated with the clinical presentation, clinical risk scores, severe outcomes, and antimicrobial treatment.
Materials and methods
Study population
The study was conducted at Oslo University Hospital, Ullevål, Norway, throughout the four consecutive influenza seasons 2014–2015, 2015–2016, 2016–2017 and 2017–2018. Potential participants were identified by daily reports from the Department of Medical Microbiology where all airway samples positive for influenza from patients in various wards were recorded. Influenza was confirmed by an in-house influenza virus A/B RNA Real Time-PCR, targeting conserved areas encoding the M-protein. Patients ≥18 years, with community-acquired influenza and laboratory confirmed influenza, were included in the study within the first 3 days of hospitalization. There were no exclusion criteria, but patients who were not able to provide a written consent were not included. Patients were only included during weekdays due to lack of study personnel during weekends and holidays.
Clinical data, risk scores and outcomes
Patient data were collected through patient interviews and from medical records. Influenza vaccination status in the season of hospitalization was based on self-report. Seven individuals with unknown vaccination status were recorded as unvaccinated.
Nine influenza-specific self-reported symptoms were recorded: Cough, fever, dyspnoea, muscle pain, sore throat, headache, rhinorrhea, chest pain and gastrointestinal symptoms. We assessed C-reactive protein (CRP) levels, pneumonia (radiological infiltrates on chest x-rays) on admission, and microbiological findings in nasopharyngeal swabs, sputum and blood cultures. In the respiratory samples, bacterial species and respiratory viruses other than influenza (detected with in-house PCR panels) were recorded. Influenza A isolates were further identified by a subtype-specific PCR targeting the HA-gene (Flu Differentiation for Influenza A©, Fast Track Diagnostics, Medical Wire), differentiating between the subtypes H1, H3, and H1N1pdm09. Antimicrobial and antiviral treatment during hospital stay was recorded.
The following four clinical risk scores on admission were evaluated retrospectively:
CRB65 score; one point to each of the following signs: confusion, respiratory rate ≥30/min, blood pressure (systolic <90 mmHg or diastolic ≤60 mmHg), and age≥65 years [Citation12]. CRB65 ≥ 2 points were defined as severe disease (CRB65high) [Citation12,Citation22].
CRB; a modified CRB65 score, without points for age. CRB ≥ 1 was defined as severe disease (CRBhigh).
Systemic inflammatory response syndrome (SIRS) score; one point to each of the following signs: temperature (>38 °C or <36 °C), heart rate >90 beats/min, respiratory rate >20/min, leucocytes (>12,000/µL or <4000/µL). SIRS ≥ 3 was defined as severe disease (SIRShigh) [Citation23].
quick Sequential Organ Failure Assessment (qSOFA) score; one point for the following clinical signs: altered mental status, respiratory rate ≥22/min, systolic blood pressure ≤100 mmHg [Citation13]. qSOFA ≥2 was defined as severe disease (qSOFAhigh).
The following severe clinical outcomes were recorded: treatment in intensive care unit (ICU), oxygen supplementation during the hospital stay, and staying ≥5 days in hospital.
Statistics
Differences in reported symptoms, clinical findings, risk scores, severe outcomes and microbial treatment according to age and influenza (sub)types were analysed using Wilcoxon rank-sum test for continuous variables, and Chi-square test for categorical variables. Logistic regression was applied to estimate odds ratios (ORs) with corresponding 95% confidence intervals (CIs) to study relevant exposures in relation to the various binary severe clinical outcomes or microbial treatment for explorative reasons. All regression analyses were adjusted for sex, having ≥2 predisposing conditions and age (except CRB65 scores). Age and having ≥2 predisposing conditions were weakly correlated (Spearman’s rank correlation coefficient = 0.30). Thus, collinearity was not a concern. Antimicrobial treatment was also adjusted for season and days between symptom onset and hospitalization. Statistical analyses were performed with Stata/SE version 15.0. The level of statistical significance was <0.05 (two-sided).
