Abstract
Background
A feared cause of bacteraemia with Gram-positives is infective endocarditis. Risk stratification scores can aid clinicians in determining the risk of endocarditis. Six proposed scores for the use in bacteraemia; Staphylococcus aureus (PREDICT, VIRSTA, POSITIVE), non-β-haemolytic streptococci (HANDOC) and Enterococcus faecalis (NOVA, DENOVA) were validated for predictive ability and the utilization of echocardiography was investigated.
Methods
Hospitalized adult patients with Gram-positive bacteraemia during 2017–2019 were evaluated retrospectively through medical records and the Swedish Death Registry. Baseline and score-specific data, definite endocarditis and echocardiographies performed were recorded. Sensitivity, specificity, negative and positive predictive values and echocardiography utilization were determined.
Results
480 patients with bacteraemia were included and definite endocarditis was diagnosed in 20 (7.5%), 10 (6.6%), and 2 (3.2%) patients with S. aureus, non-β-haemolytic streptococci and E. faecalis, respectively. The sensitivities of the scores were 80–100% and specificities 8–77%. Negative predictive values of the six scores were 98–100%. VIRSTA, HANDOC, NOVA and DENOVA identified all, the PREDICT5 score missed 1/20 and the POSITIVE score missed 4/20 cases of endocarditis. Transoesophageal echocardiography was performed in 141 patients (29%). Thus, the risk stratification scores suggested an increase of 3–63 (7–77%) investigations with echocardiography.
Conclusions
All scores had negative-predictive values over 98%, therefore it can be concluded that PREDICT5, VIRSTA, POSITIVE, HANDOC and DENOVA are reasonable screening tools for endocarditis early on in Gram-positive bacteraemia. The use of risk stratification scores will lead to more echocardiographies.
Introduction
Predicting the risk of complicated infections for each patient with bacteraemia in a hospital setting is a daily challenge for clinicians of different specialties, and indeed, the infectious diseases consultant. Staphylococcus aureus, non-β-haemolytic streptococci (NBHS) and Enterococcus faecalis are common causes of both bacteraemia and complicated infections such as infective endocarditis (IE) [Citation1–4]. In Sweden, the IE mortality rate is 10–12% [Citation5] and international estimates of health care costs have been high [Citation6]. Definite diagnosis of IE requires typical echocardiographic findings, preferably by transoesophageal echocardiography (TEE), or radiologic lesions [Citation7–9]. However, TEE is not feasible in many patients and healthcare situations [Citation10], and the identification of IE is difficult: up to 40% of S. aureus-bacteraemia (SAB) occurs in patients without obvious clinical signs of IE (heart murmur, embolic signs) [Citation11]. Symptoms and signs of IE are syndromic and so can be used for screening purposes and several risk stratification scores (RSS) have been proposed [Citation12–17]. RSS aim to aid in clinical decision making by categorizing the clinical situation into a high or low-risk of IE indicating which patients that should undergo TEE.
Three RSS meant for use in SAB (PREDICT, VIRSTA, POSITIVE) [Citation12–14], one in NBHS-bacteraemia (HANDOC) [Citation15] and two intended for E. faecalis-bacteraemia (EFB) (NOVA, DENOVA) [Citation16,Citation17] have been proposed (Appendices Citation1–3). Original papers show high sensitivities and negative predictive values (NPVs) [Citation12–17]. These RSS have been validated in some retrospective and prospective studies, which confirm the results in NBHS-bacteraemia and EFB [Citation14,Citation17–22]. For SAB, the results have been divergent [Citation14,Citation18,Citation21,Citation22]. In PREDICT5 and VIRSTA, the results from control blood cultures, drawn after 48–72 h of antibiotic treatment, are included in the score. POSITIVE estimates the bacterial load indirectly by using the time to positivity (TTP) of blood cultures and thus RSS can be used directly on the arrival of the first blood culture results, in the same way as the non-SAB RSS are used [Citation14]. Validations in different healthcare settings have been scarce, yet are needed to evaluate the utility of RSS in clinical care [Citation23].
