Abstract
Background
Pregnant women have an increased risk of developing active tuberculosis (TB). The Public Health Agency of Sweden recommends screening of active TB and latent tuberculosis infection (LTBI) among pregnant women from countries with high TB incidence at Maternal Health Care (MHC) clinics. In Östergötland County, Sweden, a screening program has been active since 2013. The aim of this study was to evaluate this screening program and the cascade of care for LTBI among pregnant women in Östergötland county.
Methods
Data were obtained from pregnant women screened for TB at MHC clinics and subsequently referred to the pulmonary medicine clinic or the clinic of infectious diseases in Östergötland County between 2013 and 2018. The Public Health Agency of Sweden’s national database for active TB was used to analyse if any women developed active TB up to two years after the screening process.
Results
A total of 439 women were included. Nine cases of active TB were discovered during the screening process and two developed active TB afterward. 177 women were recommended LTBI treatment and variables significantly associated with a decreased likelihood of being recommended treatment were increasing age, time in Sweden, and parity. 137 women received and 112 (82%) completed treatment. 14 women discontinued treatment due to adverse effects.
Conclusion
Screening of pregnant women from countries with high TB incidence at MHC clinics led to the discovery of several cases of active TB. The completion rate of LTBI treatment was high and few discontinued due to adverse effects.
Introduction
Tuberculosis (TB) is a bacterial infection caused by Mycobacterium tuberculosis. It is estimated that about a quarter of the world’s population is infected, equivalent to approximately 2 billion people [Citation1]. The majority do not develop active disease since their immune system is able to control the mycobacteria. This condition is called latent tuberculosis infection (LTBI). LTBI is defined as a state of continuous immune response stimulated by Mycobacterium tuberculosis antigen with no proof of clinically manifest active TB. [Citation2] Although the lifetime risk of developing active TB disease is small the highest risk of developing TB is during the first two years after exposure [Citation3,Citation4].
Tuberculosis during pregnancy is often associated with atypical symptoms and increased cases of extrapulmonary tuberculosis compared to non-pregnant patients. The symptoms might be mistaken for pregnancy-related symptoms, and delay in diagnosis could be a reason for increased cases of active TB during the postpartum period. [Citation5–7] Active TB during pregnancy and delayed diagnosis is a serious risk for both the mother and the child [Citation8]. To reduce the risk, systematic screening and early diagnosis are key [Citation9,Citation10].
Earlier studies looking at risk increase for women with LTBI during pregnancy and postpartum to develop active TB have not been conclusive, where some have been able to show an increased risk for active TB and some have not. However, a recent study made in Sweden showed a significant increase in risk both during pregnancy and postpartum in women from countries with high incidences of TB [Citation11].
Pregnant women from countries with high TB incidence who seek Maternal Health Care (MHC) clinics are an opportunity to screen individuals that have not been screened previously and the Public Health Agency of Sweden recommends screening for TB in this group [Citation12]. In Östergötland county such a screening program has been active since 2013. The screening criteria were pregnant women from high-endemic countries (>100 cases/100,000/year). Pregnant women with LTBI who are recently exposed to TB or have a compromised immune system are recommended to start prophylactic LTBI-treatment during the pregnancy. Otherwise, an individual assessment is done as soon as possible after childbirth [Citation12]. Factors that could influence the decision to treat are, e.g. age, parity, recent TB exposure, previous treatment for latent or active TB and time in Sweden. It is known that age over 35 years increases the risk for hepatotoxicity [Citation13] and that a recent exposure increases the risk of developing active TB [Citation14]. How the number of previous pregnancies influences the risk of developing active TB is less known. Previous studies indicate that parity does not play a role [Citation15,Citation16]. LTBI treatment options are daily isoniazid for 6 or 9 months, rifampicin for 4 months or rifampicin plus isoniazid for 3 months [Citation2]. Both rifampicin and isoniazid are safe to use during pregnancy [Citation17,Citation18]. The risk of drug-related adverse events should be balanced against the potential benefits of treatment [Citation2].