Ethics
The study was approved by The Regional Committee for Medical and Health Research Ethics in South Eastern Norway (Case number 2013/2033). Written informed consent was obtained before inclusion.
Results
Study participants
In total, 156 patients (52.6% women) were included during the four influenza seasons from 2014/2015 to 2017/2018 (seasons 1–4, respectively) (). The patients’ median age was 70 years (range 18–102) and 59.6% were ≥65 years. Conditions predisposing for severe influenza disease were common, 78.2% of patients had at least one predisposing condition, and 43.6% had ≥2 predisposing conditions. Particularly, cardiovascular and pulmonary conditions were prevalent. Only 13.5% were without any known predisposing conditions and <65 years.
Table 1. Patient characteristics and distribution of influenza (sub)types over the 4 consecutive influenza seasons.
Influenza vaccine was administered to 32.7% of the patients during the season of hospitalization. Patients ≥65 years were more often vaccinated (39.8%) as compared to younger patients (22.2%, p=.02).
More than half (52.6%) of the patients had influenza A(H3N2), followed by influenza B (32.6%) and influenza A(H1N1) (12.8%). In seasons 1 and 3, influenza A(H3N2) dominated among the patients, whereas influenza A(H1N1) was more common in season 2, and influenza B dominated in season 4.
Clinical presentation and risk scores
The median duration of self-reported symptoms before admission was 3 days (range 0–30 days). Cough (93.0%) and fever (76.9%) were the most common symptoms (). Older patients (≥65 years) reported less fever, sore throat and headache than younger patients (<65 years). The number of reported symptoms was also lower among older than younger patients.
Table 2. Symptoms, pneumonia and clinical risk scores on admission, total and by age group.
A total of 46.8% patients had radiological infiltrates on chest x-rays compatible with pneumonia on admission (). There was a trend that older patients were more likely to have pneumonia than younger patients (p =.07). CRP on admission ranged from 0.6 to 316 mg/L, with a median of 43 mg/L.
Airway pathogen bacterial strains suggesting co-infections were found in 36.7% of the 150 patients with available airway samples. Streptococcus pneumoniae was most frequently found (12.7%). Other respiratory viruses were detected in 10 patients (6.7%); respiratory syncytial virus being most frequent (4 of the 10 patients). Blood cultures were sampled from 87.2% of the patients, but all were culture negative.
Retrospectively, based on their presentation on admission, patients were scored according to four different clinical risk scoring systems: CRB65, CRB, SIRS and qSOFA. 52.6% had at least one high score, 16.0% had two high scores, while only 7.0% and 4.5% had three or four high scores, respectively. Older (≥65 years) and younger patients were equally likely to have at least one high score (p=.97). About 34.6% of patients had a high CRB score, while CRB65high (22.4%), SIRShigh (25.0%), and qSOFAhigh (14.1%) were less common (). No patients below 65 years of age had CRB65high, otherwise, the proportion of patients with the individual high clinical risk scores were not statistically different between older and younger age groups.
Clinical characteristics associated with severe clinical outcomes
Nine patients (5.8%) were treated in ICU, 43.0% received oxygen supplementation during their hospital stay and 47.4% stayed in hospital ≥5 days (). None of the clinical characteristics or laboratory data were associated with treatment in ICU, but there were few patients in this group. Age, pneumonia, CRP level and female sex were associated with increased risk of receiving oxygen supplementation during the hospital stay. Age and pneumonia were also associated with increased risk of staying ≥5 days in hospital. Being vaccinated with an influenza vaccine, was not associated with any of the outcomes.
Table 3. Severe outcomes and associations with patient characteristics, laboratory data, clinical risk scores and antimicrobial treatment.
CRBhigh on admission was the only clinical risk score associated with ICU treatment (). CRBhigh, SIRShigh and CRB65high were associated with oxygen supplementation, while CRB65high was the only score associated with staying in hospital ≥5 days. qSOFAhigh was not associated with any of the severe clinical outcomes.
