Abstract
Background
COVID-19 disease leads to prolonged hospitalisations and adverse outcomes. We describe our strategy for routine early discharge of severe COVID-19 patients with home oxygen during the Delta variant surge.
Methods
Our strategy included COVID-19 patients requiring oxygen support via nasal cannula, with stabilised but not yet improved respiration (intervention group), that followed strict criteria. Severe COVID-19 patients discharged after improved respiration were considered the control group for comparison. Outcomes included readmissions from active COVID-19 and 30-day mortality.
Results
The intervention group included 129 patients, and the control 150. The groups’ baseline characteristics were similar, although the control group had more advanced COVID-19 severity. Among the intervention group, 23 (17.8%) had readmissions secondary to active COVID-19, compared to none in the control group. The 30-day mortality rate was similar between the groups (5% vs. 7%). The intervention led to a shorter hospital stay [median 3 days (IQR 2-4) vs. 6 days (IQR 4-9), p < .01], while a very short hospitalisation was associated with readmissions (2.8 vs. 3.5 days, p = .02). A subsequent critical disease or death after the intervention occurred in old (81 years), multimorbid (3.4 ± 1.4) patients with a high percentage of acute kidney injury during their first hospitalisation (50%).
Conclusions
Our discharge strategy led to a short hospital stay, a high readmission rate, and similar long-term outcomes. Considering the difference in disease severity before discharge, this intervention cannot be considered safe for our study population. Correct patient selection is crucial to ensure patient safety when considering early discharge.
Background
Coronavirus disease 2019 (COVID-19) is a worldwide pandemic with the potential to reach nearly 60% of the population [Citation1]. It can lead to an overwhelmed healthcare system and shortage of medical staff and hospital beds. Decreasing the hospitalisation period can lower the overall disease burden, but patient safety should be prioritised. Discharging a patient only after a respiratory improvement commence might seem reasonable, as 20% of hospitalised COVID-19 patients deteriorate [Citation2–5]. However, respiratory improvement can take more than 10 days, resulting in extended hospitalisations [Citation6]. The study describes the characteristics and outcomes of patients with severe COVID-19 that were discharged early as part of a strategy to lower hospital burden on patients and medical staff.
Methods
Study definitions
Major respiratory improvement was defined for a patient that required any method of oxygen supply (nasal cannula, oxygen mask, high flow nasal cannula, etc.) and improved to the point of sustaining an oxygen saturation above 93% using a method with a lower oxygen flow supply or in room air (e.g. from oxygen mask to nasal cannula or from nasal cannula to room air). Severe COVID-19 disease was defined for patients with oxygen saturation ≤93% while breathing ambient air at resting state or with respiratory rate ≥30/min, in accordance with accepted guidelines [Citation7]. COVID-19 was defined by positive nasopharyngeal real-time polymerase chain reaction (PCR).
Study design and population
This is a retrospective study describing consecutive adult (>18 years) patients from a large tertiary hospital in the centre of Israel, after admission due to COVID-19. The study duration is a three-month period during the fourth wave of COVID-19 (between July 25 and October 25, 2021) which involved almost exclusively the Delta variant. The study inclusion process is presented in . Patients in the intervention group met all the following criteria: (1) admission due to severe COVID-19 disease, (2) requirement of intermittent or constant oxygen supplement of 2-4 litres per minute via nasal cannula to maintain oxygen saturation above 93% at discharge, (3) discharge home or to a long-term care facility with available oxygen supply (usually by oxygen concentrator device), (4) no major respiratory improvement during hospital stay.
Figure 1. Inclusion process flow chart of the study cohort. a Hospitalised to the medical centre during the study period.
