Abstract
Background
A ubiquitous human pathogen, Streptococcus pyogenes (Group A Streptococcus, GAS) causes infections from mild pharyngitis to severe septic infections. Acute kidney injury (AKI) is a condition of prompt decline of renal function. The aim of the present study was to report the incidence and outcome of AKI in GAS bacteraemia and to evaluate the diagnostic value of serum C-reactive protein as an indicator of AKI.
Methods
All adult patients with GAS bacteraemia treated at Turku University Hospital from 2007 to 2018 were identified and their patient records were scrutinised.
Results
Of 195 included patients, 38 (19.5%) had AKI stage 1, 20 (10.3%) AKI stage 2 and 26 (13.3%) AKI stage 3 and 111 (56.9%) did not have AKI. The adjusted seven-day mortality was significantly higher in AKI stages 2 and 3 compared to the non-AKI group (15% and 19% vs. 3.6%; p = .046 and .006, respectively). Of the survivors, 95.8% met the criteria of renal recovery at discharge. The higher the AKI stage, the higher was the mean serum CRP level on admission. The optimal cut-off for CRP to identify patients with AKI stage 2 or 3 was ≥244 mg/l (sensitivity 82.6% and specificity 75.8%).
Conclusions
AKI is common in patients with GAS bacteraemia and the severity of AKI correlates with the CRP level on admission. The mortality of patients with GAS bacteraemia and AKI is significantly higher than of patients without AKI. Most survivors, however, show renal recovery.
AKI is common in group A Streptococcal bacteraemia and increases mortality compared to bacteraemia alone. However, renal recovery is also common. A high CRP level on admission correlates significantly positively with the degree of severity of AKI.
Key Message
Background
A ubiquitous and well-recognized human pathogen, Streptococcus pyogenes (Group A Streptococcus, GAS), causes infections ranging from mild pharyngitis or non-necrotizing cellulitis to invasive infections (iGAS), such as bacteraemia or streptococcal toxic shock syndrome (STSS). GAS infections are associated with 500,000 deaths per year worldwide [Citation1].
Acute kidney injury denotes a prompt decline in renal function and has been defined and divided into stages by the organisation Kidney Disease Improving Global Outcomes (KDIGO) [Citation2]. Sepsis constitutes the most common aetiology for AKI, and 36–68% of septic patients have AKI [Citation3–6]. Furthermore, AKI increases the mortality rate among patients with and without sepsis [Citation4,Citation5,Citation7], but information specifically on iGAS and AKI stage are sparse.
Several serum biomarkers have been studied to identify AKI in patients with sepsis [Citation8]. Elevated levels of proenkephalin, cystatin C and Plasma Neutrophil Gelatinase Associated-Lipocalin (NGAL) are associated with AKI in septic patients [Citation8–11]. Cosentino et al. showed that in acute myocardial infarction elevated levels of high-sensitivity CRP on admission are associated with the risk of AKI and its severity [Citation12]. Studies on CRP and sepsis-associated AKI (SA-AKI) are relatively sparse. Zhou et al. reported slightly higher CRP levels in septic patients with AKI than without AKI, but the difference was not statistically significant [Citation13]. In another study, CRP levels were significantly higher among septic patients with AKI than without AKI [Citation14].
Acute post-streptococcal glomerulonephritis (APSGN) is an immune complex-mediated glomerulonephritis, the incidence of which has decreased worldwide and is a rarity in affluent societies nowadays [Citation16]. In this study, we have not investigated APSGN.
In the present study, we report the incidence of AKI and assess the impact of AKI on the clinical outcome of patients with GAS bacteraemia. We also examined serum CRP level as an early indicator of AKI.
