Abstract
Background
Cefiderocol is generally active against carbapenem-resistant Klebsiella spp. (CRK) with higher MICs against metallo-beta-lactamase producers. There is a variation in cefiderocol interpretive criteria determined by EUCAST and CLSI. Our objective was to test CRK isolates against cefiderocol and compare cefiderocol susceptibilities using EUCAST and CLSI interpretive criteria.
Methods
A unique collection (n = 254) of mainly OXA-48-like- or NDM-producing CRK bloodstream isolates were tested against cefiderocol with disc diffusion (Mast Diagnostics, UK). Beta-lactam resistance genes and multilocus sequence types were identified using bioinformatics analyses on complete bacterial genomes.
Results
Median cefiderocol inhibition zone diameter was 24 mm (interquartile range [IQR] 24–26 mm) for all isolates and 18 mm (IQR 15–21 mm) for NDM producers. We observed significant variability between cefiderocol susceptibilities using EUCAST and CLSI breakpoints, such that 26% and 2% of all isolates, and 81% and 12% of the NDM producers were resistant to cefiderocol using EUCAST and CLSI interpretive criteria, respectively.
Conclusions
Cefiderocol resistance rates among NDM producers are high using EUCAST criteria. Breakpoint variability may have significant implications on patient outcomes. Until more clinical outcome data are available, we suggest using EUCAST interpretive criteria for cefiderocol susceptibility testing.
Introduction
Carbapenem resistant Klebsiella (CRK) infections are associated with significant morbidity and mortality for the vulnerable hospitalised patients. This is partly explained by the underlying patient comorbidities, and is largely contributed by the lack of active antibiotics against CRK. Several new antibiotics have been approved recently for the treatment of OXA-48-like- and KPC-producing CRK infections. However, metallo-beta-lactamase-producing CRK remains as a challenging group with few treatment options [Citation1]. Cefiderocol is a new generation cephalosporin with in vitro activity against all clinically important carbapenemases including metallo-beta-lactamases [Citation2]. However, cefiderocol MICs were reported to be higher for the metallo-beta-lactamase producers [Citation3]. Real life data evaluating cefiderocol use for metallo-beta-lactamase-producing CRK are limited [Citation4].
Accurate antimicrobial susceptibility testing (AST) is important to guide treatment. Cefiderocol AST remains challenging due to the requirement for iron-depleted media for broth microdilution (BMD). Disc diffusion has been accepted as a robust alternative to BMD for cefiderocol AST and is the most practical method applied by the clinical laboratories [Citation5,Citation6]. Another challenging aspect of cefiderocol AST is the substantial difference between EUCAST and CLSI interpretive criteria. Isolates with a cefiderocol MIC of >2 mg/L and ≥16 mg/L are categorised as resistant according to EUCAST and CLSI, respectively () [Citation7,Citation8]. EUCAST does not have an ‘I’ (susceptible, increased exposure) category and has an area of technical uncertainty (ATU) for the isolates with a inhibition zone diameter of 18–22 mm, whereas CLSI has a range (9–15 mm) for ‘I’ (intermediate) category but no ATU. The difference between the MIC breakpoints is the reason for different zone diameter breakpoints, as the latter are calibrated to MIC breakpoints.
Table 1. Comparison of EUCAST and CLSI interpretive criteria for Enterobacterales.
The objective of this study was to test a large collection of CRK bloodstream isolates that were collected as part of a multicentre observational study, against cefiderocol to demonstrate its in vitro activity against OXA-48-like and metallo-beta-lactamase producing CRK isolates. We aimed to compare EUCAST and CLSI breakpoints, and demonstrate discrepancies that would arise from breakpoint variability.
Methods
Isolate selection
Isolates were collected as part of a multicentre observational study between June 2018 and June 2019 from 13 tertiary care centres in Turkey [Citation9]. The study was approved by the Koc University Institutional Review Board (approval number: 2018.151.IRB1.018). Carbapenem resistance was defined as being non-susceptible to at least one carbapenem according to EUCAST 2018 breakpoints (i.e. MIC >2 µg/mL for meropenem or imipenem, and >0.5 µg/mL for ertapenem). Carbapenem resistance was detected by automated methods at participating laboratories and was confirmed with Etest (bioMérieux, France) in the central laboratory (University of Queensland Centre for Clinical Research laboratories). Species identification was performed using MALDI-TOF MS (Bruker, Bremen, Germany). Whole genome sequencing was performed at Australian Centre for Ecogenomics using Illumina NextSeq500 (Illumina Inc., San Diego, CA) instrument. Carbapenemase genes were detected on whole bacterial genomes using Abricate version 0.8.10 (https://github.com/tseemann/abricate) with the NCBI database [Citation10]. Lineage STs were assigned using MLST (https://github.com/tseemann/mlst) with MLST profiles from pubMLST (https://pubmlst.org/).
