Abstract
Background
Patients with haematological malignancies have an increased susceptibility for COVID-19 and higher mortality. They may also have prolonged symptoms and viral shedding. Clinical trials have not specifically addressed the management of this patient group. We present a lymphoma patient with COVID-19 who was treated with remdesivir, and a literature review of similar cases.
Methods
SARS-CoV-2 RT-PCR, virus culture and whole-genome sequencing were performed from nasopharyngeal swabs and antibody testing from serum. In addition, SARS-CoV-2 nucleocapsid antigen was tested from serum. Medline was searched for reported cases of lymphoma and COVID-19 treated with remdesivir.
Results
The patient was undergoing lymphoma treatment including chemotherapy, rituximab and prednisolone. After diagnosis of COVID-19, broad-spectrum antibiotics were administered due to neutropenia and fever. After 20 d of fever with no signs of co-infection, remdesivir was initiated with rapid response. The treatment was continued for 4 d. Serum SARS-CoV-2 antibody tests were negative 20, 30 and 66 d from symptom onset. Before starting remdesivir, the SARS-CoV-2 PCR and virus culture from the nasopharynx and serum antigen test were positive. From earlier reports, we identified a total of eleven cases of lymphoma and COVID-19 treated with remdesivir accompanied by other antivirals and anti-inflammatory agents.
Conclusions
As shown in this and earlier reports on lymphoma patients, the clinical course of COVID-19 may be protracted and a humoral immune response may remain absent. In addition, optimal management remains undecided. The presented patient responded well to a short course of remdesivir.
Keywords:
Introduction
There is considerable variation in the risk that SARS-CoV-2 poses on different groups of people; those with haematological malignancies are more susceptible to infection and have higher mortality compared to the general population [Citation1,Citation2]. Immunocompromised patients may shed the virus for long periods of time [Citation3]. We present a lymphoma patient with COVID-19 who received remdesivir treatment, accompanied by a literature review of similar cases.
Materials and methods
All tests were performed in an accredited laboratory (HUS Diagnostic Centre, HUSLAB Clinical Microbiology, Helsinki, Finland). The SARS-CoV-2 RT-PCR test from respiratory samples and serological testing are in routine clinical use at our hospital, and have been described earlier [Citation4,Citation5]. To ensure higher specificity, a combination of two tests was used to detect antibodies targeted against both spike (SARS-CoV-2 IgG ELISA, Euroimmun, Germany) and nucleocapsid (Abbott SARS-CoV-2 IgG CLIA assay, Abbott, IL) proteins [Citation5].
A commercially available test, Salocor SARS-CoV-2 Antigen Quantitative Assay Kit© 23 (Salofa Ltd, 24 Salo, Finland), was used for nucleocapsid antigen testing from serum; a report on test performance is currently available in preprint [Citation6]. The virus was cultured in Vero E6 cells and whole-genome sequencing was performed, as described earlier [Citation4].
A systematic literature review was performed to identify reported outcomes of lymphoma patients treated with remdesivir for COVID-19. The search was conducted in Medline on 19 May 2021. Two authors (M.K and J.P) carried out the initial search using the search terms ‘covid’ and ‘lymphoma’. The exclusion criteria were (1) English translation not available; (2) insufficient information on outcomes; (3) not related to active COVID-19 or lymphoma; (4) remdesivir not used.
Results (case presentation)
A 46-year-old woman presented to hospital on the day of onset of fever. Her nasopharyngeal swab sample tested positive for SARS-CoV-2 and sequencing revealed the B.1.1.7 variant. During the preceding 4 d, she had experienced mild symptoms including sore throat, runny nose and cough. Seven weeks earlier she had been diagnosed with stage IVA CD5-negative small-cell lymphoma with suspected transformation based on extensive radiologic findings. The lymphoma had been treated with two cycles of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP), a third cycle including etoposide (R-CHOEP) and high-dose methotrexate twice. She had ongoing trimethoprim-sulfamethoxazole prophylaxis against Pneumocystis jirovecii and subcutaneous dalteparin treatment for deep vein thrombosis. She had not received a COVID-19 vaccine.
The clinical course of our patient is presented in . The COVID-19 symptoms began one day after R-CHOEP during prednisolone treatment. Upon hospital admission, she had fever and tachycardia, but was normotensive and did not develop organ dysfunction. The laboratory work-up showed severe neutropaenia, for which granulocyte colony stimulating factor treatment was administered, as well as lymphocytopaenia and thrombocytopaenia. A chest X-ray showed minor bilateral infiltrates in lower lung fields consistent with viral pneumonitis.
Figure 1. Clinical course of the SARS-CoV-2 infection following chemotherapy and rituximab treatment for lymphoma. CFP, cefepim; CFX, ciprofloxacin; VAN, vancomycin; LVX, levofloxacin; RDV, remdesivir. Neutropaenia: neutrophils <1.0 × 109/l; Lymphopaenia: lymphocytes <1.0 × 109/l. RT-PCR cycle threshold (ct) values representing an inverse of viral load given for nasopharyngeal swab samples taken on days 5 and 20.