Material and methods
Study population and data collection
In the catchment area of the Hospital District of Southwest Finland (HDSWF), there are 470,000 inhabitants and five hospitals. All adult patients with GAS bacteraemia treated in any hospital in HDSWF from January 2007 until December 2018 were retrospectively identified from the registry of antimicrobial use and infections (Sairaalan antibiootti- ja infektiojärjestelmärekisteri, SAI-registry), a database which registers all microbial findings in clinical samples, including bacteraemia. The SAI-registry includes each patient’s national identity code and the date when the specimen had been obtained. The SAI-register is maintained by the Department of Hospital Hygiene and Infection Control of the HDSWF. Eligible patients had at least one blood culture positive for GAS, concurrent clinical signs of infection, age ≥18 years and at least one contact to any of the hospitals of the HDSWF during the GAS bacteraemia episode. We have previously reported the epidemiology, clinical profiles and antimicrobial treatment choices [Citation17]. For this study, an inclusion criterion was added: the serum creatinine (SCr) value recorded simultaneously with the positive blood culture for GAS. Patients on chronic dialysis were excluded.
All data were collected manually from the electronic patient records by a specialist in infectious diseases (JVi).
Definitions
AKI was classified into stages 1–3 according to the Kidney Disease Improving Global Outcomes (KDIGO) classification system [Citation2]. We used the term ‘non-AKI’ for the cases not meeting the criteria of KDIGO AKI. Staging rested on SCr but not on urine output, since data on the urine output was not available in this retrospective study. We used the KDIGO definition of AKI: ‘Increase in SCr to ≥1.5 times baseline, which is known or presumed to have occurred within the prior 7 days’. All eligible patients had two SCr values: one on admission and one baseline value as defined below. According to KDIGO, AKI stage 1 is defined as a 1.5–1.9-fold increase and AKI stage 2 as a 2.0–2.9-fold increase of SCr above baseline. AKI stage 3 is defined as a ≥ 3.0-fold increase in SCr above baseline or as an increase to a value ≥4.0 mg/dl or as starting renal replacement therapy (RRT) [Citation2].
The baseline SCr was taken as the most recent value in the last 24 month prior to the positive blood culture for GAS, as proposed in the literature [Citation18,Citation19]. If the premorbid SCr was not available, we estimated the baseline SCr by using the Modification of Diet in renal Disease (MDRD) equation, as recommended by the Acute Disease Quality Initiative [Citation20].
Renal recovery was defined as SCr <1.5-fold above baseline without a need for renal replacement therapy [Citation21]. Renal recovery was determined at hospital discharge and one year later.
The underlying diseases were classified according to the Charlson Comorbidity Index. The index was further divided into four categories according to Charlson’s original study: 0 score is 0, 1–2 scores is 1, 3–4 scores is 2 and ≥5 scores is 3 [Citation22].
The day of admission was regarded as the day when the positive blood culture was taken.
Statistical analyses
Statistical analyses were performed with the IBM SPSS Statistics for Windows version 27 (IBM Corp., Armonk, NY). A two-sided p < 0.05 was considered statistically significant. Categorical data were analysed by the χ2 test or Fisher’s exact test. The normality of continuous variables was checked using histograms. The mean ages between AKI stages were compared by using one-way analysis of variance (ANOVA) with Tukey’s method in pairwise comparisons and the medians of the duration of hospital stay by the Kruskal-Wallis test with the Dunn-Bonferroni method in pairwise comparisons.
The difference in mean CRP values between AKI stages was tested by using analysis of covariance (ANCOVA) after adjustment for age and Charlson’s comorbidity index and Bonferroni method was used in further pairwise comparisons.
Binary logistic regression was used to compare mortality between AKI stages after adjustment for age, Charlson’s comorbidity index and ICU admission. Results are expressed as odds ratios (OR) with 95% confidence intervals (CI). The optimal cut-off value for CRP to identify the patients with AKI stage 2 or 3 was defined using the receiver-operating characteristics (ROC) curve and Youden’s index.
Results
Incidence of AKI and clinical characteristics of the stages of AKI
The data of a total of 195 adult GAS bacteraemia patients were included. Exclusions were due to chronic dialysis (n = 2) or missing SCr data simultaneously with the positive blood culture for GAS (n = 15). All included patients were hospitalized. The premorbid SCr value was not available in 59 patients (30.3%) and was estimated by the MDRD equation.