The aim of this study was to evaluate the cascade of care for LTBI among pregnant and postpartal women in Östergötland County after the start of the screening program. The evaluation included factors stated by the physicians for recommending or abstaining from LTBI treatment in postpartal women, which treatments were offered, the completion rate of those offered LTBI treatment and reasons for not completing treatment.
Materials and methods
This was a retrospective register and patient record study. The study population consists of pregnant women screened for TB at Maternal Health Care (MHC) clinics and subsequently referred to the pulmonary medicine clinic or the clinic of infectious diseases in Östergötland county between 2013 and 2018. The clinic for infectious diseases in Östergötland is present both at Linköping University Hospital and the county hospital in Norrköping whereas the clinic for pulmonary medicine is only present at Linköping University Hospital.
Pregnant women with no previous history of treatment for TB or LTBI who came from countries with high risk for TB (>100 cases per 100,000 population) and visited an MHC clinic were screened with QuantiFERON-TB Gold Plus (Qiagen inc.), an interferon-gamma releasing assay (IGRA). Health care workers at MHC clinics were informed about the screening program and had written routines regarding who was eligible for screening. If the women tested positive (⩾0.35 IU/ml) they were referred to the pulmonary medicine clinic or the clinic of infectious diseases in Östergötland county. The MHC centers in the central and western parts of Östergötland could send their referrals to either of the two clinics in Linköping whereas the MHC-centers in the eastern part of the county had to send their referrals to the Norrköping division of the infectious diseases clinic. During the visit at the MHC clinic information regarding country of birth, travel abroad, occupation, IGRA result and TST (Tuberculin Skin Test) results (if available), BCG-vaccination, former TB, immune suppression, estimated date of delivery, symptoms and the number of people living in the same household (including children) were registered and included in the referral form.
At the clinic the women met a TB nurse and were provided with information regarding LTBI and their symptoms were re-evaluated. A chest radiograph was performed during the third trimester. If they lacked symptoms of active TB and the chest radiograph was normal, the woman had a doctor’s appointment within four weeks after delivery. At the appointment, the physician decided whether to offer LTBI treatment or not. The treatment options for LTBI were monotherapy with isoniazid for six or nine months (INH6/9) or rifampicin for four months (RIF4).
After treatment for LTBI was initiated, the women visited the clinic for blood tests and follow-up every two weeks during the first month of treatment, and subsequently every four weeks.
In cases where active TB was suspected, a chest radiograph was performed followed shortly by an appointment with a physician. When pulmonary TB was suspected sputum samples were obtained for smear microscopy, PCR, and mycobacterial culture. If extrapulmonary TB was considered, samples were taken depending on the suspected site of infection. If active TB was confirmed the woman was offered standard TB treatment.
Data collection
Data was collected from the journal data system Cambio Cosmic, the laboratory databases of clinical microbiology in Östergötland (IGRA results) and The Public Health Agency of Sweden’s national database for active TB.
Referrals that were duplicates, rejected, no shows or already ongoing cases were excluded. Variables registered were age, gender, time of stay in Sweden, country of origin, former BCG vaccination, immunosuppression, IGRA results, TST result (if performed), hiv and hepatitis B status, chest radiograph results, symptoms, sputum culture, parity and if active TB was discovered. Furthermore, the proportion of pregnant women who were offered treatment for latent TB was noted, what treatment they were offered and to what extent they completed the treatment. In cases of discontinued treatment, the reason was registered, if specified. Time between the referral, first visit, and start of treatment for latent tuberculosis (LTBI) was also noted.
The Public Health Agency of Sweden’s national database for active TB was used to analyse if screened women who were referred to the pulmonary medicine clinic or clinic of infectious diseases developed active TB up to two years after completing the screening program. The Swedish communicable diseases act states that all cases of active TB in Sweden must be reported to the Public Health Agency of Sweden.
