Abstract
Background
Around 70% of all ICU patients are treated with antibiotics whereas up to 30% are suggested as unnecessary. Measuring antibiotic consumption is a prerequisite to improving its use and the purpose of the present investigation was to explore the use of antibiotics in Swedish ICUs.
Material and methods
Daily Defined Doses (DDDs) of antimicrobials delivered to Swedish ICUs, 2016–2018, were retrieved from Swedish pharmacies. From the Swedish Intensive Care Registry, we extracted data on a number of patient admissions, occupied bed days and Simplified Acute Physiology Score (SAPS)3.
Results
There was a similar annual rate of total DDDs per admission of 3.7, 3.5, 3.8 and total DDDs per 100 occupied bed days of 111, 111, and 115 but with an approximately 6-fold difference of DDDs per occupied bed days (61–366) between the ICUs. The most frequently used antibiotics were isoxazolyl penicillins (J01CF), penicillins with betalactamase-inhibitors, mainly piperacillin/tazobactam (J01CR), 3rd and 4th generation cephalosporins (J01DD + DE) and carbapenems (J01DH). Together these four classes accounted for a median of 52% of all antibiotic use. The use of carbapenems had a moderate positive correlation with the mean SAPS3 score (r = 0.6, p = .01). The use of other broad-spectrum antibiotics showed no such correlation.
Conclusion
Overall antibiotic use remained similar in Swedish ICUs during the years 2016–2018. Broad-spectrum antibiotics accounted for 50% of all DDDs but with a large inter-ICU variation which only partly can be explained by differences in patient case mix and microbial resistance. Presumably, it also reflects varying local prescribing practices.
Introduction
Approximately 70% of all patients admitted to an Intensive Care Unit (ICU) are treated with antibiotics and this level seems to be constant over the last decade [Citation1,Citation2]. Indications for an antibiotic prescription vary such as prophylaxis, pre-emptive-, empirical- and targeted treatment. The proportions of these indications can vary between different ICUs depending on the case mix, where an ICU with many postoperative patients will prescribe more prophylactic antibiotics whereas an ICU admitting patients with haematological disorders use more pre-emptive treatment.
Regardless of indication, overuse of antibiotics in the ICU is common [Citation3]. It has been estimated that up to 30% of all patients admitted to the ICU with presumed infection, and treated with antibiotics, have no, or very low likelihood of, infection [Citation4]. Apart from the difficulties in diagnosing infections in ICU patients, other reasons for overuse of antibiotics include unnecessary combination therapy with two or more antibiotics, prolonged prophylaxis and prolonged antibiotic courses beyond recommended durations.
Antimicrobial stewardships (AMS) programs are designed to improve the use of appropriately prescribed antibiotics in terms of choice, dosing, initiation and duration of therapy [Citation5–7]. This can be done either by restrictive measures such as limiting the prescription of certain antibiotics or with an educational approach, with audits and feedback [Citation8]. Benchmarking between departments or health care facilities can also be used to achieve improvement. Measuring antibiotic consumption is of key importance to evaluate change or to compare antibiotic use in different settings [Citation9,Citation10]. Daily defined doses (DDDs) are often used as a standardised measure and are defined by the WHO as the average maintenance dose per day of a drug used for its main indication in adults and are updated regularly [Citation11].
There is currently a paucity of knowledge regarding the amount of current antibiotics currently used in ICUs and the differences between smaller, primary hospitals and university-affiliated- or tertiary hospitals. The aim of the present study was to evaluate antibiotic use during the years 2016–2018 in Swedish ICUs and the relative differences between different hospital categories when adjusted for occupied bed days and case mix.
Methods
Study design and setting
This was an observational study analysing all antibiotics, compiled as DDDs, distributed to Swedish adult ICUs in the years 2016–2018 adjusted for size and type of ICU. ICUs were categorised as regional/university- (category 1), district- (category 2) or local hospital (category 3). A fourth category (category 4) with specialised ICUs was defined including neuro ICU and Sweden’s only centralised centre for extra corporeal membrane oxygenation (ECMO) unit.
Ethics approval
Since the current study does not contain any patient data, ethics approval was not deemed necessary.