Of all the cases, 43.1% exhibited AKI stage 1, 2 or 3 and 84.6% had their maximal SCr level on admission. The clinical characteristics of the cases in different AKI stages are summarized in . The mean age was lowest (51.6 years) in AKI stage 3. In AKI stage 3, 42.3% had the Charlson class 0, whereas in non-AKI, AKI stage 1 and 2 the proportions were 21.6%, 5.3%, 20.0%, respectively (global p = .012). The higher AKI stages were associated with more severe disease (considering hypotension, ICU admission and need for invasive mechanical ventilation (IMV) or RRT) and with a higher prevalence of abdominal abscess.
Table 1. Demographics and clinical characteristics of 195 patients with GAS bacteraemia and comparison of patients by KDIGO AKI stage.
Incidence of AKI among ICU-treated and non-ICU-treated patients
Altogether 46 (23.6%) patients were treated in intensive care. The reasons for ICU admission were severe septic illness with failure of at least two organs. The AKI stage distribution between ICU-treated and non-ICU-treated patients is presented in . Altogether, 32.2% patients treated outside the ICU and 78.2% treated in ICU had AKI.
Table 2. AKI stage distribution between ICU-treated and non-ICU-treated GAS bacteraemia patients (n = 195).
AKI and duration of hospital stay
The number of the hospital days varied by AKI stage. The median duration of stay was 9 (IQR 8) in non-AKI, 15 (IQR 14) in AKI stage 1, 16 (IQR 29) in AKI stage 2 and 23 (IQR 28) in AKI stage 3. There was a significant difference between non-AKI vs. AKI stage 1 (p = .020), non-AKI vs. AKI stage 2 (p = .040) and non-AKI vs. AKI stage 3 (p = .005).
AKI and mortality
Overall, hospital mortality was 10.3%. Mortality rates by AKI stage are presented in . Mortality increased by AKI stage. After adjustment for age, Charlson’s comorbidity index and ICU admission, the 7-day mortality was higher among patients with AKI stage 2 than among patients with non-AKI (p = .046). Patients with AKI stage 3 had higher 7-day (p = .006), 30-day (p = .005), 90-day (p = .012) and 1-year mortality (p = .010) than patients with non-AKI.
Table 3. Mortality rates among GAS bacteraemia patients (n = 195) by KDIGO AKI stage.
AKI and CRP
The mean serum CRP levels on admission are summarised in . The higher the AKI stage, the higher was the mean serum CRP level ( and Supplementary Figure 1). The odds of having AKI stage 2 or 3 increased 1.74-fold per every 50 mg/l increase in CRP values (95% CI 1.45-2.08). The optimal cut-off CRP value to identify patients with AKI stage 2 or 3 was ≥244 mg/l (sensitivity 82.6% and specificity 75.8%). The ROC curve is presented in .
Figure 1. Receiver-operating characteristics (ROC) curve of GAS bacteraemia patients (n = 195) presenting the ability of CRP to identify those with AKI stage 2 or 3.
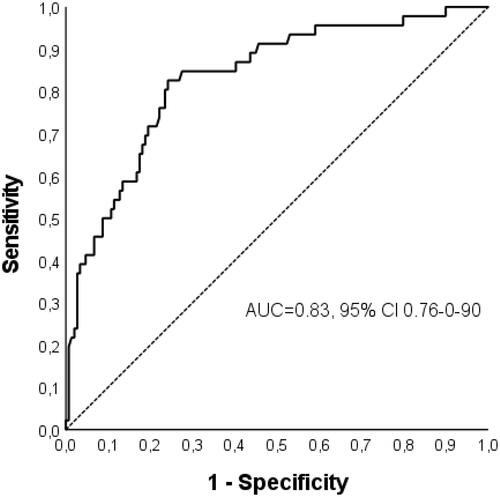
Table 4. Mean (SD) and adjusted mean (SE) CRP values (mg/ml) on admission by KDIGO AKI stage.