Statistical analysis
Descriptive data are presented as mean ± standard deviation (SD), median with range or as absolute numbers with percentage (%) depending on the type of variable. To assess differences among variables, independent samples t-test was used for normally distributed continuous variables, Mann-Whitney U for non-normally distributed continuous variables, and univariate logistic regression for categorical variables. Multiple logistic regression was performed to evaluate the association between significant variables. Chi-squared test was performed to compare treatment completion between the different treatment regimes. Results were considered statistically significant in cases of p-value <0.05. The statistical analyses were made using IBM SPSS Statistics version 28 (IBM Corp, Armonk, NY).
Results
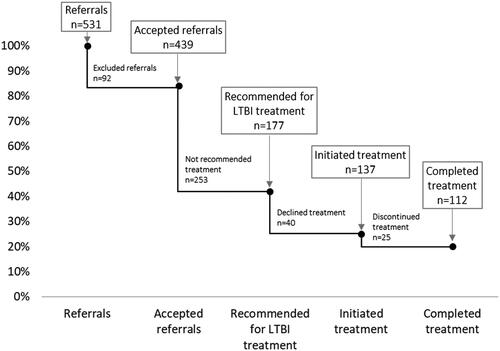
A total of 531 referrals were sent from MHC clinics in Östergötland county to the pulmonary medicine clinic or the clinic of infectious diseases during 2013–2018. Of the 531 referrals, 92 were excluded from the study of which 44 were duplicates, 16 were no-shows, 16 were written consultation responses, 11 were rejected and 5 already ongoing cases. In total 439 women were included in the study (). Altogether, nine cases of active TB were found, 177 women were recommended treatment for LTBI while 253 were not recommended. In the end, 137 initiated treatment for LTBI, and 112 completed treatment ().
Figure 1. Screening algorithm for pregnant women from countries with high TB incidence in Östergötland county presented in a flow chart. Showing excluded referrals sent from Maternal Health Care clinics to the pulmonary medicine clinic or clinic of infectious diseases, 2013-2015, as well as discovered cases of active TB and outcome of treatment decision.
TB: tuberculosis; MHC: maternal health care; IGRA: interferon gamma releasing assay; CXR: chest radiograph.
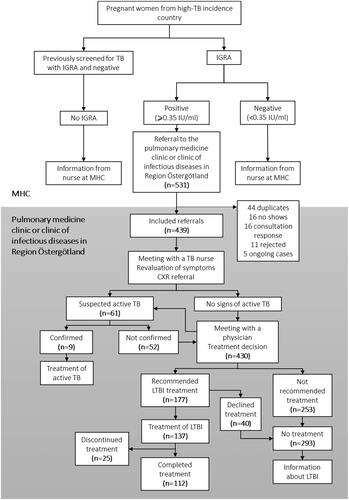
Figure 2. Cascade of Care among pregnant women screened for tuberculosis at Maternal Health Care clinics and subsequently referred to the pulmonary medicine clinic or clinic of infectious diseases in Östergötland county between 2013 and 2018.
TB: tuberculosis; LTBI: latent tuberculosis infection
The median age among the women was 29 (range 15–46) years and median time in Sweden was 42 (range 1–348) months. Median parity was 2 (range 0–12) and 135 (30.8%) women were pregnant for the first time. 409 (93.2%) were from countries with high risk for TB, 23 (5.2%) from intermediate risk countries (40–99 cases per 100,000 population), and seven (1.7%) from low-risk countries (<40 cases per 100,000 population). Most of the women, 337 (76.8%), originated from Africa. The second largest group, 93 (21.2%), came from Asia and the remaining nine women originated from Europe or the Americas. The screening routine at the MHC-clinics stated that only women from high-incidence countries should be screened. Risk factors noted among the participants included 17 women with Hepatitis B, one with HIV and one with insulin treated diabetes (). Hepatitis B is not a risk factor for developing active TB but may increase the risk of liver related adverse events during LTBI-treatment and was therefore included as a parameter.