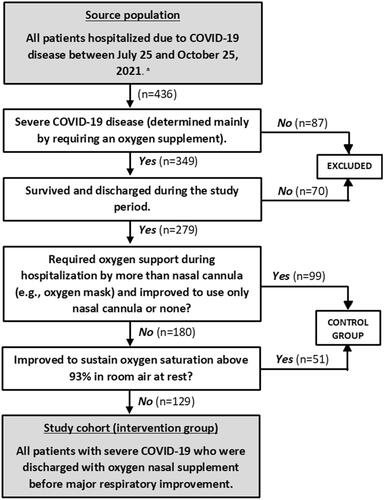
To properly evaluate our cohort’s characteristics and outcomes we used a control group. The control group included all severe COVID-19 patients that were discharged from our hospital during the study period after major respiratory improvement ().
The study was approved by the Sourasky Medical Centre review board (TLV-0876-20) and conducted in accordance with the declaration of Helsinki.
General discharge policy
Part of our daily routine in the COVID-19 department includes an evaluation of each patient’s ability to be safely discharged. Early discharge was a high priority to avoid any hospital-related adverse events for the patient, prevent the opening of more COVID-19 departments and lower the workload of medical staff. Before discharge, the hospital notified the patients’ health maintenance organisation (HMO) to ensure the continuity of care in the ambulatory setting.
In general, before patients’ discharge, all the following had to be met: (1) at least 24 h of respiratory stability (no need of higher oxygen supply), (2) at least 24 h of hemodynamic stability, (3) maintaining oxygen saturation above 93% at rest in room air or by nasal cannula (with oxygen flow of up to 4 litres per minute), (4) ability to self-manage or manage with assistance of a caregiver (in specialised long term care facility if needed), and (5) no other active medical issues that require continued acute inpatient care.
Treatment protocol during hospitalisation for severe COVID-19 included dexamethasone (6 mg every 24 h, for 10 days) and daily prophylaxis dose of enoxaparin, based on previous studies and guidelines [Citation7,Citation8]. Remdesivir was given per case based on infectious disease expert opinion (200 mg loading dose proceeded with 100 mg per day for 4 days) [Citation6]. Upon discharge, patients were prescribed for the completion of the treatment with dexamethasone and enoxaparin.
Because patient safety was our top priority, we ensured all the following before discharge: (1) home treatment, oxygen monitoring and isolation instructions were given by a physician to the patient and a family member, (2) home oxygen generator was installed and instructions for its use were given to the patient and a family member, (3) available home oxygen oximeter, (4) available 24-hour consult centre operated by the HMO for medical advice (patients were instructed to report any deterioration in saturation), and (5) routine follow up calls by a physician from the HMO (usually the patients’ family primary care physician) during the isolation period and at the end.
Study outcomes and variables
To evaluate the outcomes of early discharge, we examined the incidence of readmissions secondary to an active COVID-19 disease, progression to critical COVID-19 disease or death from any cause. The follow-up period was 30 days from hospital discharge. Readmissions were reviewed from the hospital medical electronic files. Death was collected from our centre database which is updated based on the Israel National death registry.
We evaluated baseline characteristics of patients based on previous literature [Citation3,Citation4,Citation9]. Immunodeficiency was termed for a definition of active haematologic malignancy or treatment with immune-suppressive drugs (if steroids, equivalent dose of prednisone 15 mg for more than one month). Clinical data included length of stay (continuous variable in days), length of symptoms before admission (continuous variable in days), treatment given and any extra-pulmonary complications. COVID-19 vaccine status was divided into three: non-vaccinated, patients after the first two doses of the BNT162b2 vaccine and patients after the booster dose. Patients were considered vaccinated if the last vaccine dose was given at least 10 days before the first positive nasopharyngeal PCR test for SARS-CoV-2, as previous studies showed a protective effect starting 7 to 12 days after vaccinations [Citation10]. The booster vaccination was offered to the general Israeli population in mid-July 2021 (two weeks before the study period).
Statistical analysis
Data were analysed with IBM SPSS statistics software version 28.0. The significance levels were set at 0.05. Data were presented as mean and standard deviation (with median and interquartile range when needed) for continuous variables and as frequency and percentage for categorical variables. Median and interquartile range was used in cases of non-normally distributed continuous variables. Chi-square tests and independent t-tests were performed to compare categorical and continuous variables, respectively.
