To the Editor,
In a recent report in this journal, risk factors for recurrence of tuberculosis (TB) were identified [Citation1]. By multivariate logistic regression, authors found diabetes, weight gain ≤ 5%, non-adherence to therapy, smoking, presence of lung cavitation and unemployment to be risk factors of TB recurrence. In a study of adult male and female patients with TB, we here focussed on height as a marker of nutritional status and thereby a putative predictor of survival and treatment outcome during TB treatment.
It has been suggested that adult body height can be used as a prognostic marker for survival during antituberculous therapy [Citation2]. Faurholt-Jepsen et al. found that mortality among taller patients with TB was lower than among shorter statured patients. This study was conducted in a cohort of patients with TB living in Bissau, Guinea-Bissau, to examine body height as a prognostic factor of TB treatment outcome.
This prospective, cohort study was conducted at the Bandim Health Project (BHP), a health and demographic surveillance site located in Bissau, the capital of Guinea-Bissau, West Africa [Citation3]. The BHP covers a well-defined study area of six suburbs in Bissau with a population of approximately 102,000. A surveillance system identifies TB cases in the study area receiving treatment.
Individuals living in the study area and diagnosed with TB by WHO criteria between October 2003 and July 2017 were eligible for this study [Citation4]. Inclusion criteria were (1) initiation of TB treatment less than 1 month before inclusion in the cohort, (2) fulfilment of growth maturity (age ≥ 18 years) and (3) no age-related declining body height (age ≤ 70 years). Exclusion criteria were (1) rifampicin-resistant TB, (2) skeletal TB (due to potential influence on body height) and (3) missing body height measurement.
At inclusion, patients with TB voluntarily gave information on sex, age, birth region and ethnicity. Body height was measured, and patients underwent HIV quick testing using Determine HIV-1/2 assay (Abbott Laboratories, Tokyo, Japan). Treatment outcome was assessed at the end of TB treatment [Citation4]. Body heights were measured to the nearest cm using a roll-up tape measure with the individual barefoot.
All patients with TB were divided into sex-stratified height terciles; low, medium and tall body height. The terciles cut-offs were defined mathematically (containing 33.33% of patients) and then adjusted manually by allocating individuals with equal height into the same tercile.
Successful treatment outcome included cured or completed treatment, while non-successful treatment outcome included failed treatment, lost to follow-up or death during treatment. Patients who transferred to a treatment site outside of the study area and patients with missing information about treatment outcome were ‘Not-evaluated’ [Citation4].
Data were entered in Dbase version 5.0 (dataBased Inc., Vestal, NY), and statistical analyses were performed in Stata IC version 16 (Stata Corp, College Station, TX). Adult body height across covariates was compared by t-test (mean comparison test) and one-way ANOVA analysis where applicable. Successful versus non-successful treatment outcome was both tabulated and evaluated using multivariate logistic regression. Mortality was assessed using multivariate Cox proportional hazards model. Both regression models were adjusted for age and HIV-status. For the Cox-proportional hazards model, exit date was defined as the date of death, curation, treatment completion, treatment failure or lost to follow-up, whichever came first. All analyses were stratified by sex.
As a first sensitivity analysis, the association between adult height and mortality during antituberculous treatment was investigated with linear regression, using COX proportional hazards model adjusted for age and HIV-status. Second, we alternatively divided the study population into terciles with similar cut-offs as Fauerholt-Jepsen et al.; 157 and 162.5 cm for female patients, and 166.1 and 172.3 cm for male patients [Citation2]. Third, we also allocated individuals into height groups with 10 cm intervals.
Faurholt-Jepsen et al. found patients with TB to be taller than healthy controls and hypothesized that low adult body height is a risk factor for active TB disease. We tested by t-test comparison whether patients with TB was taller than controls in our study population. In this study, we pooled together data from three other studies carried out in the surveillance site (Haraldsdottir et al. [Citation5], Patsche et al. [Citation6] and Seegert et al. (data unpublished)). Data regarding sex, age and ethnicity were collected at inclusion (data on HIV-status and birth region was not available). Body height was measured at inclusion, and the same age criteria were applied as for patients with TB. Exclusion criteria for healthy controls were: (1) suspected TB disease if reporting cough for 14 day or more, (2) known TB diagnosis within 2 years following participation in cross-sectional study, (3) pregnancy and (4) missing body height measurement.
The study abided by the Declaration of Helsinki. Written informed consent by signature or fingerprint was provided before inclusion. The TB cohort studies were permitted by the National Committee of Ethics in Health in Bissau, Guinea-Bissau (MINSAP 220405).
Of the 2597 patients diagnosed with TB, 1894 patients (65% men) met the eligibility criteria. Height deficits of patients with TB co-infected with HIV counted −1.3 cm for males and −1.0 cm for females compared with patients with TB not co-infected with HIV. Patients born in the capital Bissau had a taller mean height than patients with TB born outside the city. The sex-stratified inter-ethnical differences varied with up to 2.4 cm for male patients and 1.3 cm for female patients.
Height terciles cut-off points were at 169 and 175 cm for males, and 159 and 164 cm for females. The sex-stratified frequency of treatment outcomes was approximately equal across height terciles. Among both sexes, medium and tall heighted patients with TB had a similar adjusted OR for an unsuccessful treatment outcome compared with patients in the lowest height tercile (). Male patients in the medium and the tallest height tercile had similar mortality as the male patients in the lowest tercile. Among females, mortality at the end of treatment of the medium statured was lower than the mortality of the shortest statured female patients, while it was similar for the tallest female patients ( and ).
Figure 1. Kaplan–Meier survival curves for patients with tuberculosis stratified by sex and body height.