Table 1. Demographics and clinical characteristics of women screened for tuberculosis at Maternal Health Care clinics and subsequently referred to the pulmonary medicine clinic or clinic of infectious diseases in Östergötland County between 2013 and 2018.
To evaluate determining factors important for recommending treatment of LTBI in recently pregnant women, univariate analyzes were made to assess differences among numerical variables between the group that was recommended LTBI treatment (n = 177) and the group that was not (n = 253). The mean age was significantly different between the groups (p<.001), with a median age of 28 years in the group recommended for LTBI treatment and 31 years in the group not recommended for treatment. Parity was also significantly different (a median of two children compared to three, p<.001) as well as time in Sweden (median 24 versus 55 months, p<.001, ). No women were started on LTBI-treatment prior to delivery.
Table 2. Multiple binary logistic regression between group recommended for LTBI treatment and not recommended.
To assess differences among the categorical variables regarding country of origin (with high, intermediate, or low TB incidence), CXR (not done, normal or with abnormal findings), symptoms (positive or negative), and performed sputum sample (not done or negative) between the group of women recommended for LTBI treatment and the group not recommended, univariate logistic regression was performed. No difference among country of origin was noted between the groups (Odds Ratio (OR) 1.12; 95% Confidence interval (CI) 0.62–2.00; p = 0.72), nor was there any difference regarding CXR results (OR 1.23; 95% CI 0.72–2.11; p = 0.45), symptoms (OR 0.82; 95% CI 0.38–1.76; p = 0.60) or performed sputum sample (OR 1.57; 95% CI 0.88–2.80; p = 0.12).
The significant variables age, parity and time in Sweden were subsequently included in a multiple logistic regression. The regression was made to calculate adjusted OR and to ascertain the effects of age, parity and time in Sweden on the likelihood that patients were recommended LTBI treatment. The variables were all still significant and the regression showed a decreasing probability of being recommended treatment with increasing age, time in Sweden and parity. The multiple logistic regression analysis correctly classified 68.5% of the cases and explained 18.8% (Nagelkerke R2) of the variance in recommendation for LTBI treatment (). No significant difference could be found regarding age (p = 0.29), parity (p = 0.55) or time in Sweden (p = 0.88) between the group that discontinued LTBI treatment (n = 25) and the group that completed treatment (n = 112).
Among the women not treated for LTBI (n = 293), 55 (19%) were not treated due to several years of residence in Sweden, 40 (14%) women declined treatment, 39 (13%) were not recommended treatment due to previous treatment for active TB and 29 (10%) due to previous treatment for LTBI ().
Table 3. Reasons for not being treated for latent tuberculosis, in descending order, among women screened for tuberculosis at Maternal Health Care clinics and subsequently referred to the pulmonary medicine clinic or the clinic of infectious diseases in Östergötland County between 2013 and 2018.
Out of the 137 women treated for LTBI, 112 (82%) completed treatment and 25 (18%) discontinued treatment. Reasons for discontinued treatment were in seven (5.1%) cases due to liver-related adverse effects, seven (5.1%) due to other adverse effects, six (4.4%) due to a new pregnancy, three (2.2%) due to loss to follow-up, one (0.7%) due to relocation and one (0.7%) due to investigation for active TB (). Out of 17 women with hepatitis B only four were recommended treatment out of which two declined, one completed and one discontinued treatment due to adverse events (other than hepatotoxicity).
Table 4. Treatment regimens and outcome among women screened for tuberculosis at Maternal Health Care clinics and later treated for latent tuberculosis at the pulmonary medicine clinic or clinic of infectious diseases in Östergötland county between 2013 and 2018.