There was a trend that receiving antiviral treatment was associated with being treated in ICU (p=.052) (). Antibiotic therapy was associated with oxygen supplementation and staying ≥5 days in hospital.
Clinical characteristics associated with influenza (sub)types
Patients with influenza A(H3N2) were significantly older (median age 72 years) than those with influenza B (median 64 years, p= .032), or influenza A(H1N1) (median age 55 years, p=.025). Cough was less common with influenza A(H1N1) than the other subtypes ( and Supplementary Table 1). Patients with influenza B reported more rhinorrhea and were more likely to report ≥5 symptoms than patients with A(H1N1) and they reported a sore throat more often than patients with influenza A(H3N2). Pneumonia was found equally frequent with the three influenza (sub)types (data not shown).
Figure 1. Symptoms, clinical risk scores and outcomes of disease and according to influenza (sub)types. (a) Percentage of patients self-reporting specific symptoms prior to admission. (b) Percentage of patients with high clinical risk scores on admission defined by CRB, CRB65, SIRS and qSOFA criteria. CRB is a modified CRB65 score, where age of the patients no longer is part of the score. (c) Percentage of patients with various severe outcomes. Oxygen: oxygen supplementation and ICU: intensive care unit. *indicates p<.05 and **indicates p < 0.01 (chi2 analyses).
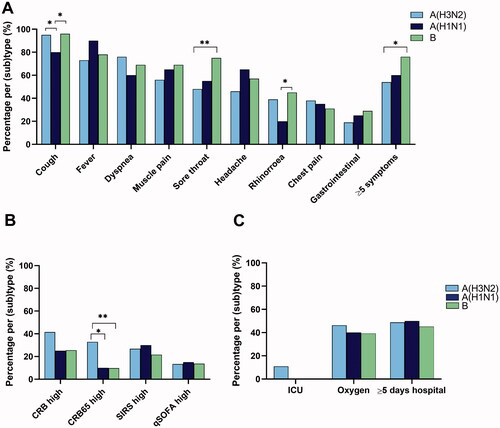
The proportion of patients with high CRB, qSOFA or SIRS scores, was similar for the influenza sub(types) (). In contrast, more patients with influenza A(H3N2) had CRB65high compared to influenza B (p=.002) and influenza A(H1N1) (p=.042). When adjusting for sex and having ≥2 predisposing conditions in multiple regression analyses (Supplementary Table 2), the difference in CRB65high was only significant between influenza B and A(H3N2) patients, probably due to the difference in age for these patients.
Influenza A(H3N2) was the only (sub)type found in patients with treatment in ICU ( and Supplementary Table 3), whereas the proportion of patients who received oxygen supplementation or stayed in hospital ≥5 days did not differ between influenza (sub)types.
Antimicrobial treatment
During their hospital stay, 37.2% of patients received antiviral treatment (oseltamivir). Patients with influenza A(H3N2) received more antivirals than patients with influenza B (46.3% vs. 25.5%, respectively) ( and Supplementary Table 4).
Figure 2. Use of antimicrobial treatment according to influenza season and influenza (sub)types. (a) Percentage of patients receiving antiviral and antibiotic treatment over the four consecutive influenza seasons. For each treatment type, season 1 is compared with the other seasons. (b) Percentage of patients receiving antiviral and antibiotic treatment according to their influenza (sub)types. *indicates p<.05 and **indicates p<.01 (chi2 analyses).
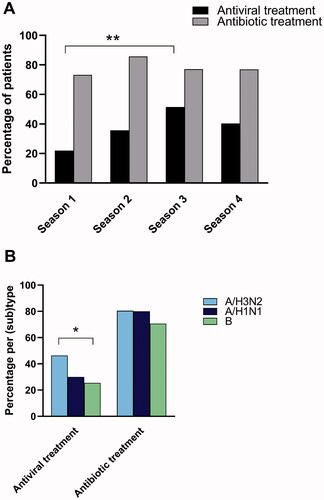
Table 4. Antiviral and antibiotic treatment and associations with patient characteristics, laboratory data and risk scores.