Previous studies have focussed on one bacterial species at a time, but in clinical practice, several bacterial species and groups are associated with a risk for IE. In this study, we retrospectively evaluated six previously described RSS in bacteraemia with three Gram-positive groups of bacteria in a Swedish healthcare setting and compared to the ESC 2015 modified criteria for diagnosis of IE for definite IE. The primary endpoint was the predictive ability for IE and the secondary aim was to describe the pattern of echocardiography utilization in our setting.
Method
Design
A retrospective observational study of hospitalized patients with blood culture findings of three groups of Gram-positive bacteria – S. aureus, non-β-haemolytic streptococci (NBHS) and E. faecalis – was conducted between March 2017 and March 2019.
Participants
All hospitalized patients in the two sites of Halland’s hospital, with one or more positive blood cultures of S. aureus, NBHS or E. faecalis, were eligible for inclusion. Halland is a region comprising 330,000 people and has one primary and one secondary hospital. Exclusion criteria were: age under 18 years, blood culture bottles with growth from other body fluids than blood and polymicrobial growth in blood culture bottles. Only the first episode of positive cultures during the study period was recorded.
Ethics
Ethical approval for the study was obtained from the Swedish Ethical Review Authority (2019-5946).
Procedures
The BACT/ALERT VIRTUO blood culture system (Biomeriéux, Lyon, France), located at both hospital sites, was used and bottles were incubated at all hours. The bacterial findings were identified with MALDI-TOF MS (Bruker Daltonics, Bremen, Germany) using MALDI Biotyper version 4.0 software with MBT Compass Library, DB-5989 and later DB-6903 MSP for species determination. A score of ≥2.0 was required for identification except for NBHS, where a score of >1.7 was used. NBHS were categorized into six groups, S. mitis, S. anginosus, S. mutans, S. bovis and S. salivarius group, or other NBHS. Patients were identified in the laboratory database according to inclusion criteria. Since HANDOC score divides the S. mitis group into the S. mitis and the S. sanguinis group and gives a point for S. sanguinis, isolates determined by the laboratory belonging to the S. mitis-group were manually assessed and defined as S. sanguinis if all the top three MALDI-TOF results were S. sanguinis or S. gordonii [Citation15] and otherwise kept in the S. mitis group. Data of premorbid conditions, patient history, signs of disease at presentation, laboratory and microbiology data needed to calculate RSS were obtained from the electronical medical record (see Appendix for list of variables). Mortality data were extracted from the Swedish Death Registry. An ID specialist (HL) performed the data extraction.
Definitions
Bacteraemia was defined as growth in one or more blood culture bottles. Follow-up cultures were defined as cultures drawn 48–72 h after treatment of antibiotics were initiated. TTP was defined as the shortest time between the start of incubation and the signal of positivity. Definitions of the RSS values followed the original papers with a change in NOVA from three to two positive cultures as proposed by Dahl et al. [Citation12–15]. Predisposing cardiac conditions were defined by the original papers and any condition specified as medium or high-risk by Dajani et al. [Citation24]. IE was defined according to the European Society of Cardiology (ESC 2015) modified criteria for diagnosis of IE [Citation8] and was the reference standard throughout the study. Community, healthcare and the nosocomial acquisition were defined according to Friedman [Citation25] and comorbidities according to Charlson updated comorbidity index [Citation26,Citation27]. Severe sepsis and septic shock were defined by Sepsis-3 criteria [Citation28]. Echocardiographic evidence of IE was vegetation, perivalvular abscesses, new insufficiency or dehiscence of a prosthetic valve [Citation7,Citation8]. The absence of clinical data was interpreted and registered as a lack of that feature.
Analysis
The prespecified cut-off points suggested by the authors of the six RSS were used to dichotomize the patients into high or low-risk of IE (see Appendices Citation1–3). The RSS were calculated at the point of the positive blood culture observation. PREDICT and VIRSTA were evaluated at the point of notice of the follow-up cultures and early PREDICT was not assessed. No imputations of missing values were made and missingness is given in tables when appropriate. The echocardiographic and radiologic examinations during the hospital stay or in the follow-up of the actual period of infection were noted. Positive and negative predictive values of the RSS’s were calculated, and SAB RSS were compared by Fisher’s exact test. Statistical analysis of sensitivity, specificity and predictive values were performed using SPSS Statistics version 27 (IBM, Armonk, NY).