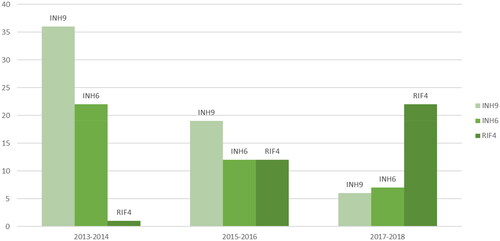
The treatments given were INH9 (n = 61, of which 11 discontinued), INH6 (n = 41, of which 11 discontinued) and RIF4 (n = 35, of which three discontinued) ( and ). No association was found between completion rate and treatment regime (p = 0.12). The pulmonary medicine clinic treated 39 women with INH9, seven with INH6 and 13 with RIF4. The clinic of infectious diseases treated 22 women with INH9, 34 with INH6 and 22 with RIF4.
Figure 3. Treatment regimens among pregnant women treated for latent tuberculosis at the pulmonary medicine clinic or clinic of infectious diseases in Östergötland County between 2013 and 2018.
INH9: isoniazid nine months; INH6: isoniazid six months; RIF4: rifampicin four months.
Nine cases of active TB were found during the screening process which was 5% of all cases of active TB in Östergötland county during the studied period (2013–2018) (15). All patients with active TB in our study originated from countries with a high incidence of TB. Among the nine cases with active TB, time in Sweden ranged from seven months to eight years. Three of the women were diagnosed with pulmonary TB and six with lymph node TB. See for further details.
Table 5. Details of cases with active tuberculosis (TB) among pregnant women detected in Östergötland county between 2013 and 2018.
A search in the Public Health Agency of Sweden’s national database for active TB showed that two women developed active TB after finishing the screening cascade of care. They had not been offered treatment for LTBI. Both had changes on CXR and were therefore subject to follow-up with CXR until normalisation. One of the two also left repeated negative sputum samples.
Discussion
The aim of this study was to evaluate the cascade of care for pregnant women in Östergötland county during the first six years after its introduction (2013–2018). Nine cases of active TB were discovered through the screening process and two women developed active TB afterwards. 177 women were recommended for LTBI treatment, 137 women received treatment and 112 (82%) completed treatment. Further, only three out of 137 treated patients were lost to follow up.
There are few studies examining the screening process of pregnant women [Citation15,Citation19]. One of the existing few evaluated the TB screening program for pregnant women in Stockholm, Sweden, and discovered several women with active TB and previously unknown LTBI and concluded that the screening program was justified to perform [Citation15]. In our study, we see similar results although we found an even higher proportion of active TB based on the number of examined women.
During the study period, a total of 180 cases of active TB were discovered in Östergötland county, which means that the nine cases of detected active TB during the screening accounted for 5% of the total number of cases in the region during the period studied. Our study was not designed to investigate if the screening program reduces the risk of active TB in pregnant women from high endemic areas, but our data suggests that the screening was effective in identifying active cases in a risk group although further studies are needed to properly address this question. The cost-effectiveness of the screening program at MHC clinics in low-endemic countries has not been evaluated previously and it was not the scope of this study. However, when evaluating screening programs for migrants it has been demonstrated to be cost-effective when screening individuals from high TB burden countries [Citation20–23]. The pregnant women who are subject to screening for LTBI come from areas with high TB incidence. Furthermore, pregnant women have an extra risk of developing active TB [Citation5,Citation11,Citation24]. Considering this and that 5% of the cases with active TB in Östergötland county were pregnant women during the studied period, indicates that it could be cost-effective to screen this group of women, but further studies are needed.
It is important to remember that finding and preventing active TB among pregnant women is of high value not only because of the increased risk for the mother, but also for the foetus or infant [Citation8,Citation25]. Pregnancy and appointments to MHC clinics represent an occasion for immigrant women to access the health care system, which is important since this group often has limited health-seeking behaviour, this makes pregnancy an opportunity to screen for LTBI [Citation26]. Regardless of if LTBI treatment is offered or not, information is given about the symptoms of active TB and to seek healthcare if necessary [Citation12].
Up until 2015 the MHC-clinics in the central and eastern part of Östergötland county could choose whether to send their referral to the department of pulmonary medicine or the department for infectious diseases. For some reason, unknown to us, more referrals were sent to the department for pulmonary medicine. In the eastern region of Östergötland county, it was only possible to send the referrals to the department of infectious diseases since there was no department of pulmonary medicine at that hospital. We found no difference between the two departments regarding how many women were recommended for treatment.