A total of 77.6% of the patients received antibacterial therapy (). Benzylpenicillin was ordinated to most of the patients receiving antibiotics (75.2%). In 14 patients, benzylpenicillin was combined with gentamicin. Only 8.3% of patients were treated with a second or third generation cephalosporin. There was no association between the influenza (sub)types and antibiotic treatment. A combination of antiviral and antibiotic treatment was given to 31.4% of the patients.
The likelihood of receiving antiviral therapy decreased with increasing duration between onset of symptoms and admission to hospital (measured in days, p<.001). In contrast, no association with symptom duration was observed for antibiotic treatment (). Pneumonia, CRP level and detection of bacterial isolates in airway samples were associated with receiving antibiotic therapy. Antiviral treatment was significantly higher in season 3 (51.4%) than in season 1 (22.0%), while the use of antibiotics was constant during the four seasons ( and Supplementary Table 4).
Patients with qSOFAhigh were less likely to receive antiviral treatment than patients with a corresponding low score (p=.025), whereas patients with SIRShigh were more likely to receive antivirals and antibiotics than patients with a low SIRS score (p=.060 and .047, respectively). No statistically significant difference in antimicrobial treatment was found between patients with high or low CRB65/CRB scores.
Discussion
Here, we describe the clinical picture and treatment practice in adult influenza patients during four consecutive influenza seasons (2014–2018) and explore whether clinical risk scores or influenza (sub)types are associated with severe clinical outcomes. Approximately half of the patients had at least one high clinical risk score on admission indicating severe disease. The clinical score CRBhigh showed an association with treatment in ICU and oxygen supplementation irrespective of the age of the patients. However, the number of patients was limited, and the present study was not designed to answer if such a score can be used to predict clinical outcomes in influenza patients.
Possible differences in disease severity due to different influenza (sub)types have been addressed in several previous studies with conflicting results [Citation10,Citation24–28]. In our study, we found no differences in occurrence of pneumonia or CRBhigh, SIRShigh and qSOFAhigh scores between the influenza (sub)types. There were more patients with CRB65high among patients with A(H3N2) and all of the nine patients in ICU had A(H3N2), possibly explained by influenza A(H3N2) patients being older. When studying the other defined outcomes, no differences between influenza (sub)types were found. Contrary to this, Delgado-Sanz et al, recently reported more severe outcome in patients hospitalized with A(H1N1) compared to A(H3N2) and B, while Wang et al. found that hospitalized patients with influenza B had less severe disease than influenza A patients [Citation7]. Contradictory findings may partly be explained by methodological differences both in terms of patient inclusion and criteria for assessment of disease severity, and more studies on this are warranted.
Most of the patients in this study had increased risk of severe influenza disease, either due to their age or other predisposing conditions. We found that patients infected with influenza A(H3N2) were significantly older than patients with influenza B, who again were older than patients with influenza A(H1N1). This age distribution pattern has also been reported by others [Citation9,Citation10,Citation26]. In studies comparing the age of hospitalized patients with either influenza A or influenza B, some reported no difference in age, while others found that patients with influenza B were younger than those with influenza A. [Citation7,Citation8,Citation28]. It is not known why the elderly population seems to be more at risk for severe outcome from infection with influenza A(H3N2). Contributing factors may be the combined effects of ageing of the immune system, the A(H3N2) virus evolving more rapidly than the other sub(types) and immune imprinting of today’s elderly patients with A(H1N1) [Citation29–32].