Patients with acute RRT
Altogether 16 (8.21%) patients needed RRT during the bacteraemia episode. All patients who needed RRT were ICU treated; 3 died during the hospital period. The mean age of patients with RRT was 53.6 years (SD 14.33) and the mean number of treatment days was 14.3 (SD 10.6). The RRT was started within two days of admission in all cases. The most common indication for RRT was anuria with or without acidosis. None of the patients needed RRT at discharge from hospital. See Supplementary Table 1 for details.
Renal recovery
The patients who died during the hospital period (n = 20), were excluded from this analysis. SCr status was available at discharge for 167 patients. 160 of these (95.8%) met the criteria signifying renal recovery. Recovery was significantly (p = .003) more common in the milder AKI stages: 93.8% in AKI stage 1, 100% in AKI stage 2 and 80.0% in AKI stage 3. One patient in the non-AKI group developed AKI later during the hospital period and had higher SCr at discharge than on admission, but data on urinalysis or on a control SCr were not available. One year after discharge the SCr status was available for 135 patients. Only one did not meet the renal recovery criteria at one year, although SCr at discharge indicated recovery.
Discussion
The main observation in this study was a high incidence of AKI among patients with GAS bacteraemia, but on the other hand, a high renal recovery rate among survivors. There was a statistically significant association between AKI stage and duration of stay and between AKI stage and mortality. We also found that an increased CRP level on admission provides predictive information on the severity of AKI.
The overall incidence of AKI was 43.1% and among non-ICU-treated patients, the incidence was 32.2%. Fiorentino et al. reported that 15% of patients with community-acquired pneumonia who survived to hospital discharge had stage 2– 3 AKI [Citation18]. Chertow et al. reported an incidence of 18% of AKI among hospitalised patients with infectious diseases [Citation23]. In earlier studies, the prevalence of AKI among septic ICU-treated patients has varied between 53% and 68% [Citation4,Citation5,Citation24]. In our study, the incidence of AKI among ICU-treated patients was even higher, 78.2%.
Björck et al. found that iGAS patients had higher levels of SCr at ICU admission compared to septic patients with a non-iGAS infection (median 173 vs. 133 µmol/l) and a higher Maximal Acute Kidney Injury Network classification score during the first 10 days after admission (median 3 vs. 0). They also found that iGAS patients with emm1, compared to other serotypes, more often had renal failure [Citation25]. Similarly, Bruun et al., who compared patients with necrotizing soft-tissue infections caused by GAS and Streptococcus dysgalactiae (SD), found that the patients in the GAS-group had higher preoperative SCr values than the patients in the SD group (178 vs. 103 µmol/l) [Citation26].
In earlier studies, SA-AKI has been associated with higher age [Citation4,Citation24], a higher number of co-morbid diseases [Citation4,Citation27], chronic kidney disease [Citation24,Citation28], hypertension [Citation24,Citation28], cardiovascular disease [Citation28] and diabetes mellitus [Citation24,Citation28]. However, in our study, those associations were not seen. Conversely, the mean age was lowest in AKI stage 3 and a majority of patients displaying AKI stage 3 had chronic kidney disease stage 1 or 2. However, these earlier studies have not focussed on SA-AKI caused by GAS. Our results are in line with a Swedish study in which the iGAS group was younger but developed still more often AKI than non-iGAS septic patients [Citation25]. GAS is known to cause severe invasive infections, for example, STSS and necrotising fasciitis, in younger adults and in patients without underlying disease [Citation29].
The duration of hospital stay was significantly longer in each AKI stage than in the non-AKI group. This is in line with earlier studies in which AKI has been related to longer ICU and hospital stays among septic and non-septic patients [Citation5,Citation30].