Out of the 439 referred women, two individuals developed active TB within two years after being screened and subsequently referred to the pulmonary medicine clinic or the clinic of infectious diseases in Östergötland County. Neither had been offered treatment for LTBI during screening. Both women had abnormalities on CXR discovered during the screening process and were followed until normalisation of the CXR. One of the women also left repeated negative sputum samples but developed active TB after follow-up ended. We consider two out of 439 (0.5%) women as few. When analysing these cases, we cannot be completely sure of the connection to the pregnancy, or a possible re-exposure and we cannot be sure that there were other reasons for not giving LTBI treatment to the two women that were not mentioned in the patient records.
Pregnant women have an increased risk of developing active TB up to six months postpartum [Citation5,Citation11,Citation16,Citation24], however, we chose to control for active TB up to two years after the screening. The reason for this was to not miss any cases and because the risk of falling ill with active TB is highest within two years of exposure [Citation14,Citation27]. Interestingly, both women who developed TB after completing the screening program did so after more than six months post-partum.
In our study, significant determining factors for recommending treatment of LTBI in pregnant women were age, time in Sweden and parity. Accordingly, our study showed a decreasing likelihood of being recommended treatment with increasing age, time in Sweden and parity. Age is an important factor to consider since the risk for treatment-related adverse effects such as hepatotoxicity increases with age and thus the indication for treatment decreases with age over 35 years [Citation12,Citation13]. In addition, most cases of active TB also occur in the age group 15–39 years [Citation28].
When reasoning whether to treat LTBI or not, time in Sweden is usually a factor that physicians consider since the risk of exposure in Sweden is very small. As previously stated, the highest risk of falling ill is within two years of infection [Citation14,Citation27]. In a study examining factors that predict physicians’ decision not to treat LTBI, immigration over two years ago was such a factor [Citation29]. However, when deciding if to treat LTBI in pregnant women, the question is whether to place equivalent importance on time spent in Sweden since there is no data to support that the risk of reactivation decreases with the number of previous pregnancies [Citation15,Citation16]. Thus, time without re-exposures is most likely not important in pregnant women with LTBI regarding their risk of reactivation. In our study, the cases with active TB had a range of time spent in Sweden between seven months and eight years. However, the number of cases with active TB were too few to do statistical analyzes to corroborate previous data regarding the risk of developing TB and time spent in Sweden in pregnant women. Also, we do not know why time spent in Sweden was stated as a risk factor in the journal records since there was seldom a thorough explanation following the statement.
Another significant factor in our study was parity. Women with higher parity were not offered LTBI treatment as often as women with a lower parity. One reason for this could be that increased parity might be associated with older age. However, parity was still significant in the multiple linear regression with adjusted OR. When looking at the cases of active TB in our study, the parity ranged from one to seven. In the study by Fröberg and co-workers [Citation15], parity among cases with active TB was between one and three, and five out of nine had their first child. Considering the few cases with active TB discovered during the screening process in our study it was not possible to do a statistical analysis concerning parity and the risk of developing active TB. However, the women with active TB displayed a wide spread of parity and earlier studies have shown no correlation between parity and the risk of developing active TB [Citation16]. Treatment decisions based on this factor can therefore be considered questionable.
When deciding whether to offer treatment for LTBI or not, it is an assessment based on the individual’s risk of developing TB, estimated adherence to treatment, and the risk of drug-related adverse events. The most reported reasons for not recommending treatment for LTBI were several years of residence in Sweden or declining treatment. Furthermore, few women with hepatitis B were recommended LTBI treatment. Nonetheless, the clinical assessment is always a balance between several factors which is not always described in detail in the medical record and thus can the exact reason for not treating a specific patient be difficult to comprehend.