Cough and fever were the most common self-reported symptoms, as observed in other studies in hospitalized influenza patients [Citation8,Citation15,Citation26,Citation33]. Patients ≥65 years reported fewer typical influenza symptoms than the younger patients, supporting that elderly patients present with a less specific clinical picture of influenza disease [Citation26,Citation33,Citation34]. For symptom presentation, we found small differences between the influenza (sub)types, with influenza B patients reporting more rhinorrhea and cough than A(H1N1) patients. Other studies also reported more rhinorrhea and less fever in patients with influenza B than influenza A (sub)types [Citation8,Citation11,Citation35], yet other studies did not identify such differences [Citation24]. The different influenza symptoms are probably correlated to each other, and clinical implications of possible differences between influenza (sub)types are uncertain.
Antibiotic therapy was given to almost 80% of the patients, which corresponds well with previous studies on hospitalized influenza patients [Citation15,Citation33]. Clinical assessments using scoring systems could possibly be useful to guide antibiotic treatment in influenza patients. In our study, only patients with SIRShigh on admission were more likely to receive antibiotics. Surprisingly, patients with high CRB65/CRB were not more likely to receive antibiotics, as CRB65 was designed to assess severity of community-acquired pneumonia [Citation12]. However, antibiotic treatment was more frequent in patients with pneumonia, having higher CRP levels or bacterial findings in airway samples, all factors that are indicative of bacterial coinfections. While CRP is a widely used biomarker to discriminate between bacterial and viral infection, CRP unfortunately discriminates poorly between pure viral and bacterial coinfections in influenza disease [Citation36,Citation37].
In our study, less than 40% of the patients received antiviral therapy. International guidelines recommend antiviral treatment for all hospitalized patients with suspected or proven influenza disease [Citation19]. Moreover, antiviral treatment was part of the national recommendation at the time when the study started. Other studies from the same time have shown a great variation of antiviral prescription rates in hospitalized patients, from 12% to 93% [Citation8–10,Citation26,Citation27]. Starting antiviral therapy within 48 h of symptom onset has been associated with best outcome in hospitalized influenza patients, but beneficial effects have also been seen in patients with symptom durations up to 5–7 days [Citation19,Citation38]. In our patient population, patients with fewer days between symptom onset and hospitalization were more likely to receive antivirals, suggesting that the clinicians weighted this information as important when deciding upon treatment. We found a trend of increased use of antivirals from the 2014/15 and 2015/16 seasons to the 2016/17 season, suggesting increased awareness of the beneficial effect of antiviral treatment during the study period. Interestingly, we found that patients with influenza B received less antiviral therapy than patients with A(H3N2). Similar differences were also reported in other studies [Citation9,Citation27,Citation28]. Possibly the clinicians deemed that patients with influenza B would benefit less from antiviral therapy or the observed difference could be explained by patients with influenza A(H3N2) being older and more fragile which lowered the threshold for an aggressive therapeutic approach.
Our study has several limitations. First, the overall number of patients was low, and the study was not designed to conclude on risk scores. Second, few patients with influenza A(H1N1) were included. Third, we did not discriminate between the influenza B lineages, which may differ in their clinical presentation and severity [Citation24]. Fourth, we were not able to include all the patients that were admitted to the hospital over the four seasons, potentially creating a bias in the patient population. Patients with a relatively mild disease who were admitted during weekends or holidays and rapidly discharged could be missed due to study logistics, as well as the most critically ill patients who could be missed if they were not able to consent.
A strength of this study is that all patients had PCR-confirmed influenza. The influenza (sub)type distribution among the patients coincided with the virus (sub)types circulating in Norway and in Europe in this period [Citation1,Citation9,Citation10,Citation39]. We also present adjusted results which are lacking in many studies on influenza (sub)types and severity [Citation24]. By including patients over several seasons, we could study possible differences between influenza (sub)types and potential changes in antimicrobial treatment with time.
To conclude, the patients in our study were mostly elderly and/or with conditions predisposing for severe influenza. Clinical scoring systems were associated with severe outcomes, but whether such scores can be used to predict severe outcomes in influenza patients, needs to be studied further in larger studies. The different influenza (sub)types were not associated with differences in clinical outcomes, but we observed more influenza A(H3N2) in older than in younger individuals and that patients with influenza B received less antiviral therapy.