Data collection
Pharmacists in the 21 regional councils of Sweden, who were responsible for antibiotic purchase and distribution, provided data, from the pharmacy registry, expressed as DDDs, on aggregate yearly deliveries of antibiotics to the ICUs of the hospitals in the regions.
The number of yearly admissions, patient days and simplified acute physiology score (SAPS)3 were retrieved from the open website of the Swedish Intensive care registry (SIR) Derived variables (i.e. patient days, SAPS3 scores) were calculated by SIR based on the raw data entered into local software in each ICU. Antibiotics were grouped according to the Anatomical Therapeutic Chemical (ATC) classification on a 5-digit level.
Outcomes
The main outcome was DDDs normalised to number of patient admissions per ICU and year and DDDs per 100 occupied bed-days (OBD). Bed days were truncated by dates, meaning that for a patient admitted before midnight and discharged the day after, there were two OBDs. SAPS3 was used to address differences in ICU case mix between hospitals.
Broad-spectrum antibiotics were defined as 3rd 4th generation cephalosporins (J01DD + DE), carbapenems (J01DH), penicillins with betalactamase inhibitors (J01CR) and fluoroquinolones (J01MA).
To evaluate the use of carbapenems in relation to other antibiotics we defined two categories of relative antibiotic use: the ratio of carbapenem in relation to total antibiotic use (J01DH/J01) and the ratio of carbapenem use to penicillins with betalactamase inhibitors plus third and fourth generation cephalosporins (J01DH/J01CR + (J01DD + DE)). We also evaluated the ratio of aminoglycosides to all antibiotics (J01MA/J01).
Statistics
Results are presented as numbers or percentages. Summary data are shown as median with interquartile range (IQR) where appropriate. Kruskal-Wallis test was used to evaluate differences between years and hospital categories. All data management and calculations were performed in R, primarily using the “tidyverse” package (R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
Results
We analysed data from 63 of Sweden’s 84 ICUs after excluding 21 ICUs (4 paediatric ICUs with no adult admissions, 2 burns and 2 infectious disease ICUs because they reported antibiotic delivery together with an attached general ward, 8 cardiothoracic ICUs because they primarily covered the perioperative setting and 5 ICUs unable to report antibiotic delivery. With these ICUs excluded, there were 36368, 35942 and 35031 admissions, for the years 2016–2018 with a median patient stay of 2 days (). This corresponds to approximately 90% of the total yearly adult admissions to Swedish ICUs during the years studied (). The median age was 56, 58, 61 and 55 years for categories 1, 2, 3 and 4 ICUs, respectively. The median SAPS3-score was 57 for category 1, 2 and 4 hospitals and 53 for category 3 hospitals (p < .01).
Table 1. Antibiotic consumption in Swedish ICUs 2016–2018.
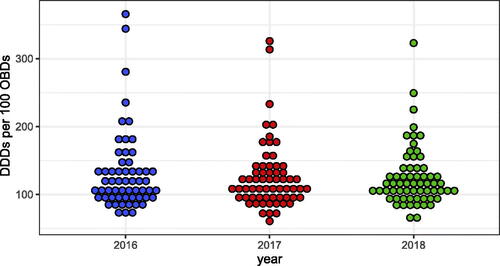
There was a similar yearly rate of total DDDs per admission (∼3.7) and DDDs per 100 OBDs (∼112) for all antibiotics (J01) (). As shown in , there was an approximately 6-fold difference in DDDs per OBDs (61–366) between hospitals, similar over the years studied.
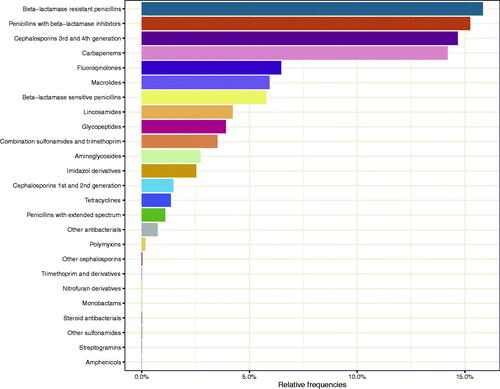
The most common antibiotic classes were beta-lactamase resistant penicillins (16%) followed by penicillins with betalactamase inhibitors, main piperacillin with tazobactam (15.2%), and 3rd and 4th generation cephalosporins (14.7%), carbapenems (14.2%) and fluoroquinolones (6.5%) (). This pattern was the same during the three years.