In a previous systematic review analysing migrant screening in Europe, it was estimated that only 54% of migrants with diagnosed LTBI completed treatment after screening [Citation30]. Another systematic review and meta-analysis showed that migrants who initiated and completed treatment for LTBI were only 52% in cases where data from the entire treatment cascade were included [Citation31]. In our study, the completion rate was 82% which in comparison to the previous studies is high. Of the 25 women who discontinued treatment, 14 did this because of adverse events seven of which were liver-associated (). Although when compared to the study by Fröberg et al. [Citation15], who investigated LTBI treatment among pregnant women in Stockholm, Sweden, our completion rate was considerably lower than theirs (94%). One reason for this could be that most women in their study were treated with RIF (90%), while majority in our study was treated with INH. In the group treated with RIF in our study, treatment completion was considerably higher compared to INH ().
The majority of the women in this study were treated with isoniazid for six or nine months. Today the recommended LTBI treatment regimens in Sweden are shorter and rifamycin based, even though isoniazid for six or nine months still are alternatives [Citation2,Citation32]. In addition to being shorter the rifamycin-containing regimens are non-inferior in preventing active TB disease, well tolerated with potentially less adverse events and have a higher rate of treatment completion compared to isoniazid monotherapy [Citation32,Citation33]. Even though the majority were treated with isoniazid, treatment with rifampicin for four months increased during the study period while isoniazid decreased ().
We found no association between treatment completion rate and treatment regime. The most common cause for non-completion was side effects (n = 14/25). Interestingly the second most common cause for non-completion was a new pregnancy (n = 6) even if there is no risk to continue the treatment when pregnant. When we investigated those cases, we found that four of the women had interrupted the treatment on their own initiative, one had had liver side effects before treatment why she discontinued during her new pregnancy and restarted shortly after delivery and in the last case, it was unclear why she stopped her treatment.
A strength of the study was the relatively large cohort of 439 women which allowed for the investigation of indications for treatment. Another strength was the low loss to follow-up, only three out of 137 treated women. A limitation is that a larger number of cases with active TB would be necessary to study the correlation between different patient factors and the risk of developing active TB. Further, to be able to study adverse events correlated to LTBI treatment, the treatment groups would need to be bigger.
Conclusion
The described cascade of care for screening pregnant women from countries with high TB incidence at MHC clinics seems to be a successful and useful tool for assessing pregnant women with LTBI and led to the discovery of several cases of active TB. The study showed a decreasing likelihood of being recommended treatment for LTBI with increasing age, time in Sweden and parity. In our cohort, few women discontinued offered treatment or were lost to follow up. Further studies are needed to be certain the screening program has led to fewer cases of active TB.
Ethical approval
All collected data were anonymized and presented in aggregated form. Ethical approval was granted from Linköping Regional Ethics committee (approval number 2019–0095).
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- WHO. Global Tuberculosis Report 2021. Geneva: WHO; 2021. Available from: https://www.who.int/publications/i/item/9789240037021.
- WHO. Latent tuberculosis infection updated and consolidated guidelines for programmatic management. Geneva (Switzerland): World Health Organization; 2018. English.
- Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the national tuberculosis controllers association and CDC. MMWR Recomm Rep. 2005; 54(Rr-15):1–47.
- Horsburgh CR, Jr., Rubin EJ. Clinical practice. Latent tuberculosis infection in the United States. N Engl J Med. 2011;364(15):1441–1448. doi: 10.1056/NEJMcp1005750.
- Zenner D, Kruijshaar ME, Andrews N, et al. Risk of tuberculosis in pregnancy: a national, primary care-based cohort and self-controlled case series study. Am J Respir Crit Care Med. 2012;185(7):779–784. doi: 10.1164/rccm.201106-1083OC.
- Kothari A, Mahadevan N, Girling J. Tuberculosis and pregnancy–results of a study in a high prevalence area in London. Eur J Obstet Gynecol Reprod Biol. 2006; 126(1):48–55. doi: 10.1016/j.ejogrb.2005.07.025.