Susceptibility testing
Cefiderocol susceptibility testing was performed using 30 µg cefiderocol discs (Mast Diagnostics, UK) according to EUCAST 2021 criteria [Citation7]. Briefly, isolates were grown on non-selective blood agar at 35 ± 1 °C for 18 ± 2 h. A single colony was suspended in 0.9% NaCl for a McFarland of 0.5. This was streaked onto standard Mueller-Hinton agar (Thermo Fisher Scientific, Swindon, UK) and plates were incubated in aerobic air at 35 ± 1 °C for 16–18 h. Quality control organisms (i.e. Escherichia coli ATCC 25922) were prepared each day of testing. All experiments were performed in duplicates on the same day. In the event of a discordance between the results of the first two experiments, a third experiment was performed. Discordance was defined as discrepancies resulting in assignment of bacterial isolates to adjacent interpretative category (i.e. susceptible to intermediate, intermediate to susceptible, intermediate to resistant, resistant to intermediate, susceptible to resistant, resistant to susceptible). Inhibition zone diameters were read using the innermost colony-free zone when pinpoint colonies were observed within the zone. Results were interpreted by applying EUCAST and CLSI breakpoints ().
Results
Isolate characteristics
There were 254 Klebsiella spp. isolates (92% Klebsiella pneumoniae, 6% Klebsiella variicola, 0.4% Klebsiella quasipneumoniae and 0.4% Klebsiella oxytoca). Fifty (20%) isolates were found to be carbapenem susceptible in the central laboratory and did not harbour any carbapenemases. The majority of the remaining isolates were OXA-48-like producers (145/204, 70%), followed by metallo-beta-lactamase/OXA-48-like co-producers (31/204, 15%), single metallo-beta-lactamase producers (11/204, 5%), no-carbapenemase producers (13/204, 6%) and KPC producers (4/204, 2%). All metallo-beta-lactamases, except a single VIM, were NDM.
SHV was the main extended spectrum beta-lactamase (ESBL) class detected in this collection (232/254, 91%), followed by CTX-M (196/254, 77%) and TEM (109/254, 43%). Predominant SHV, CTX-M and TEM types were SHV-106 (90/232, 36%), CTX-M-15 (184/196, 73%) and TEM-150 (105/109, 96%), respectively (data not shown).
ST2096 was the main multilocus sequence type (66/254, 26%) followed by ST101 (37/254, 15%) and ST14 (28/254, 11%). The majority (58/66, 88%) of ST2096 strains carried OXA-232 and the majority (20/28, 71%) of ST14 strains carried an NDM and an OXA-48-like concomitantly.
Inhibition zone diameters
Median cefiderocol disc zone diameter was 24 mm (interquartile range [IQR] 24–26 mm) for all isolates, 24 mm (IQR 23–26 mm) for OXA-48-like producers, 18 mm (IQR 15–21 mm) for metallo-beta-lactamase producers, 25 mm (IQR 22–26 mm) for KPC producers, 26 mm (IQR 24–27 mm) for none-carbapenemase-producers and 28 mm (IQR 26–28 mm) for carbapenem susceptible isolates (, ).
Figure 1. Box plot graph of cefiderocol inhibition zone diameter according to carbapenem resistance mechanism. S: susceptible.
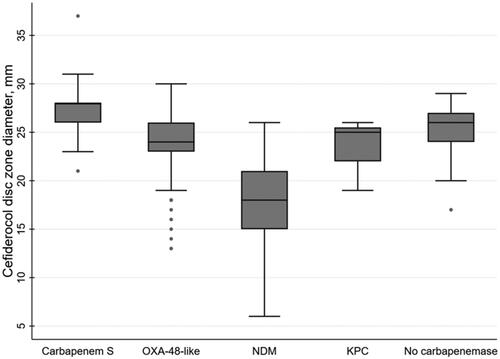
Table 2. Cefiderocol inhibition zone diameter according to the carbapenemase type.