A high level of CRP on admission was associated, with a significant positive correlation, with an increased incidence of the higher severity stages of AKI, despite adjustment for patient age and Charlson’s comorbidity index. This suggests that the inflammatory reaction caused by GAS may play a more important role than comorbidities and age in the pathogenesis of SA-AKI caused by GAS. Nie et al. showed a correlation between, on the one hand, high serum procalcitonin (PCT) and CRP levels and, on the other hand, AKI among inpatients with infection symptoms. In the same study, PCT predicted more precisely than CRP the emergence of AKI in patients with suspected infection [Citation15]. In a retrospective observational study among 514 septic patients, CRP levels were significantly higher in the AKI-group than non-AKI-group (137 vs. 105 mg/l) [Citation14]. In our study, a CRP cut-off level of ≥ 244 mg/l identified the patients with stage 2 or 3 AKI with a sensitivity of 82.6% and a specificity of 75.8%. However, as both CRP and SCr are readily available and widely used in clinical practice, and as we did not study whether the CRP elevation precedes SCr elevation (which would enable earlier prediction of AKI), the clinical utility of this finding is limited.
In our study, almost all patients (95.8%) who survived to hospital discharge recovered from AKI. Patients with AKI stage 3, compared to lower stages, had a slightly lower renal recovery rate (80.0%) but the rate was still high compared to earlier studies. Fiorentino et al. showed that 42.4% of patients hospitalised with community-acquired pneumonia and AKI stage 2–3 recovered [Citation18] and Kellum et al. found that half of the patients with septic shock and AKI had regained their renal function by hospital discharge [Citation7]. In our study, mean age of patients with AKI stage 2 and 3 was lower compared to study by Fiorentino et al. Also, the Charlson class was relatively low among AKI stage 2 and 3 patients. Younger age and milder comorbidity may be one explanation for the better AKI recovery in our patients.
In recent years, the pathophysiology of SA-AKI has been shown to be multifactorial involving microcirculatory flow abnormalities and inflammation. Circulating cytokines may amplify endothelial inflammatory responses. An adaptive response of tubular epithelial cells to the changes of the local environment (i.e. downregulation of the cell function in order to ensure cell survival) is also recognised [Citation31,Citation32]. On the other hand, various GAS toxins may act as superantigens, stimulating immune-cells and inducing cytokine expression, which results in tissue damage and organ dysfunction [Citation33,Citation34]. Thus, we propose that the causative pathogen, or even the strain of this pathogen, may have an impact on the development of AKI among septic patients. However, iGAS-induced AKI seems generally to be reversible due to patient characteristics, e.g. younger age and less comorbidities.
In earlier studies, mortality has been related to the severity of AKI [Citation5,Citation7,Citation24], and AKI stage 3 has been shown to be independently associated with 90-day mortality [Citation24]. In the present study, the 7-day mortality was higher in AKI stage 2 and 3 than in the non-AKI group, and the 30-day mortality was higher in AKI stage 3 than in the non-AKI group. However, the 7-day and 30-day mortality rates in AKI stage 3 (19.2% and 23.1%) and AKI stage 2 (15.0% and 20.2%) were lower than in previous studies, where mortality among septic AKI patients ranged from 27.7% [Citation7] to 38.1% [Citation24] and to 51.7% [Citation5]. In these previous studies, patients were critically ill, but the pathogen was not specified, whereas in our study 73.1% of GAS bacteraemia patients with AKI stage 3 and 50.0% of AKI stage 2 were admitted to the ICU. Thus, our results are more in line with the earlier study of Björck et al [Citation25], in which 30-day mortality among ICU-treated iGAS patients was only 13%, and significantly lower than among non-iGAS septic patients, albeit renal failure was more common in the iGAS group.
A recent study has shown that the timing of the highest SCr value during a single admission correlates with mortality. If the highest SCr value occurred before the lowest (i.e. resolving AKI), rather than the reverse, mortality was lower [Citation35]. In our study, most patients with AKI could be categorised as resolving, thus in agreement with the low mortality noted.
Our study has some limitations. The retrospective dataset is a weakness. The premorbid SCr value was estimated by the MDRD equation in 33% of cases, which may overestimate the AKI stage in these patients. The proportion of patients without data on the premorbid SCr in our study was, nevertheless, markedly lower than in the FINNAKI study where it was 69% [Citation6]. Also, since 17 patients were excluded, the incidence of AKI should be interpreted with caution.