Results
Patients
From March 2017 to March 2019, 585 patients presented with bacteraemia of S. aureus, NBHS or E. faecalis. After exclusion, 480 were included in the analysis. The exclusion were mainly due to polymicrobial culture (41 patients) or referral to another hospital, i.e. lost to follow-up (21 patients) (). The majority had SAB (n = 266 patients), whereof four had methicillin-resistant S. aureus (MRSA), followed by bacteraemia with NBHS (n = 152) and with E. faecalis (n = 62). The patient cohort characteristics are shown in . Of the cohort, 66% were male and acquisition of infection were mostly non-nosocomial and 2.3% (n = 11) had thoracic surgery performed (five valve replacements, five removals of pacemaker, one both procedures). Over-all mortality after 30 days was 16% and 1 year 40%, see . In 25 patients, a new episode of bacteraemia with the same species were recorded within one year, 17 with S. aureus (6.4% of episodes) and 8 with E. faecalis (12.9% of isolates), Time to recurrent bacteraemia was in median 70 days for S. aureus and 48 days for E. faecalis. IE was diagnosed in only one patient with recurrent E. faecalis bacteraemia.
Figure 1. Flowchart of inclusion of cases of Gram-positive bacteraemia.
Comment: SAB: S. aureus bacteraemia; NBHS: non-β-hemolytic streptococci bacteraemia; EFB: E. faecalis bacteraemia.
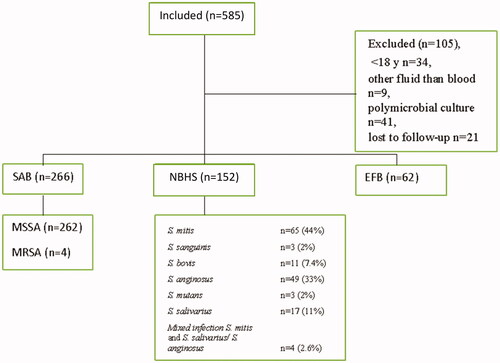
Table 1. Baseline data.
Table 2. Procedures.
IE prevalence and predictive ability
Definite IE was diagnosed according to the ESC 2015 modified criteria in 20 patients (7.5%) with SAB (none was MRSA), in 10 (6.6%) with NBHS-bacteraemia and in 2 (3.2%) with EFB. Data showing the performance of RSS in predicting IE are shown in . VIRSTA identified all patients with IE caused by S. aureus whereas PREDICT5 missed one and POSITIVE missed four cases of IE among patients with SAB. The sensitivity of HANDOC, DENOVA and NOVA was 100% and thus sensitivity ranged from 80% to 100% and specificity from 46% to 77%, except for NOVA, which stood out with low specificity (8%). The NPV of the six RSS were 98–100%. No statistically significant differences in the sensitivity between SAB RSS were demonstrated.
Table 3. Sensitivity, specificity, PPV, NPV.
Echocardiography
TEE was performed in 141 (29%) patients and TTE in 211 (44%), see . A higher proportion of patients were investigated with TEE and TTE in the high-risk group than in the low-risk group. The distributions of TEE and TTE in the high and low-risk groups are shown in . Investigating all SAB patients with high risk would have led to a total increase of TEE investigations with 45% (PREDICT5), 69% (VIRSTA) and 6.6% (POSITIVE). For HANDOC, the increase would have been 57% and for EFB RSS 23% (DENOVA) and 77% (NOVA).
Table 4. Echocardiography frequency in high-risk vs low-risk groups and consequence of RSS use.
Discussion
The RSS studied showed sensitivities of 80% or higher. The specificities were lower, in particular, NOVA with 8% specificity. The evaluation of predictive ability demonstrated similar results to the original papers with NPV’s over 98%. Implementation of TEE for all high-risk patients would lead to an increase in echocardiographies performed. We conclude that PREDICT5, VIRSTA, POSITIVE, HANDOC and DENOVA are reasonable to use as aid in the clinical decision to perform echocardiography.