Supplemental Material
Download MS Word (14.6 KB)Supplemental Material
Download MS Word (14.9 KB)Supplemental Material
Download MS Word (15.3 KB)Supplemental Material
Download MS Word (19.2 KB)Acknowledgements
The authors thank the patients that participated in this study. The authors also wish to thank the staff at the Department of Infectious Diseases and the Department of Microbiology, Oslo University Hospital for handling of biological samples and laboratory analyses.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Influenza (seasonal). Geneva, Switzerland: World Health Assosiation; 2018. [updated 2018.11.06. Available from: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
- Gran JM, Iversen B, Hungnes O, et al. Estimating influenza-related excess mortality and reproduction numbers for seasonal influenza in Norway, 1975–2004. Epidemiol Infect. 2010;138(11):1559–1568.
- Czaja CA, Miller L, Alden N, et al. Age-related differences in hospitalization rates, clinical presentation, and outcomes among older adults hospitalized with influenza—U.S. Influenza hospitalization surveillance network (FluSurv-NET). Open Forum Infect Dis. 2019;6(7):ofz225.
- Hauge SH, Bakken IJ, de Blasio BF, et al. Burden of medically attended influenza in Norway 2008–2017. Influenza Other Respir Viruses. 2019;13(3):240–247.
- Oliva J, Delgado-Sanz C, Larrauri A, et al. Estimating the burden of seasonal influenza in Spain from surveillance of mild and severe influenza disease, 2010–2016. Influenza Other Respi Viruses. 2018;12(1):161–170.
- Su S, Chaves SS, Perez A, et al. Comparing clinical characteristics between hospitalized adults with laboratory-confirmed influenza a and B virus infection. Clin Infect Dis. 2014;59(2):252–255.
- Wang Y, Fan G, Horby P, et al. Comparative outcomes of adults hospitalized with seasonal influenza a or B virus infection: application of the 7-Category ordinal scale. Open Forum Infect Dis. 2019;6(3):ofz053.
- Cohen R, Babushkin F, Geller K, et al. Characteristics of hospitalized adult patients with laboratory documented influenza A, B and respiratory syncytial Virus - A single center retrospective observational study. PLoS One. 2019;14(3):e0214517.
- Delgado-Sanz C, Mazagatos-Ateca C, Oliva J, et al. Illness severity in hospitalized influenza patients by virus type and subtype, Spain, 2010–2017. Emerg Infect Dis. 2020;26(2):220–228.
- Martinez A, Soldevila N, Romero-Tamarit A, et al. Risk factors associated with severe outcomes in adult hospitalized patients according to influenza type and subtype. PLoS One. 2019;14(1):e0210353.
- Wie SH, So BH, Song JY, et al. A comparison of the clinical and epidemiological characteristics of adult patients with laboratory-confirmed influenza a or B during the 2011–2012 influenza season in Korea: a multi-center study. PLoS One. 2013;8(5):e62685.
- Bauer TT, Ewig S, Marre R, et al. CRB-65 predicts death from community-acquired pneumonia. J Intern Med. 2006;260(1):93–101.
- Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):762–774.
- Mulrennan S, Tempone SS, Ling IT, et al. Pandemic influenza (H1N1) 2009 pneumonia: CURB-65 score for predicting severity and nasopharyngeal sampling for diagnosis are unreliable. PLoS One. 2010;5(9):e12849.
- Akers IE, Weber R, Sax H, et al. Influence of time to diagnosis of severe influenza on antibiotic use, length of stay, isolation precautions, and mortality: a retrospective study. Influenza Other Respir Viruses. 2017;11(4):337–344.
- Riquelme R, Jimenez P, Videla AJ, et al. Predicting mortality in hospitalized patients with 2009 H1N1 influenza pneumonia. The international journal of tuberculosis and lung disease: the official journal of the international union against. Int J Tuberc Lung Dis. 2011;15(4):542–546.