The median DDDs of the respective antibiotic classes were similar and did not change significantly from 2016 to 2018, except for a decrease in aminoglycosides from 53 (IQR= 59) in 2016 to 28 (IQR = 50) in 2018 (p = .04) and of co-trimoxazol 75 (IQR = 85) in 2016 and 40 (IQR = 63) in 2018 (p = .04).
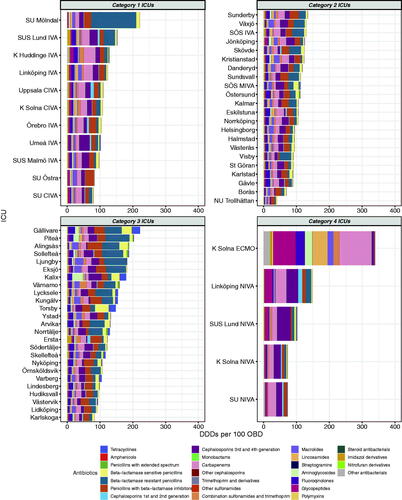
As depicted in , the total DDDs per 100 OBD varied widely between hospitals, from 40 to 340 DDDs per 100 OBD. The largest variation was seen in category 4 hospitals (73 to 340) and the smallest in category 2 hospitals (40 to 134).
Figure 3. DDDs per 100 OBD of all antibiotic classes according to hospital category. Mean of 2016–2018.
The use of systemic broad-spectrum antibiotics (3rd 4th generation cephalosporins, carbapenems, penicillins with betalactamase inhibitors, and fluoroquinolones) accounted for a median of 52% of all antibiotics, ranging from 21% to 73% and with a large variation in a class of broad-spectrum antibiotics used (). This was further explored by analysing the relative use of carbapenems that varied from 4% to 34% (median 12%) of total antibiotic use ().
Figure 4. Relative use of broad-spectrum (J01DH + J01CR + J01DD_DE + J01MA) antibiotics to all antibiotics (J01). Arranged according to hospital category.
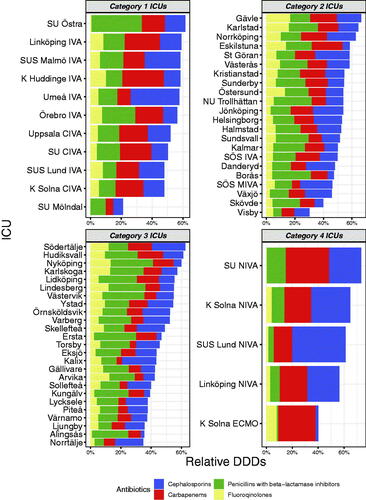
Figure 5. (a) Carbapenem use in relation to total antibiotic use (J01DH/J01). (b) Aminoglycoside use in relation to total antibiotic use (J01GB/J01).
Note. Dashed line indicates the median.
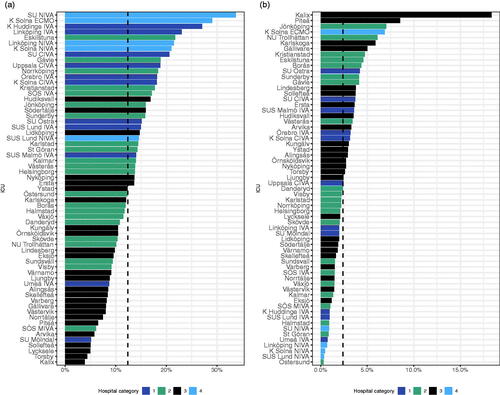
Aminoglycosides are often used in combination with a beta-lactam antibiotic to provide additional and/or double coverage of gram-negative bacteria. This class showed the most extensive variety of all antibiotic classes with a 600-fold difference ranging from 0.3% to 18% (median 2.4%) of total antibiotic use ().