The prevalence of IE in SAB was low in our study even though the total number of follow-up cultures was high [Citation14] and the proportion of patients undergoing TEE was comparable to other validating studies [Citation14,Citation18,Citation21]. Previous validations of RSS for SAB have shown diverging results in different clinical settings [Citation14,Citation18,Citation21,Citation22]. All RSS in our setting reached NPV of 98%, which Van der Vaart et al. stipulated as a limit of clinical value [Citation21]. VIRSTA had the highest sensitivity (100%) in our study whereas PREDICT5 and POSITIVE had sensitivities of 95% and 80% respectively. Other validating studies have also shown VIRSTA to be the most sensitive RSS in SAB [Citation21,Citation22] though the differences seen between the RSS in our study were not statistically significant. POSITIVE failed to identify four patients with SAB and IE, of whom two had a pacemaker. POSITIVE does not include intracardiac devices in the RSS, which is a weakness since in the clinical context the presence of a pacemaker should lead to a TEE. Another weakness of POSITIVE is that it might be sensitive to differences in TTP generated by differences in logistics and blood culture routines. However, POSITIVE was developed in a similar healthcare setting as in this study and the range of TTP was similar [Citation14]. PREDICT5 failed to identify one patient with IE, who had a negative control culture.
If the RSS had been implemented on the whole SAB population, VIRSTA would have demanded TEE on 58%, PREDICT5 on 50% and POSITIVE on 36% of patients, reflecting the higher specificity of POSITIVE. In this cohort, TEE was performed on 34% of the patients. RSS-guided use of TEE would have led to a total increase of 63 (VIRSTA), 41 (PREDICT) and 6 (POSITIVE) investigations. The optimal management of patients in the low-risk category remains to be determined but screening with TTE or merely clinical investigations are feasible options. However, complicated SAB should always be suspected in cases of slow clinical amelioration and follow-up cultures should be drawn generously to search for complicated SAB also in the early low-risk scenario if POSITIVE is used.
The validation of HANDOC demonstrated a sensitivity of 100% and specificity of 66%, consistent with the original paper and an earlier validation in a small cohort [Citation15,Citation20]. Most demographic features of our NBHS cohort were similar compared to the study by Sunnerhagen et al. [Citation15]. However, the proportion of S. mitis isolates was higher (44% versus 30%) and of S. sanguinis lower (2% vs 15%), possibly representing misclassification since S. sanguinis was not identified in laboratory routine during the study period. The IE prevalence of 6.6% was lower than former studies (8.5% [Citation15] and 24% [Citation20]) and it is possible that cases of uncomplicated IE were missed since TEE examinations were only performed in 24% of episodes and TTE in 32%. However, HANDOC appears to be a sensitive method in detecting patients with NHBS-IE. Through guiding the use of TEE with HANDOC, the number of TEE required would have increased.
We studied monobacterial EFB, and the incidence of IE was much lower than in previous reports [Citation4,Citation29]. Only two cases of IE (3.2%) were found and NOVA and DENOVA could not be evaluated further. The IE incidence reported by Bouza et al. was 4.3%, by Berge et al. 11% and by Dahl et al. 26% [Citation16,Citation17,Citation19]. The risk for IE among patients with E. faecalis bacteraemia, therefore, remains unclear and is possibly different in different clinical settings. In this study, the patients in the EFB group were older than in the previous reports but not burdened with more diseases as measured by the updated Charlson Comorbidity Index [Citation16,Citation17,Citation19]. Nosocomial acquisition was low, 23%, compared to earlier studies. Among the included patients who experienced a later episode of bacteraemia (n = 8), one case of definite IE was identified. Thus, we cannot rule out that non-diagnosed IE’s were at hand in some of the patients who experienced new bacteraemia during the study period. Even though NOVA and DENOVA could not be evaluated due to the low number of IE, both of the RSS correctly identified our two definite cases. Interestingly both cases of IE had short TTP (1.2 h, 8.3 h). The association between a short TTP has been previously reported [Citation30]. TTP might be a way to increase the diagnostic accuracy of the RSS intended for EFB in the future, but this needs to be further studied. Only five patients were defined as low-risk by NOVA, which led to TEE suggestion in 92% of EFB episodes while DENOVA suggested TEE in 26%. In conclusion, NOVA is not useable as a screening tool but DENOVA can be of some value in defining patients at higher risk of IE.