Results
During the study period, 436 patients were admitted to any of the COVID-19 departments in our facility. Overall, 349 patients had severe COVID-19 disease, 70 (20%) died during hospitalisation, 129 (37%) were included in the intervention group and 150 (43%) were included as the control group (). Of the intervention group, 23 patients (17.8%) were re-admitted secondary to COVID-19 disease. Additional three patients (2.3%) died after discharge during the 30-days follow-up period and were included in the analysis (overall 30-day mortality 5%). Among the control group, eleven patients (7%) died during the 30-days follow-up period and none of the patients had a readmission secondary to COVID-19 disease ().
Table 1. Characteristics of the intervention and control groups during the first hospitalisation.a
Characteristics of the cohort population during the first hospitalisation are presented in . Compared with the control group, the intervention group had similar baseline characteristics and duration from symptoms or swab to hospital admission (). Dexamethasone was prescribed to 99% of the patients. During the first hospitalisation, the intervention group had lower rates of critical COVID-19 disease (0 vs. 27%, p < .001), extra-pulmonary involvement (p = .03) and other proven infections (p < .01) than the control group. On the other hand, 30 days after discharge, the groups had a similar rate of critical disease or death (6% vs. 7%). The mean hospital stay duration was significantly longer among the control group (8.67 ± 5.5 vs. 3.33 ± 1.7 p < .01).
presents the characteristics of patients with and without the primary outcomes among the intervention group. Overall, death or critical COVID-19 disease 30-days following discharge occurred in 8 patients (6%) in the intervention group. Their age was 81 ± 11, and they had a high number of comorbidities (3.4 ± 1.4), cognitive decline (50%), and AKI during their first hospitalisation (50%). Still, all baseline characteristics were not statistically different between patients with and without any of the outcomes 30 days after discharge. Of note, the vaccine status was also similar between the groups (p = .655). The only clinical difference between these groups was a shorter hospital stay among patients that had a readmission or died (2.85 ± 1.38 vs 3.46 ± 1.78, p = .03).
Table 2. Characteristics of the intervention group divided by the different outcomesa.
Outcomes of the second hospitalisation among readmitted patients are presented in . Respiratory deterioration was the main reason for readmission and was either subjective (e.g. complaints of dyspnoea without needing higher oxygen support, 10 of 22 patients, 45%) or objective (e.g. requirement of higher oxygen level than that supplied by nasal cannula already available at home, 12 of 22 patients, 55%). One patient was readmitted due to COVID-19 related encephalopathy. All readmitted patients complied with the prescribed treatment throughout their home stay.
Table 3. Characteristics of readmitted patients and outcomes of second hospitalisation.
Discussion
In this cohort, we describe a selected group of stable severe COVID-19 patients that were discharged early before an improvement in their oxygen support needs. This group consisted of 129 patients, which were 46% of the discharged patients after severe COVID-19 disease in the study period (). Our clinical experience and results show that once major respiratory improvement occurs, patients do not tend to have a second deterioration related to COVID-19 pneumonia. For this reason, findings of previous studies regarding discharge outcomes may present an underestimation for patients that are discharged before major respiratory improvement. An early discharge of a stable patient carries a benefit potential for both the patient and the healthcare system, especially during a pandemic with its constant threat of reaching hospital insufficiency. Taking that into consideration, patient safety should come first to avoid any complications or delay in treatment. Previous studies have already described the general readmission rates among COVID-19 patients [Citation11–13]. Few studies have also described the ambulatory care and outcomes of hospitalised COVID-19 patients that were discharged with oxygen support [Citation14,Citation15]. However, these studies addressed patients with an initial in-hospital respiratory improvement. To our knowledge, this is the first study to address this specific patient population and level of severity.