The use of carbapenems (expressed both as per OBDs and relative to total DDDs) was moderately correlated with the mean SAPS3 score (r = 0.4, p ≤ .001). This correlation was not seen for other broad-spectrum antibiotics, (penicillins with beta-lactamase inhibitors (J01CR), fluoroquinolones (J01MA) and 3rd and 4th generation cephalosporins (J01DD + DE).
Discussion
In the present study, we show that the total amount of antibiotics in Swedish ICUs expressed as DDDs per 100 patient days or per admission, remained fairly constant during the years 2016–2018 and that there was a large variation between hospitals. The overall consumption of antibiotics, both in the out-patient and hospital setting, decreased slightly during the same period [Citation12]. Our study can not explain why such a trend was not seen in the ICU setting.
The most frequently used antibiotic class was beta-lactamase resistant penicillins (J01CF). Beta-lactamase resistant penicillins are widely used as prophylaxis in orthopaedic surgery and are recommended for the treatment of methicillin-susceptible Staphylococcus aureus. We found that units with large orthopaedic post-operative care, were associated with antibiotic consumption of beta-lactamase resistant penicillins.
Broad-spectrum antibiotics, defined as 3rd 4th generation cephalosporins (J01DD + DE), carbapenems (J01DH), penicillins with betalactamase inhibitors (J01CR) and fluoroquinolones (J01MA), accounted for approximately 50% of total DDDs. In a recent report on antibiotic consumption in acute care hospitals in Sweden, broad-spectrum antibiotics accounted for approximately 30% of all DDDs (www.sva.se/swedres-svarm/) reflecting that ICUs have the highest use of these classes of antibiotics. Most guidelines recommend broad-spectrum antibiotics as initial empirical treatment for critically ill patients and, thus, the data is not surprising. The same guidelines also recommend de-escalation for targeted treatment. In a recently published international study on antibiotics in the ICU, the most common de-escalation was a cessation of one of two antibiotics in combination therapy where glycopeptides, aminoglycoside and macrolides were the most frequently discontinued antibiotics [Citation13]. When de-escalation was done by changing antibiotics, most commonly piperacillin-tazobactam was switched to a third-generation cephalosporin and piperacillin-tazobactam to penicillin combined with a beta-lactamase inhibitor [Citation13]. The current practice of de-escalation in Sweden is not known. In fact, the safety of de-escalation has been questioned since it may include a stepdown to an antibiotic with inferior pharmacokinetics and pharmacodynamics. A shorter course of the initial antibiotic might be a better option in e.g. uncomplicated gram-negative bacteriemia [Citation14,Citation15].
Carbapenems are often a last resort antibiotic for infections with multi-drug resistant bacteria and are therefore proposed to be spared when there are other alternatives. According to our results, the use of carbapenems varied widely between the different hospital categories as well as between individual hospitals within the same category. Some of the variations could be explained by higher disease severity as there was a moderate but significant correlation between mean ICU SAPS3 score and carbapenem use. However, this does not fully explain the differences. Multi-drug resistance in Swedish ICUs does not vary enough to explain the differences (https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistik-a-o/sjukdomsstatistik/extended-spectrum-beta-lactamase-esbl/?t=county#statistics-nav). Thus, local variations, traditions and risk factors of infections caused by extended-spectrum beta-lactamase-producing bacteria, even in a low resistance setting, are likely part of decisions to prescribe-, and whether to de-escalate, a carbapenem. We had no information regarding the consultation with infectious disease specialists and regarding local antimicrobial stewardship programs in the ICUs. Such measures may have influenced the antibiotic choice, dosing and duration [Citation16].
Aminoglycosides are still widely used worldwide in combination therapy in patients with severe gram-negative sepsis [Citation17]. They are still a recommended part of empirical antibiotic therapy in the most recent guidelines from the Society of Swedish infectious disease physicians (https://infektion.net/vardprogram/svar-sepsisseptisk-chock/#). However, aminoglycosides remain under debate as there is no definite evidence of improved outcomes and several studies report harmful side effects, especially renal toxicity [Citation18–20]. In Sweden, aminoglycosides are usually administered as single dose in patients with sepsis [Citation21]. The extreme variation in our study cannot be explained by case mix, disease severity or variation in microbial resistance patterns. Rather, it probably reflects the lack of firm evidence of efficacy, varying interpretation of published data, and local traditions [Citation21].