- Blyth CC, Webb SA, Kok J, et al. The impact of bacterial and viral co-infection in severe influenza. Influenza Other Respir Viruses. 2013;7(2):168–176.
- Clark TW, Medina MJ, Batham S, et al. Adults hospitalised with acute respiratory illness rarely have detectable bacteria in the absence of COPD or pneumonia; viral infection predominates in a large prospective UK sample. J Infect. 2014;69(5):507–515.
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the infectious diseases society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa. Clin Infect Dis. 2019;68(6):e1–e47.
- Wolkewitz M, Schumacher M. Neuraminidase inhibitors and hospital mortality in British patients with H1N1 influenza A: a re-analysis of observational data. PLoS One. 2016;11(9):e0160430.
- Katzen J, Kohn R, Houk JL, et al. Early oseltamivir after hospital admission is associated with shortened hospitalization: a 5-Year analysis of oseltamivir timing and clinical outcomes. Clin Infect Dis. 2019;69(1):52–58.
- Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382.
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644–1655.
- Caini S, Kroneman M, Wiegers T, et al. Clinical characteristics and severity of influenza infections by virus type, subtype, and lineage: a systematic literature review. Influenza Other Respir Viruses. 2018;12(6):780–792.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186.
- Loubet P, Samih-Lenzi N, Galtier F, et al. Factors associated with poor outcomes among adults hospitalized for influenza in France: a three-year prospective multicenter study. J Clin Virol. 2016;79:68–73.
- Chagvardieff A, Persico N, Marmillot C, et al. Prospective comparative study of characteristics associated with influenza a and B in adults. Med Mal Infect. 2018;48(3):180–187.
- Sharma Y, Horwood C, Hakendorf P, et al. Clinical characteristics and outcomes of influenza a and B virus infection in adult Australian hospitalised patients. BMC Infect Dis. 2020;20(1):913.
- Ferguson NM, Galvani AP, Bush RM. Ecological and immunological determinants of influenza evolution. Nature. 2003;422(6930):428–433.
- Gostic KM, Bridge R, Brady S, et al. Childhood immune imprinting to influenza a shapes birth year-specific risk during seasonal H1N1 and H3N2 epidemics. PLoS Pathog. 2019;15(12):e1008109.
- Lessler J, Riley S, Read JM, et al. Evidence for antigenic seniority in influenza A (H3N2) antibody responses in Southern China. PLoS Pathog. 2012;8(7):e1002802.
- Nikolich-Žugich J. The aging immune system: challenges for the 21st century. Semin Immunol. 2012;24(5):301–302.
- Babcock HM, Merz LR, Fraser VJ. Is influenza an influenza-like illness? Clinical presentation of influenza in hospitalized patients. Infect Control Hosp Epidemiol. 2006;27(3):266–270.
- Falsey AR, Baran A, Walsh EE. Should clinical case definitions of influenza in hospitalized older adults include fever? Influenza Other Respir Viruses. 2015;9(1):23–29.
- Gutierrez-Pizarraya A, Perez-Romero P, Alvarez R, et al. Unexpected severity of cases of influenza B infection in patients that required hospitalization during the first postpandemic wave. J Infect. 2012;65(5):423–430.
- Haran JP, Beaudoin FL, Suner S, et al. C-reactive protein as predictor of bacterial infection among patients with an influenza-like illness. Am J Emerg Med. 2013;31(1):137–144.
- Ingram PR, Inglis T, Moxon D, et al. Procalcitonin and C-reactive protein in severe 2009 H1N1 influenza infection. Intensive Care Med. 2010;36(3):528–532.
- Dominguez A, Romero-Tamarit A, Soldevila N, et al. Effectiveness of antiviral treatment in preventing death in severe hospitalised influenza cases over six seasons. Epidemiol Infect. 2018;146(7):799–710.
- Bragstad C, Hungnes O, Knudsen TH, et al. Årsrapporter for influensa. Oslo, Norway: Norwegian Institute of Public Health; 2019.