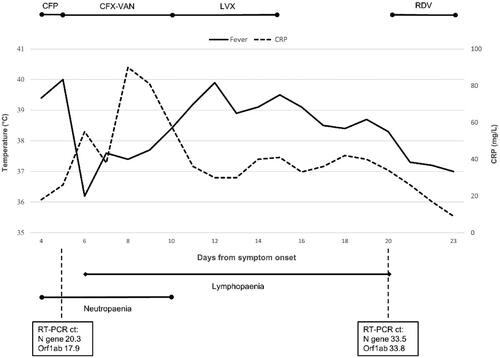
Empirical antimicrobial therapy with cefepime was initiated due to fever and neutropaenia, followed by vancomycin and ciprofloxacin after anaphylaxis, these were later changed to levofloxacin. Physical examinations showed no signs of bacterial foci, and cultures of blood and urine were negative. A computed tomography scan of the chest, abdomen and pelvis showed residual lymphoma findings and bilateral, peripheral, ground-glass opacities consistent with COVID-19 pneumonia, without suspicion of bacterial superinfection. Levofloxacin was discontinued.
The patient had persistent fever for over 2 weeks, accompanied by fatigue, dyspnoea on exertion and diarrhoea. She had mild hypoxaemia with intermittent need for supplemental oxygen. A SARS-CoV-2 PCR test from a nasopharyngeal swab sample 19 d after the onset of symptoms was positive, but based on cycle threshold values and virus culture, the viral load was decreasing (Supplementary material). A serum antigen test for SARS-CoV-2 was positive before remdesivir treatment 20 d from the onset of symptoms. SARS-CoV-2 antibodies were negative after 20, 30 and 66 d of symptoms.
Intravenous remdesivir was commenced after 20 d of symptoms with a 200 mg loading dose and the patient soon became afebrile. The treatment was continued 100 mg daily for three more days, after which she was discharged in good overall condition. Although 5 d treatment duration was generally recommended, in this case 4 d was considered adequate due to rapid recovery.
In the literature review, a total of 227 articles were found and eleven cases were included () [Citation7–16].
Table 1. Previously reported lymphoma patients treated with remdesivir for COVID-19.
Discussion
There is a wide range of clinical manifestations of COVID-19, and the risk of mortality depends on age, sex, comorbidities and individual immune responses [Citation2,Citation17,Citation18]. In comparison to immunocompetent patients, those with haematological malignancies can have more protracted symptoms, including delayed respiratory failure or relapse even months later [Citation7,Citation16,Citation18–20]. In a recent study, anti-CD20 therapy such as rituximab was associated with a longer hospital stay and increased mortality [Citation21].
Our patient had a positive serum SARS-CoV-2 antigen test consistent with viraemia 20 d after the onset of symptoms, although we were not able to test for live virus in the blood. However, virus culture from a nasopharynx swab was positive. In earlier reports, immunocompromised patients have shed live virus for months [Citation3,Citation7]. Therefore, COVID-19 in severely immunocompromised hosts may present both with persistent viral replication and an atypical clinical course.
A functioning adaptive immune response including humoral and cellular immunity is considered important for efficient clearance of SARS-CoV-2 infection. Circulating antibodies are usually detected within 15 d after infection onset, but our patient failed to develop a humoral response probably due to prior rituximab therapy [Citation17]. However, recovery is achievable without seroconversion and in earlier studies 1–9% of PCR-confirmed cases have remained seronegative, which underlines the role of T cell responses in clearing the virus [Citation17].
Remdesivir has been shown to inhibit the replication of coronaviruses including SARS-CoV-2 in vitro [Citation22]. The ACTT-1 trial demonstrated a shorter time to recovery in the remdesivir group compared to placebo among hospitalized COVID-19 patients [Citation23]. Currently there is, however, a lack of evidence from clinical trials for mortality benefit in the treatment of hospitalized patients [Citation22,Citation24]. Timing may be important, as antivirals are expected to be efficacious only in the viral replication phase [Citation22]. Indeed, a recent randomized controlled trial showed remdesivir to be efficacious in preventing hospitalization when given to outpatients within 7 d of symptom onset [Citation25]. Remdesivir has been granted a conditional marketing authorization for COVID-19 treatment by the European Medicines Agency, but as of now is not in routine clinical use in Finland.
We are not aware of randomized controlled trials addressing COVID-19 management in patients with haematologic malignancies, and such data may be difficult to obtain. Efficient treatment options would be crucial, especially due to inadequate COVID-19 vaccine responses in this subgroup [Citation26]. In case reports, lymphoma patients have appeared to benefit from treatment with convalescent plasma or antivirals such as remdesivir, some requiring repeated treatment cycles () [Citation7–9,Citation20]. Although we cannot rule out spontaneous recovery of our patient in due course, we did observe an excellent response to remdesivir. A shortened duration of infection may also be valuable, especially in patients urgently needing next cycles of lymphoma treatment.