Interpretive criteria
Cefiderocol susceptibility was demonstrated for 189 (74%) and 236 (93%) of all isolates using EUCAST and CLSI interpretive criteria, respectively (p < 0.001) (). About 90% (47/52) of those that were categorised as resistant by EUCAST were categorised as susceptible by CLSI. Susceptible percentages using EUCAST and CLSI interpretive criteria for OXA-48-like, metallo-beta-lactamase and KPC producers were 81% and 97%, 19% and 67%, and 75% and 100%; respectively. Majority of the non-carbapenemase producers and all carbapenem susceptible isolates remained susceptible to cefiderocol using EUCAST and CLSI interpretive criteria, respectively (). Forty-nine (19%) isolates fell into EUCAST ATU category (). The percentage of isolates in the ATU category were higher for the metallo-beta-lactamase producers (16/42, 38%).
Table 3. Comparison of interpretive categories when using EUCAST and CLSI inhibition zone diameter breakpoints for cefiderocol according to carbapenem resistance mechanism.
Cefiderocol susceptibility according to MLST
ST14 and ST101 were the sequence types with the highest and lowest cefiderocol resistance rates, respectively, according to EUCAST interpretive criteria (27/28, 96% and 1/37, 3%; respectively). Cefiderocol resistance rate was 29% (19/66) for the predominant sequence type ST2096 (Supplementary Figure).
Cefiderocol susceptibility according to ESBL type
ESBL presence was more common among cefiderocol resistant isolates as compared to cefiderocol susceptible isolates according to EUCAST criteria. CTX-M, SHV and TEM were present in 94%, 100% and 58% of the cefiderocol resistant isolates, and 71%, 88% and 38% of the cefiderocol susceptible isolates, respectively (). SHV and TEM carriage rates were similar for cefiderocol susceptible and resistant OXA-48-like producers, whereas CTX-M carriage rate was higher for cefiderocol resistant OXA-48-like producers as compared to their susceptible counterparts (100% vs. 80%). CTX-M and TEM carriage rates were similar for cefiderocol susceptible and resistant metallo-beta-lactamase producers, whereas SHV carriage was higher for cefiderocol resistant metallo-beta-lactamase producers as compared to their susceptible counterparts (100% vs. 75%) ().
Table 4. ESBL type according to cefiderocol susceptibility as per EUCAST.
Discussion
In this study, majority of OXA-48-like producers remained susceptible to cefiderocol using disc diffusion, in accordance with the previous reports [Citation2]. There was a striking variation in resistance rates, particularly for metallo-beta-lactamase producers, using EUCAST vs. CLSI breakpoints, with significantly higher resistance rates with the former. The range of inhibition zones was large (e.g. 14–26 mm for metallo-beta-lactamase producers), suggesting the presence of additional resistance mechanisms contributing to cefiderocol resistance.
Elevated cefiderocol MICs for NDM producers were previously demonstrated in in vitro surveillance studies and the co-presence of an ESBL, particularly CTX-M, was found to be more frequent among cefiderocol-resistant metallo-beta-lactamase producers [Citation3]. In this study, copresence of an ESBL, particularly SHV, was more frequent among cefiderocol-resistant metallo-beta-lactamase producers. We also observed an association between the MLST type and cefiderocol resistance, such that all except one of the ST14 isolates and all except one of the ST101 isolates were cefiderocol resistant and susceptible, respectively. This may partially be explained by the carbapenemase types, as 75% of the isolates in the ST14 group harboured a metallo-beta-lactamase, whereas only 5% of the ST101 isolates harboured a metallo-beta-lactamase.
More recently, cefiderocol resistance development under cefiderocol exposure was demonstrated in a clinical isolate recovered from a patient who was treated with cefiderocol for an intra-abdominal infection caused by NDM-5 producing E. coli [Citation11]. Cefiderocol resistance development in this study was demonstrated to be associated with an increase in the blaNDM-5 copy number. A number of other mechanisms were found to be associated with cefiderocol MIC increase in Enterobacterales, such as the deletions of the bacterial iron transport system components, mutations in the signal transduction and energy transduction systems [Citation12,Citation13], and mutations in the chromosomal AmpC enzymes [Citation14,Citation15]. The molecular resistance mechanisms contributing to cefiderocol resistance in this study are yet to be described.