Strengths of this study were the coverage of an unselected regional population and the similarities in data extraction to ordinary infectious diseases consultant work. Patients were easily found and followed as they have a Swedish Social Security code, which is kept for life for all citizens. Study limitations were due to the retrospective design and bias due to missing data was inevitable. The lack of echocardiography in many patients might have led to an underestimation of the true number of IE and thus misclassification. Moreover, control cultures were missing in many patients possibly hampering the sensitivity of PREDICT5 and VIRSTA. New episodes with bacteraemia were few in the SAB cohort and no patient had IE at the recurrent episode. Among new episodes with EFB, one episode of IE was diagnosed but not all patients were subjected to TEE even at recurrence. Defining the conditions of heart and valve disease according to different classification scores imply a risk of bias of classification. The variable of severe sepsis and septic shock was hard to define due to many missing values of the SOFA score for a large proportion of patients,
Bacteraemia with Gram-positives and complicated intracardiac infections in healthcare settings are increasing [Citation1,Citation2,Citation31,Citation32]. Safe diagnostics means both finding disease and minimizing risks and resources of the diagnostic procedures [Citation33]. RSS have the potential capability to identify low-risk patients who, thereby, do not have to undergo investigations. However, implementation of the RSS investigated in this work would lead to increased total numbers of TEE investigations. Whether it is safe to omit echocardiography completely in low-risk patients with SAB remains to be answered.
In summary, we found the six RSS to have NPV over 98% concluding that they are reasonable to use as aid in the clinical decision on whether to perform echocardiography. POSITIVE, HANDOC and DENOVA offer instantaneous and simple instruments to define a patient to be of high-risk or low risk of IE. However, scores can never replace thorough clinical evaluation and observation. The introduction of RSS will shift echocardiography utilization towards the high-risk group and the total TEE frequency would increase between 7% and 69%, depending on chosen RSS. Since this study, we have implemented three RSS in clinical practice in the Halland Region and aim to evaluate this in 2024.
Acknowledgement
The authors thank Erik Gunnarsson and Christoffer Lindsten at the Department of Microbiology, Halmstad, for their work with the microbiological data extraction in this study, Anders Holmén for the support in SPSS-files, Ingrid Larsson for important discussions, and Sienna Linden for generous help proofreading the text.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Olmos C, Vilacosta I, Fernández-Pérez C, et al. The evolving nature of infective endocarditis in Spain: a population-based study (2003 to 2014). J Am Coll Cardiol. 2017;70(22):2795–2804.
- Asgeirsson H, Thalme A, Weiland O. Staphylococcus aureus bacteraemia and endocarditis – epidemiology and outcome: a review. Infect Dis (Lond). 2018;50(3):175–192.
- Kim SL, Gordon SM, Shrestha NK. Distribution of streptococcal groups causing infective endocarditis: a descriptive study. Diagn Microbiol Infect Dis. 2018;91(3):269–272.
- Østergaard L, Bruun NE, Voldstedlund M, et al. Prevalence of infective endocarditis in patients with positive blood cultures: a Danish nationwide study. Eur Heart J. 2019;40(39):3237–3244.
- Ternhag A, Cederström A, Törner A, et al. A nationwide cohort study of mortality risk and long-term prognosis in infective endocarditis in Sweden. PLoS One. 2013;8(7):e67519.
- Franklin M, Wailoo A, Dayer M, et al. The cost-effectiveness of antibiotic prophylaxis for patients at risk of infective endocarditis. Circulation. 2016;134(20):1568–1578.
- Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30(4):633–638.
- Habib G, Lancellotti P, Antunes MJ, ESC Scientific Document Group, et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European society of cardiology (ESC). endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075–3128.
- Bai AD, Agarwal A, Steinberg M, et al. Clinical predictors and clinical prediction rules to estimate initial patient risk for infective endocarditis in Staphylococcus aureus bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect. 2017;23(12):900–906.
- Holden E, Bashir A, Das I, et al. Staphylococcus aureus bacteraemia in a UK tertiary referral centre: a 'transoesophageal echocardiogram for all' policy. J Antimicrob Chemother. 2014;69(7):1960–1965.
- Le Moing V, Alla F, Doco-Lecompte T, VIRSTA study group, et al. Staphylococcus aureus bloodstream infection and endocarditis – a prospective cohort study. PLoS One. 2015;10(5):e0127385.
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2015;61(1):18–28.
- Tubiana S, Duval X, Alla F, VIRSTA/AEPEI Study Group, et al. The VIRSTA score, a prediction score to estimate risk of infective endocarditis and determine priority for echocardiography in patients with Staphylococcus aureus bacteremia. J Infect. 2016;72(5):544–553.