In conclusion, patients admitted for GAS bacteraemia met very often the criteria for AKI, but their renal recovery rate was high. A high CRP-level on admission was associated with a higher occurrence of stage 2 or 3 AKI. Development of severe AKI in bacteraemic GAS patients may be related to an acute septic inflammatory reaction rather than underlying diseases and patient characteristics. Patients with GAS bacteraemia and severe AKI may have higher rates of survival and recovery than SA-AKI patients in general. More studies are needed to investigate the association between the causative pathogen and SA-AKI. Regarding the pathogenesis of SA-AKI, the inflammatory pathways and AKI categories induced by sepsis and specific pathogens present interesting and important avenues for further clinical research.
Ethical approval
This retrospective registry-based study was approved by the Hospital District of Southwest Finland (decision numbers T05/014/17, T05/047/17 and T05/032/18, the latest dated 10 Sept 2018).
Author contributions
The material is original and has not been published elsewhere. Parts of this study were presented as a poster at the Annual meeting of the Nordic Society of Clinical Microbiology and Infectious Diseases (NSCMID) in Turku, Finland in September 2021.
All authors have made substantial intellectual contributions to the study conception, design and manuscript and approved the final version for submission and are able to account for its content.
Supplemental Material
Download MS Word (33.8 KB)Acknowledgements
The authors thank Robert Paul for language revision.
Disclosure statement
JVi reports a travel grant from Gilead and the Nordic Society of Clinical Microbiology and Infectious Disease and a lecture fee from the Finnish Medical Society Duodecim.
JVu reports a lecture fee from the Finnish Medical Association and has acted as book editor for the Finnish Medical Society Duodecim.
JO has been a scientific advisor (review panel or advisory committee) to Gilead Sciences Finland, GlaxoSmithKline and MSD Finland and received lecture honoraria from Biocodex, Orion, GlaxoSmithKline and MSD Finland.
NK and TV report no conflicts.
Data availability statement
The datasets generated during the current study are not publicly available as they contain health related data but limited datasets (without any identifiable, person-related data) are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Carapetis JR, Steer AC, Mulholland EK, et al. The global burden of group A streptococcal diseases. The Lancet Infectious Diseases. 2005;5(11):685–694.
- Kellum JA, Lameire N, Aspelin P, et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney International Supplements. 2012;2:1–138.
- Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818.
- Bagshaw SM, Lapinsky S, Dial S, The Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group, et al. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med. 2009;35(5):871–881.
- Peters E, Antonelli M, Wittebole X, et al. A worldwide multicentre evaluation of the influence of deterioration or improvement of acute kidney injury on clinical outcome in critically ill patients with and without sepsis at ICU admission: results from The intensive care Over nations audit. Crit Care. 2018;22(1):188.
- Poukkanen M, Wilkman E, Vaara ST, FINNAKI Study Group, et al. Hemodynamic variables and progression of acute kidney injury in critically ill patients with severe sepsis: data from the prospective observational FINNAKI study. Crit Care. 2013;17(6):R295.
- Kellum JA, Chawla LS, Keener C, ProCESS and ProGReSS-AKI Investigators, et al. The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. 2016;193(3):281–287.
- Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ. 2019;364:k4891.
- Md Ralib A, Mat Nor MB, Pickering JW. Plasma neutrophil Gelatinase-Associated lipocalin diagnosed acute kidney injury in patients with systemic inflammatory disease and sepsis. Nephrology. 2017;22(5):412–419.
- Dai X, Zeng Z, Fu C, et al. Diagnostic value of neutrophil gelatinase-associated lipocalin, cystatin C, and soluble triggering receptor expressed on myeloid cells-1 in critically ill patients with sepsis-associated acute kidney injury. Crit Care. 2015;19(1):223
- Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–1761.
- Cosentino N, Genovese S, Campodonico J, et al. High-Sensitivity C-Reactive protein and acute kidney injury in patients with acute myocardial infarction: a prospective observational study. JCM. 2019;8(12):2192. Available at:
- Zhou X, Liu J, Ji X, et al. [Predictive value of inflammatory markers for acute kidney injury in sepsis patients: analysis of 753 cases in 7 years]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018;30:346–350.