The rate of readmission secondary to active COVID-19 disease in the intervention group was 17.8%, while it did not occur for any of the patients in the control group. This rate is also higher compared to other studies of discharged COVID-19 patients, with reported ranges of 4.2%-9.2% [Citation12,Citation13,Citation15]. Our intervention group included patients with respiratory failure (all required oxygen supply) that were discharged before major evidence of improvement in their need of supplemental oxygen, possibly causing a higher likelihood for readmissions. The rate of critical disease among readmitted patients (22%) is consistent with the rate among the control group (27%) and with rates of respiratory deterioration reported in previous studies [Citation3,Citation4,Citation16–19]. The 30-day mortality rate of our intervention group was 5%, similar to the 7% rate among the control group and among the mentioned previous studies.
While similar long-term outcomes were noted between the groups, none of the patients in the intervention group had a critical disease during their first hospitalisation compared to the 27% in the control group, and none required a higher oxygen support than that obtained by nasal cannula. Therefore, a lower rate of mortality was expected among the intervention group. We did not find evidence to indicate a direct harm by early discharge, such as discontinuation of treatment among readmitted patients. Still, when considering the difference in disease severity during the first hospitalisation, our intervention cannot be considered safe as a general strategy for the study population. Among the intervention group, patients with 30-day mortality or critical COVID-19 disease seemed to be older, with high rates of cognitive decline, comorbidities, and AKI during their first hospitalisation. These characteristics should be taken into consideration for future implementation of an early discharge to avoid possible harm.
Patients’ baseline characteristics and rates of comorbidities were in accordance to previous studies of hospitalised COVID-19 patients [Citation2,Citation5,Citation9,Citation16]. As mentioned in the results, they were also similar between the intervention and control groups. These findings show that discharging severe COVID-19 patients early was not limited to young, healthy, and independent patients. Of note, extra-pulmonary disease or other concurrent infections occurred more in the control group. This can be the result of a worse COVID-19 infection or general condition (which can possibly withhold any early discharge). However, concurrent infections might also be the result of a longer hospital stay duration.
The unique Health system in Israel, where all citizens are insured and receive similar ambulatory and non-ambulatory services by the HMOs, enabled a high-quality community care for COVID-19 patients. This resulted in a fast arrangement of home oxygen generator, 24-hour available consultants, long-term care facilities for those in need and regular follow-ups until recovery by a general physician. We emphasise the need to ensure a safe community environment with suitable support measures to react early if any clinical deterioration occurs and prevent delays in treatment or readmission.
Considering only the intervention group, we found a shorter duration of hospital stay among readmitted patients (2.78 ± 1.02 vs 3.46 ± 1.78, p = .02). Previous results showed a similar relationship [Citation11,Citation13], while others did not [Citation12]. The median duration of hospital stay among the intervention group was 3 days (interquartile range 2-4 days) and to our knowledge it is the shortest reported compared with previous studies, ranging 4.5-17 days [Citation2,Citation11,Citation14,Citation16]. It is also significantly shorter than the hospital stay duration of our control group (median 6, IQR 4-9, p < .01). To translate our observations into a widely accepted agenda, more research that focuses on methods to lower the disease burden is needed, with patient safety as their main primary outcome. As mentioned above, ensuring a safe environment after discharge is crucial for the implementation of a similar discharge method. Understanding that we can often treat severe COVID-19 disease in the community setting will help lower the immense disease burden.
Our study has several limitations. A selection bias is possible by addressing only patients with early discharge. While the intervention group had similar baseline characteristics as the control group and as described in previous studies, they also had lower rates of critical disease, extra-pulmonary COVID-19 involvement and other proven infections during their first admission. Therefore, the comparison between the groups is limited. Any readmission secondary to active COVID-19 disease was evaluated using the hospital databases and we cannot account for any missed readmissions. We addressed improvement in supplemental oxygen as the only exclusion criteria while other clinical and non-clinical factors, such as improvement in respiratory rate and social status may had an impact on the decision to discharge a patient. Risk factors for readmission among COVID-19 patients are known and were described in several studies [Citation11,Citation13]. The unique nature of our study population resulted in a limited sample size which prevented similar analysis. Additional studies with prospective designs and larger sample size are needed prior to a general use of our presented discharge method.