To conclude, a prolonged duration of viral replication among immunocompromised patients is noteworthy from the perspective of infectiousness and may indicate benefit from antiviral therapy at a later stage than for other patients. With respect to COVID-19, patients with haematological malignancies are clearly a vulnerable group requiring special attention.
Supplemental Material
Download MS Word (13.4 KB)Acknowledgements
The authors thank Hanna Jarva for advice and help with the microbiological analyses.
Disclosure statement
V-J.A. has received lecture fees from Pfizer, MSD, Astellas, Unimed, Roche, Biogen, and participated in studies supported by Pfizer and GSK. E.K. has received lecture and consultation fees from Merck, Janssen-Cilag and AstraZeneca. None of the interests listed above are relevant to the current manuscript. M.K. and J.P. declare no conflict of interest.
References
- Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7(2):220–227.
- Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436.
- Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383(26):2586–2588.
- Haveri A, Smura T, Kuivanen S, et al. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro Surveill. 2020;25(11):2000266.
- Jääskeläinen AJ, Kuivanen S, Kekäläinen E, et al. Performance of six SARS-CoV-2 immunoassays in comparison with microneutralisation. J Clin Virol. 2020;129:104512.
- Ahava M, Kurkela S, Kuivanen S, et al. Detection of SARS-CoV-2 nucleocapsid antigen from serum can aid in timing of COVID-19 infection. Preprint, MedRxiv. 2021.
- Baang JH, Smith C, Mirabelli C, et al. Prolonged severe acute respiratory syndrome coronavirus 2 replication in an immunocompromised patient. J Infect Dis. 2021;223(1):23–27.
- Camprubí D, Gaya A, Marcos MA, et al. Persistent replication of SARS-CoV-2 in a severely immunocompromised patient treated with several courses of remdesivir. Int J Infect Dis. 2021;104:379–381.
- Malsy J, Veletzky L, Heide J, et al. Sustained response after remdesivir and convalescent plasma therapy in a B-cell depleted patient with protracted COVID-19. Clin Infect Dis. 2020;73:e4020–e4024.
- Ormazabal Vélez I, Induráin Bermejo J, Espinoza Pérez J, et al. Two patients with rituximab associated low gammaglobulin levels and relapsed covid-19 infections treated with convalescent plasma. Transfus Apher Sci. 2021;60(3):103104.
- Fujii H, Tsuji T, Sugitani M, et al. Prolonged persistence of SARS-CoV-2 infection during a + AVD therapy for classical hodgkin’s lymphoma: a case report. Curr Probl Cancer. 2021;45(6):100739.
- Rnjak D, Ravlić S, Šola AM, et al. COVID-19 convalescent plasma as long-term therapy in immunodeficient patients? Transfus Clin Biol. 2021;28:264–270.
- Simioli F, Martino M, Annunziata A, et al. Therapeutic approach for severe COVID-19 and immunocompromised patients. A case series. Respir Med Case Rep. 2021;33:101397.
- Kenig A, Ishay Y, Kharouf F, et al. Treatment of B-cell depleted COVID-19 patients with convalescent plasma and plasma-based products. Clin Immunol. 2021;227:108723.
- Pelcovits A, Pandita A, Farmakiotis D, et al. Lymphocyte-depleting chemotherapy for aggressive hematologic malignancies in two patients with positive SARS-CoV-2 PCR. Leuk Res. 2021;100:106473.
- Reuken PA, Stallmach A, Pletz MW, et al. Severe clinical relapse in an immunocompromised host with persistent SARS-CoV-2 infection. Leukemia. 2021;35(3):920–923.
- Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062.
- Hoffmann MS, Ganguly S. Delayed COVID-19 respiratory failure in patients with lymphoma on rituximab-based chemoimmunotherapy. Clin Lymphoma Myeloma Leuk. 2021;21:e548–e550.
- Betrains A, Godinas L, Woei AJF, et al. Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies. Br J Haematol. 2021;192(6):1100–1105.
- Duléry R, Lamure S, Delord M, et al. Prolonged in-hospital stay and higher mortality after covid-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am J Hematol. 2021;96(8):934–944.
- Aleissa MM, Silverman EA, Paredes Acosta LM, et al. New perspectives on antimicrobial agents: remdesivir treatment for COVID-19. Antimicrob Agents Chemother. 2020;65(1):e01814–20.
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid-19 - Final report. N Engl J Med. 2020;383(19):1813–1826.
- Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for covid-19 - Interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511.
- Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe covid-19 in outpatients. N Engl J Med. 2021. DOI:https://doi.org/10.1056/NEJMoa2116846
- Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778.