- Katayama S, Nunomiya S, Koyama K, et al. Markers of acute kidney injury in patients with sepsis: the role of soluble thrombomodulin. Crit Care. 2017;21(1):229.
- Nie X, Wu B, He Y, et al. Serum procalcitonin predicts development of acute kidney injury in patients with suspected infection. Clin Chem Lab Med. 2013;51(8):1655–1661. Available at Accessed 13 January 2022.
- Rodriguez-Iturbe B, Musser JM. The current state of poststreptococcal glomerulonephritis. JASN. 2008;19(10):1855–1864.
- Vilhonen J, Vuopio J, Vahlberg T, et al. Group A streptococcal bacteremias in Southwest Finland 2007-2018: epidemiology and role of infectious diseases consultation in antibiotic treatment selection. Eur J Clin Microbiol Infect Dis. 2020;39(7):1339–1348.
- Fiorentino M, Tohme FA, Wang S, et al. Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLoS One. 2018;13(6):e0198269.
- Mildh H, Pettilä V, Korhonen A-M, FINNAKI Study Group, et al. Three-year mortality in 30-day survivors of critical care with acute kidney injury: data from the prospective observational FINNAKI study. Ann Intensive Care. 2016;6(1):118.
- Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. In: Critical care (London, England)2004.
- Chawla LS, Bellomo R, Bihorac A, Acute Disease Quality Initiative Workgroup 16., et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. 2017;13(4):241–257.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370.
- Poukkanen M, Vaara ST, Pettilä V, FINNAKI study group, et al. Acute kidney injury in patients with severe sepsis in finnish intensive care units. Acta Anaesthesiol Scand. 2013;57(7):863–872.
- Björck V, Påhlman LI, Bodelsson M, et al. Morbidity and mortality in critically ill patients with invasive group A streptococcus infection: An observational study. Crit Care. 2020;24(1):302.
- Bruun T, Rath E, Madsen MB, INFECT Study Group, et al. Risk factors and predictors of mortality in streptococcal necrotizing soft-tissue infections: a multicenter prospective study. Clinical Infectious Diseases. 2021;72(2):293–300.
- Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. CJASN. 2007;2(3):431–439.
- Liu J, Xie H, Ye Z, et al. Rates, predictors, and mortality of sepsis-associated acute kidney injury: a systematic review and Meta-analysis. BMC Nephrol. 2020;21(1):318.
- Nelson GE, Pondo T, Toews K-A, et al. Epidemiology of invasive group A streptococcal infections in the United States, 2005-2012. Clin Infect Dis. 2016;63(4):478–486.
- Xu X, Nie S, Liu Z, et al. Epidemiology and clinical correlates of AKI in chinese hospitalized adults. Clin J Am Soc Nephrol. 2015;10(9):1510–1518.
- Umbro I, Gentile G, Tinti F, et al. Recent advances in pathophysiology and biomarkers of sepsis-induced acute kidney injury. Journal of Infection. 2016;72(2):131–142.
- Zarbock A, Gomez H, Kellum JA. Sepsis-induced AKI revisited: pathophysiology, prevention and future therapies. Curr Opin Crit Care. 2014;20(6):588–595.
- Lappin E, Ferguson AJ. Gram-positive toxic shock syndromes. The Lancet Infectious Diseases. 2009;9(5):281–290.
- Shannon BA, McCormick JK, Schlievert PM. Toxins and superantigens of group A streptococci. Microbiol Spectr. 2019;7(1). DOI:10.1128/microbiolspec.GPP3-0054-2018
- Warnock DG, Neyra JA, Macedo E, et al. Comparison of static and dynamic baseline creatinine surrogates for defining acute kidney injury. Nephron. 2021;145(6):664–674.
- Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. American Journal of Kidney Diseases. 2014;63(5):713–735.
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System (NNDS). Streptococcal Toxic Shock Syndrome | 2010. Case Definition.