In vitro studies and earlier PK/PD studies demonstrated favourable outcomes with cefiderocol against metallo-beta-lactamase-producers with MICs <16 mg/L [Citation16,Citation17]. In the CREDIBLE-CR trial, there were 23 patients infected with metallo-beta-lactamase-producing pathogens (including the non-fermenters) and the clinical cure rates were higher with cefiderocol as compared to the best available therapy (75% vs. 29%, respectively) [Citation17]. A recent analysis of the outcomes of the patients with metallo-beta-lactamase producing CRK infections from the APEKS-NP and CREDIBLE-CR trials demonstrated numerically higher clinical cure for NDM infections treated with cefiderocol [Citation18]. It is important to note, however, that the greatest cefiderocol MIC was 4 mg/L for the isolates randomised to the cefiderocol arm, and the majority of the patients with elevated cefiderocol MICs had an APACHE score of <15. The numbers are too small to draw strong conclusions and more clinical outcome data are required to assess the effectiveness of cefiderocol for metallo-beta-lactamase producing CRK infections, particularly in patients with higher disease severity. However, it is important to note that breakpoint variability may have significant implications on patient outcomes. If CLSI breakpoints are more clinically relevant, using the more conservative EUCAST criteria would limit the use of a potent active treatment option, whereas if it is the other way around, using cefiderocol might result in treatment failure.
The major limitation of this study is that disc diffusion results were not compared to BMD. Previous studies demonstrated good correlation between disc diffusion and BMD, with 90% categorical agreement and no very major errors [Citation5]. There may be a resistance overcall by disc diffusion as major error rate was reported as 14% in a previous study comparing disc diffusion and BMD [Citation5]. However, our results were in line with the previous studies, where high susceptibility rates were observed against OXA-48-like producers using CLSI criteria. Another limitation of this study is that genomic mechanisms behind cefiderocol resistance were not elucidated.
In conclusion, this, to our knowledge is the largest collection of CRK BSI isolates tested against cefiderocol. Cefiderocol resistance rate varied widely, particularly for metallo-beta-lactamase producers, depending on the interpretive criteria used. The majority of the metallo-beta-lactamase producers were categorised as cefiderocol resistant using the more conservative EUCAST criteria. More clinical outcome data are required to ascertain which breakpoints are more clinically relevant. Until such data are available, we advise using the more cautious approach for this group of infections.
Author contributions
Conceptualisation, B.I., S.K., O.D., O.E., F.C., P.H., D.P.; Methodology, B.I., B.O., C.V., C.F., B.F., O.D., F.C.; Software, V.B. and M.G.; Formal Analysis, B.I. C.F., B.F.; Investigation and data curation, B.I., B.O., C.V., G.C., A.T.A, F.S., N.T., H.D., S.M., H.A., I.I.B., M.A., E.T.T., S.K.D., M.K., S.K., O.D., C.A., S.Y., G.H., N.S., A.A., O.A., M. A.; Resources, B.I., P.H., O.E., D.P. F.C.; Data Curation, B.I.; Writing – Original Draft Preparation, B.I., Writing – Review & Editing, B.I., B.O., C.V., A.S., G.C., A.T.A, C.F., B.F., P.S., F.S., N.T., H.D., S.M., H.A., I.I.B., M.A., E.T.T., S.K.D., M.K., S.K., O.D., C.A., S.Y., G.H., N.S., A.A., O.A., M.A., P.H., O.E., D.P., F.C.; Supervision, P.H., O.E., D.P., F.C.; Project Administration, B.I., B.O., C.V., O.D., F.C., O.E.; Funding Acquisition, B.I., D.P.
Institutional Review Board statement
The study was approved by the Koc University Institutional Review Board (approval number: 2018.151.IRB1.018).
Supplemental Material
Download MS Word (14.6 KB)Disclosure statement
Dr. Isler received honoraria from Pfizer. Dr. Paterson reports research grants from Merck, Pfizer and Shionogi. David Paterson has received honoraria for advisory board membership from Merck, Pfizer, Shionogi, GSK, QPex, Entasis, VenatoRx, BioMerieux and Accelerate. Dr Harris has received research grants from Sandoz, Merck/MSD and Shionogi, speaker’s fees from Pfizer and honoraria for advisory board membership from Merck/MSD, OpGen and Sandoz, paid to the University of Queensland. All others have no conflict of interest to declare.
Data availability statement
The genomic dataset generated during this study is available in the public database [i.e. US National Library of Medicine (NLM) National Centre for Biotechnology Information (NCBI), PRJNA789336].
Additional information
Funding
References
- Isler B, Aslan AT, Akova M, et al. Treatment strategies for OXA-48-like and NDM producing Klebsiella pneumoniae infections. Expert Rev Anti Infect Ther. 2022;20(11):1389–1400. doi:10.1080/14787210.2022.2128764.