- Kahn F, Resman F, Bergmark S, et al. Time to blood culture positivity in Staphylococcus aureus bacteremia to determine risk of infective endocarditis. Clin Microbiol Infect. 2020 27(9):1345.e7–1345.e12.
- Sunnerhagen T, Törnell A, Vikbrant M, et al. HANDOC: a handy score to determine the need for echocardiography in non-β-hemolytic streptococcal bacteremia. Clin Infect Dis. 2018;66(5):693–698.
- Bouza E, Kestler M, Beca T, for the Grupo de Apoyo al Manejo de la Endocarditis, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2015;60(4):528–535.
- Berge A, Krantz A, Östlund H, et al. The DENOVA score efficiently identifies patients with monomicrobial Enterococcus faecalis bacteremia where echocardiography is not necessary. Infection. 2019;47(1):45–50.
- Abu Saleh O, Fida M, Asbury K, et al. Prospective validation of PREDICT and its impact on the transesophageal echocardiography use in management of Staphylococcus aureus bacteremia. Clin Infect Dis. 2021;73(7):e1745–e1753.
- Dahl A, Lauridsen TK, Arpi M, et al. Risk factors of endocarditis in patients with Enterococcus faecalis bacteremia: external validation of the NOVA score. Clin Infect Dis. 2016;63(6):771–775.
- Sunnerhagen T, Højgaard Andersen M, Bruun NE, et al. External validation of the HANDOC score – high sensitivity to identify patients with non-beta-haemolytic streptococcal endocarditis. Infect Dis. 2020;52(1):54–54.
- Van Der Vaart TW, Prins JM, Soetekouw R, et al. Prediction rules for ruling out endocarditis in patients with Staphylococcus aureus bacteremia. Clin Infect Dis. 2021;ciab632. DOI:10.1093/cid/ciab632
- Peinado-Acevedo JS, Hurtado-Guerra JJ, Hincapié C, et al. Validation of VIRSTA and predicting risk of endocarditis using a clinical tool (PREDICT) scores to determine the priority of echocardiography in patients with Staphylococcus aureus bacteremia. Clin Infect Dis. 2021;73(5):e1151–e1157.
- Lambregts MMC, Molendijk EBD, Meziyerh S, et al. Early differentiation between uncomplicated and complicated Staphylococcus Aureus bacteremia: potential value and limitations of a clinical risk score. Int J Clin Pract. 2020;74(11):e13601.
- Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis: recommendations by the American Heart Association. Clin Infect Dis. 1997;25(6):1448–1458.
- Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137(10):791.
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and Score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- Ternavasio-de la Vega HG, Castaño-Romero F, Ragozzino S, et al. The updated Charlson comorbidity index is a useful predictor of mortality in patients with Staphylococcus aureus bacteraemia. Epidemiol Infect. 2018;146(16):2122–2130.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA: J Am Med Assoc. 2016;315(8):801–810.
- Dahl A, Iversen K, Tonder N, et al. Prevalence of infective endocarditis in Enterococcus faecalis bacteremia. J Am Coll Cardiol . 2019;74(2):193–201.
- Oldberg K, Thorén R, Nilson B, et al. Short time to blood culture positivity in Enterococcus faecalis infective endocarditis. Eur J Clin Microbiol Infect Dis. 2021;40(8):1657–1664.
- Pinholt M, Østergaard C, Arpi M, Danish Collaborative Bacteraemia Network (DACOBAN), et al. Incidence, clinical characteristics and 30-day mortality of enterococcal bacteraemia in Denmark 2006–2009: a population-based cohort study . Clin Microbiol Infect. 2014;20(2):145–151.
- Urien J, Camus C, Leclercq C, et al. The emergence of Staphylococcus aureus as the primary cause of cardiac device-related infective endocarditis. Infection. 2021;49(5):999–1006.
- Sainathan S, Andaz S. A systematic review of transesophageal echocardiography-induced esophageal perforation. Echocardiography. 2013;30(8):977–983.
Appendix 1. Variables for three RSS – S. aureus
Appendix 2. Variables for three RSS – NBHS
Appendix 3. Variables for two RSS – E. faecalis
Appendix 4. List of variables