A recent international multidisciplinary consensus program suggested 12 evidence-based quantity metrics for antibiotic use in hospitals in order to standardise surveillance methods and increase the accuracy of benchmarking [Citation22]. We chose to present our data as DDDs per admission and DDDs per 100 OBDs which were two of the suggested metrics. As internal normalisation we chose to evaluate various ratios of antibiotic DDDs as an indicator between hospitals thereby increasing comparability. The median DDDs per 100 OBDs over the years studied of approximately 112 is in accordance with recent data from a German antibiotic surveillance program [Citation23]. In their report, they presented an increase in antibiotic consumption of 19%, from 118 per 100 OBDs in 2001 to 141 per 100 OBDs in 2015. Thus, our data is in the lower span 20 years later. Antibiotic consumption in our study was also lower than that in a recent systematic review of eighty studies representing 3130 hospitals, in which the consumption in the ICU was 156 DDDs per 100 hospital days [Citation24].
Limitations
DDD does not necessarily correspond to the prescribed daily dose. Individual patients, such as the obese or patients at risk of underdosing due to augmented renal clearance can be prescribed higher doses. Conversely, in patients with renal failure, the dose will be reduced to compensate for reduced clearance. We had no information regarding such adjustments. The DDD data were derived from deliveries from the pharmacies to the ICUs which is a proxy of consumption. The relationship between sales and consumption has previously been described as acceptable and can thus serve as an accessible marker for consumption [Citation24,Citation25]. Perioperative care is integrated to a greater or lesser extent in different ICUs and some of the variation seen in our results likely reflects not only antibiotics used for treatment but also for prophylaxis. Beta-lactamase resistant penicillins (J1CF) are most frequently used for prophylaxis in Sweden. As a sensitivity analysis, all results were calculated with and without the inclusion of J01CF which did not alter any of the main conclusions except that the most commonly used class was then penicillins with betalactamase inhibitors. We used hospital category and SAPS3 as indicators of case mix [Citation26]. SAPS3 is well correlated with outcome and has been customised to Swedish ICUs [Citation27]. Even so, SAPS3 does not cover all case mix factors which might influence the use of antibiotics.
Conclusion
A relatively stable level of antibiotic use might be a positive finding but since it is estimated that up to 30% of antibiotic use in the ICUs is redundant there is likely room for improvement and the educational challenge to change antibiotic prescription in ICUs remain [Citation28]. The current study provides data that can be used to benchmark longitudinal antibiotic consumption mainly within individual ICUs but also between hospitals with the limitations discussed. Case mix and microbial resistance only partly explain the large variation in DDDs in different ICUs. The variation likely also reflects differences in local prescribing practices. Future research should be aimed toward a better understanding of differences in consumption patterns.
Disclosure statement
The authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329.
- Vincent JL, Sakr Y, Singer M, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323(15):1478–1487.
- Denny KJ, De Waele J, Laupland KB, et al. When not to start antibiotics: avoiding antibiotic overuse in the intensive care unit. Clin Microbiol Infect. 2020;26(1):35–40.
- Klein Klouwenberg PM, Cremer OL, van Vught LA, et al. Likelihood of infection in patients with presumed sepsis at the time of intensive care unit admission: a cohort study. Crit Care. 2015;19:319.
- Pickens CI, Wunderink RG. Principles and practice of antibiotic stewardship in the ICU. Chest. 2019;156(1):163–171.
- Lanckohr C, Boeing C, De Waele JJ, et al. Antimicrobial stewardship, therapeutic drug monitoring and infection management in the ICU: results from the international A- TEAMICU survey. Ann Intensive Care. 2021;11(1):131.
- Dyar OJ, Huttner B, Schouten J, et al. What is antimicrobial stewardship? Clin Microbiol Infect. 2017;23(11):793–798.
- Schoffelen T, Schouten J, Hoogerwerf J, et al. Quality indicators for appropriate antimicrobial therapy in the emergency department: a pragmatic Delphi procedure. Clin Microbiol Infect. 2021;27(2):210–214.