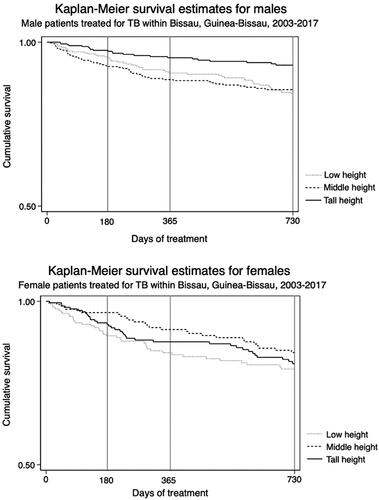
Table 1. Odds ratio (OR) for non-successful treatment outcome and hazard ratio (HR) for mortality during tuberculosis treatment within sex-specific height terciles.
With a linear function approach, the mortality was similar for each increasing cm in body height among male patients (aHR = 0.99 [95% CI: 0.95–1.03], crude HR = 0.98 [0.94–1.01]) and among female patients (aHR = 1.00 [95% CI: 0.96–1.04] and crude HR = 0.99 [0.95–1.03]).
Using similar height tercile cut-offs as done previously (females 157 and 162.5 cm, males 166.1 and 172.3 cm), no association was found between height and treatment outcome and mortality (data not presented). Similar results were found when repeating the analyses after allocating the study population into height groups with 10 cm intervals (data not presented).
A total of 2073 healthy controls were enrolled and pooled together as a comparison cohort. Hereof, 829 (40%) were men. Median age among controls was 30 [26–42] years for both male and female controls. Mean body height was 172.5 (±6.9) cm for men and 160.6 (±6.3) cm for women.
Male patients with TB were on average −0.7 [−1.4;−0.1] cm shorter than male healthy controls (p value = .018). After adjustment for age and ethnicity, male patients with TB were not shorter than controls (−0.2 cm [−1.0;0.5], p value = .510). Female patients with TB were on average same height as female controls (0.3 cm [−0.3;0.9], p value = .322). After adjustment for age and ethnicity, female patients with TB were 1.2 [0.6;1.8] cm taller than controls (p value < .001).
We did not find an association between body height tercile and TB treatment outcome or mortality. Our estimates pointed in opposite directions, implying no overall association. The sensitivity analysis supported this conclusion. Our findings are conflicting with the findings by Faurholt-Jepsen et al. [Citation2]. Some of the differences in results may be caused by differences in confounder adjustment. Multiple prognostic factors during the course of antituberculous treatment have been suggested previously. It has been heavily debated whether malnutrition has a significant influence on treatment outcome in TB patients, and nutritional status has been suggested as prognostic marker [Citation1,Citation7–9]. These possible prognostic factors may cause residual confounding, but exploring these were beyond the scope of this study.
This study included a relatively large study population. Both TB incidence and mortality in Guinea-Bissau are among the highest worldwide. The prevalence of undernourishment in the total population of Guinea-Bissau was 28.0% in 2016–2018, while stunting among children under 5 years old counted a prevalence of 32.2% in 2012 [Citation10]. Therefore, a considerable number of patients in our cohort must have been exposed to these factors, even though we did not have information on which individuals had been exposed to childhood malnutrition and who was stunted.
In conclusion, among patients with TB, we found no correlation between adult body height with antituberculous treatment outcome or mortality during treatment. We therefore find that body height is an unsuitable marker for treatment outcome and mortality during antituberculous therapy.
Acknowledgements
We thank the community in Bissau and the Bandim Health Project (BHP) study area for participating in our research projects. We thank the nurses, doctors and other health staff for their assistance. Also, we thank authors of the cross-sectional studies within BHP for allowing us to borrow their data for the sensitivity analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Saif Anaam M, Alrasheedy AA, Alsahali S, Alfadly SO, et al. Rate and risk factors of recurrent tuberculosis in Yemen: a 5-year prospective study. Infect Dis (Lond). 2020;52(3):161–169.
- Faurholt-Jepsen D, Range N, Praygod G, et al. Height as a prognostic marker for survival during antituberculous therapy. Infect Dis (Lond). 2015; 47(7):515–516.
- University of Southern Denmark and Projecto de Saúde Bandim. Website of the Bandim Health Project. 2022 [cited 2022 Feb 22]. Available from: https://www.bandim.org.
- World Health Organization. Definitions and reporting framework for tuberculosis. Geneva, Switzerland: World Health Organization; 2013. (updated 2014).
- Haraldsdottir TL, Rudolf F, Bjerregaard-Andersen M, et al. Diabetes mellitus prevalence in tuberculosis patients and the background population in Guinea-Bissau: a disease burden study from the Capital Bissau. Trans R Soc Trop Med Hyg. 2015;109(6):400–407.
- Patsche CB, Rudolf F, Mogensen SW, et al. Low prevalence of malnourishment among household contacts of patients with tuberculosis in Guinea-Bissau. Int J Tuberc Lung Dis. 2017;21(6):664–669.
- Lee N, White LV, Marin FP, et al. Mid-upper arm circumference predicts death in adult patients admitted to a TB ward in the Philippines: a prospective cohort study. PLoS One. 2019;14(6):e0218193.
- Nguyen DT, Graviss EA. Development and validation of a prognostic score to predict tuberculosis mortality. J Infect. 2018;77(4):283–290.
- Kawai K, Meydani SN, Urassa W, Wu D, et al. Micronutrient supplementation and T cell-mediated immune responses in patients with tuberculosis in Tanzania. Epidemiol Infect. 2014;142(7):1505–1509.
- Food and Agriculture Organization, International Fund for Agricultural Development, The United Nations Children's Fund, World Food Programme, and World Health Organization. The State of Food Security and Nutrition in the World 2019. Safeguarding against economic slowdowns and downturns. Rome: Food and Agriculture Organization; 2019.