We conclude that early discharge of severe COVID-19 patients with supplemental oxygen resulted in a shorter hospital stay, similar overall critical disease or death 30 days after discharge, and a high rate of readmissions due to subjective or objective worsening of COVID-19. Given the large difference in oxygen support levels and critical COVID-19 disease during the first hospitalisation between the groups, early discharge could not be considered safe for our study population. To avoid any harm, the medical team needs to thoroughly evaluate the patients’ baseline characteristics, clinical condition, available support systems and available ambulatory medical care before deciding on early discharge.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in wuhan, China: a modelling study. Lancet. 2020;395(10225):689–697.
- Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369(March):1–12.
- Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York city: a prospective cohort study. Lancet. 2020;395(10239):1763–1770.
- Lewnard JA, Liu VX, Jackson ML, et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ. 2020;369(9):m1923
- Cao J, Tu W-J, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in wuhan, China. Clin Infect Dis. 2020;71(15):748–755.
- Beigel JH, Tomashek KM, Dodd LE, ACTT-1 Study Group Members, et al. Remdesivir for the treatment of covid-19 — final report. N Engl J Med. 2020;383(19):1813–1826.
- National Institutes of Health. COVID-19 Treatment Guidelines. 2021. https://www.covid19treatmentguidelines.nih.gov/
- Group RC. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693–704.
- Lu Y, Jiao Y, Graham DJ, et al. Risk factors for COVID-19 deaths among elderly nursing home medicare beneficiaries in the prevaccine period. J Infect Dis. 2022;225(4):567–577.
- Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400.
- Somani SS, Richter F, Fuster V, Mount Sinai COVID Informatics Center, et al. Characterization of patients who return to hospital following discharge from hospitalization for COVID-19. J Gen Intern Med. 2020;35(10):2838–2844.
- Ramos-Martínez A, Parra-Ramírez LM, Morrás I, et al. Frequency, risk factors, and outcomes of hospital readmissions of COVID-19 patients. Sci Rep. 2021;11(1):1–10.
- Donnelly JP, Wang XQ, Iwashyna TJ, et al. Readmission and death after initial hospital discharge among patients with COVID-19 in a large multihospital system. JAMA - J Am Med Assoc. 2021;325(3):304–306.
- Dinh A, Mercier J-C, Jaulmes L, AP-HP/Universities/INSERM COVID-19 Research Collaboration, et al. Safe discharge home with telemedicine of patients requiring nasal oxygen therapy after COVID-19. Front Med (Lausanne). 2021;8:703017.
- Banerjee J, Canamar CP, Voyageur C, et al. Mortality and readmission rates among patients with COVID-19 after discharge from acute care setting with supplemental oxygen. JAMA Netw Open. 2021;4(4):e213990–6.
- Richardson S, Hirsch JS, Narasimhan M, the Northwell COVID-19 Research Consortium, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA - J Am Med Assoc. 2020;323(20):2052–2059.
- Bouzid D, Visseaux B, Kassasseya C, IMProving Emergency Care (IMPEC) FHU Collaborators Group, et al. Comparison of patients infected with Delta versus omicron COVID-19 variants presenting to paris emergency departments : a retrospective cohort study. Ann Intern Med. 2022;175(6):831–837.
- Rzymski P, Pazgan-Simon M, Kamerys J, et al. Severe breakthrough COVID-19 cases during six months of Delta variant (B.1.617.2) Domination in Poland. Vaccines. 2022;10(4):557.
- Jassat W, Abdool Karim SS, Mudara C, et al. Clinical severity of COVID-19 in patients admitted to hospital during the omicron wave in South Africa: a retrospective observational study. Lancet Glob Heal. 2022;10(7):e961–e969.