- Davey P, Marwick CA, Scott CL, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2017;2:CD003543.
- Kallen MC, Natsch S, Opmeer BC, et al. How to measure quantitative antibiotic use in order to support antimicrobial stewardship in acute care hospitals: a retrospective observational study. Eur J Clin Microbiol Infect Dis. 2019;38(2):347–355.
- WHO. ATC/DDD Index 2021 2021 [Available from: https://www.whocc.no/atc_ddd_index/.
- Swedres-Svarm. Consumption of antibiotics and occurrence of resistance in Sweden. Uppsala: Solna; 2018.
- De Bus L, Depuydt P, Steen J, et al. Antimicrobial de-escalation in the critically ill patient and assessment of clinical cure: the DIANA study. Intensive Care Med. 2020;46(7):1404–1417.
- Huttner B, Pulcini C, Schouten J. De-constructing de-escalation. Clin Microbiol Infect. 2016;22(12):958–959.
- Molina J, Montero-Mateos E, Praena-Segovia J, et al. Seven-versus 14-day course of antibiotics for the treatment of bloodstream infections by enterobacterales: a randomized, controlled trial. Clin Microbiol Infect. 2022;28(4):550–557.
- Raineri E, Pan A, Mondello P, et al. Role of the infectious diseases specialist consultant on the appropriateness of antimicrobial therapy prescription in an intensive care unit. Am J Infect Control. 2008;36(4):283–290.
- Roger C, Louart B, Elotmani L, et al. An international survey on aminoglycoside practices in critically ill patients: the AMINO III study. Ann Intensive Care. 2021;11(1):49.
- Paul M, Lador A, Grozinsky-Glasberg S, et al. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. 2014;1:CD003344.
- Deelen JWT, Rottier WC, Buiting AGM, et al. Short-course aminoglycosides as adjunctive empirical therapy in patients with gram-negative bloodstream infection, a cohort study. Clin Microbiol Infect. 2021;27(2):269–275.
- Ong DSY, Frencken JF, Klein Klouwenberg PMC, et al. Short-course adjunctive gentamicin as empirical therapy in patients with severe sepsis and septic shock: a prospective observational cohort study. Clin Infect Dis. 2017;64(12):1731–1736.
- Liljedahl Prytz K, Prag M, Fredlund H, et al. Antibiotic treatment with one single dose of gentamicin at admittance in addition to a beta-lactam antibiotic in the treatment of community-acquired bloodstream infection with sepsis. PLOS ONE. 2020;15(7):e0236864.
- Stanic Benic M, Milanic R, Monnier AA, et al. Metrics for quantifying antibiotic use in the hospital setting: results from a systematic review and international multidisciplinary consensus procedure. J Antimicrob Chemother. 2018;73(suppl_6):vi50–vi8.
- Remschmidt C, Schneider S, Meyer E, et al. Surveillance of antibiotic use and resistance in intensive care units (SARI). Dtsch Arztebl Int. 2017;114(50):858–865.
- Bitterman R, Hussein K, Leibovici L, et al. Systematic review of antibiotic consumption in acute care hospitals. Clin Microbiol Infect. 2016;22(6):561 e7–e19.
- Olsen MH, Anhoj J, Knudsen JD, et al. Comparison of methods for measuring antibiotic consumption in an intensive care unit. APMIS. 2019;127(1):33–40.
- Metnitz PG, Moreno RP, Almeida E, et al. SAPS 3-From evaluation of the patient to evaluation of the intensive care unit. Part 1: objectives, methods and cohort description. Intensive Care Med. 2005;31(10):1336–1344.
- Engerstrom L, Kramer AA, Nolin T, et al. Comparing time-fixed mortality prediction models and their effect on ICU performance metrics using the simplified acute physiology score 3. Crit Care Med. 2016;44(11):e1038–e44.
- Gursoy G, Uzun O, Metan G, et al. Do antimicrobial stewardship programs improve the quality of care in ICU patients diagnosed with infectious diseases following consultation? Experience in a tertiary care hospital. Int J Infect Dis. 2022;115:201–207.