- Kazmierczak KM, Tsuji M, Wise MG, et al. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 study). Int J Antimicrob Agents. 2019;53(2):177–184. doi:10.1016/j.ijantimicag.2018.10.007.
- Kohira N, Hackel MA, Ishioka Y, et al. Reduced susceptibility mechanism to cefiderocol, a siderophore cephalosporin, among clinical isolates from a global surveillance programme (SIDERO-WT-2014). J Glob Antimicrob Resist. 2020;22:738–741. doi:10.1016/j.jgar.2020.07.009.
- Bassetti M, Echols R, Matsunaga Y, et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis. 2021;21(2):226–240. doi:10.1016/S1473-3099(20)30796-9.
- Morris CP, Bergman Y, Tekle T, et al. Cefiderocol antimicrobial susceptibility testing against Multidrug-Resistant Gram-Negative bacilli: a comparison of disk diffusion to broth microdilution. J Clin Microbiol. 2020;59(1):e01649-20. doi:10.1128/JCM.01649-20.
- Matuschek E, Longshaw C, Takemura M, et al. Cefiderocol: EUCAST criteria for disc diffusion and broth microdilution for antimicrobial susceptibility testing. J Antimicrob Chemother. 2022;77(6):1662–1669. doi:10.1093/jac/dkac080.
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters, version 11.0. 2021. [accessed 2022 Feb 10]. Available from: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf
- Clinical and laboratory standards institute (CLSI). M100-ED31. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed. Table 2A. Zone Diameter and MIC Breakpoints for Enterobacterales. 2021.
- Isler B, Özer B, Çınar G, et al. Characteristics and outcomes of carbapenemase harbouring carbapenem-resistant Klebsiella spp. bloodstream infections: a multicentre prospective cohort study in an OXA-48 endemic setting. Eur J Clin Microbiol Infect Dis. 2022;41(5):841–847. doi:10.1007/s10096-022-04425-4.
- Feldgarden M, Brover V, Haft DH, et al. Validating the AMRFinder tool and resistance gene database by using antimicrobial resistance Genotype-Phenotype correlations in a collection of isolates. Antimicrob Agents Chemother. 2019;63(11):e00483-19. doi:10.1128/AAC.00483-19.
- Simner PJ, Mostafa HH, Bergman Y, et al. Progressive development of cefiderocol resistance in Escherichia coli during therapy is associated with increased blaNDM-5 copy number and gene expression. Clin Infect Dis. 2021;75:47–54.
- Ito A, Sato T, Ota M, et al. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-Negative bacteria. Antimicrob Agents Chemother. 2018;62(1):e01454-17. doi:10.1128/AAC.01454-17.
- Yamano Y, Takemura M, Nakamura R, et al. Frequency of resistance acquisition and resistance mechanisms to cefiderocol. Presented at: ID Week Annual Meeting; 2020 October 22–25; Online conference; Poster 1455.
- Kawai A, McElheny CL, Iovleva A, et al. Structural basis of reduced susceptibility to Ceftazidime-Avibactam and cefiderocol in Enterobacter cloacae due to AmpC R2 loop deletion. Antimicrob Agents Chemother. 2020;64(7):e00198-20. doi:10.1128/AAC.00198-20.
- Shields RK, Iovleva A, Kline EG, et al. Clinical evolution of AmpC-Mediated Ceftazidime-Avibactam and cefiderocol resistance in Enterobacter cloacae complex following exposure to cefepime. Clin Infect Dis. 2020;71(10):2713–2716. doi:10.1093/cid/ciaa355.
- Katsube T, Echols R, Wajima T. Pharmacokinetic and pharmacodynamic profiles of cefiderocol, a novel siderophore cephalosporin. Clin Infect Dis. 2019;69(7):S552–S558. doi:10.1093/cid/ciz828.
- Tan X, Kim HS, Baugh K, et al. Therapeutic options for metallo-β-Lactamase-Producing enterobacterales. Infect Drug Resist. 2021;14:125–142. doi:10.2147/IDR.S246174.
- Timsit JF, Paul M, Shields RK, et al. Cefiderocol for the treatment of infections due to Metallo-Beta-Lactamase-Producing pathogens in the CREDIBLE-CR and APEKS-NP phase 3 randomized studies. Clin Infect Dis. 2022;75:1081–1084.