- Knight M, Kurinczuk JJ, Nelson-Piercy C, et al. Tuberculosis in pregnancy in the UK. BJOG. 2009;116(4):584–588.rdoi: 10.1111/j.1471-0528.2008.02097.x.
- Jana N, Barik S, Arora N, et al. Tuberculosis in pregnancy: the challenges for South Asian countries. J Obstet Gynaecol Res. 2012;38(9):1125–1136. Sepdoi: 10.1111/j.1447-0756.2012.01856.x.
- Doveren RF, Block R. Tuberculosis and pregnancy–a provincial study (1990–1996). Neth J Med. 1998;52(3):100–106. doi: 10.1016/s0300-2977(98)00004-7.
- Carter EJ, Mates S. Tuberculosis during pregnancy. The Rhode Island experience, 1987 to 1991. Chest. 1994; 106(5):1466–1470. doi: 10.1378/chest.106.5.1466.
- Jonsson J, Kuhlmann-Berenzon S, Berggren I, et al. Increased risk of active tuberculosis during pregnancy and postpartum: a register-based cohort study in Sweden. Eur Respir J. 2020;55(3):1901886. doi: 10.1183/13993003.01886-2019.
- Folkhälsomyndigheten. Rekommendationer för preventiva insatser mot tuberkulos – Hälsokontroll, smittspårning och vaccination. Stockholm: Folkhälsomyndigheten; 2022. Available from: https://www.folkhalsomyndigheten.se/publikationer-och-material/publikationsarkiv/r/rekommendationer-for-preventiva-insatser-mot-tuberkulos-halsokontroll-smittsparning-och-vaccination/.
- Kunst H, Khan KS. Age-related risk of hepatotoxicity in the treatment of latent tuberculosis infection: a systematic review. Int J Tuberc Lung Dis. 2010; 14(11):1374–1381.
- Sloot R, Schim van der Loeff MF, Kouw PM, et al. Risk of tuberculosis after recent exposure. A 10-year follow-up study of contacts in Amsterdam. Am J Respir Crit Care Med. 2014;190(9):1044–1052. doi: 10.1164/rccm.201406-1159OC.
- Fröberg G, Jansson L, Nyberg K, et al. Screening and treatment of tuberculosis among pregnant women in Stockholm, Sweden, 2016–2017. Eur Respir J. 2020;55(3):1900851. doi: 10.1183/13993003.00851-2019.
- Espinal MA, Reingold AL, Lavandera M. Effect of pregnancy on the risk of developing active tuberculosis. J Infect Dis. 1996; 173(2):488–491. doi: 10.1093/infdis/173.2.488.
- Kucers’ The Use of Antibiotics. In Grayson ML, editor. A clinical review of antibacterial, antifungal, antiparasitic, and antiviral drugs. 6 ed. Vol. 1. Boca Raton, FL: CRC Press 2010.
- Nahid P, Dorman SE, Alipanah N, et al. Official American thoracic society/centers for disease control and prevention/infectious diseases society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147–e195. doi: 10.1093/cid/ciw376.
- Bullarbo M, Barnisin M, Vukas Radulovic N, et al. Low prevalence of active tuberculosis among high-risk pregnant and postpartum women in Sweden: a retrospective epidemiological cohort study using and evaluating TST as screening method. Infect Dis Obstet Gynecol. 2018;2018:3153250. doi: 10.1155/2018/3153250.
- Pareek M, Watson JP, Ormerod LP, et al. Screening of immigrants in the UK for imported latent tuberculosis: a multicentre cohort study and cost-effectiveness analysis. Lancet Infect Dis. 2011;11(6):435–444. doi: 10.1016/S1473-3099(11)70069-X.
- Schwartzman K, Menzies D. Tuberculosis screening of immigrants to low-prevalence countries. A cost-effectiveness analysis. Am J Respir Crit Care Med. 2000; 161(3 Pt 1):780–789. doi: 10.1164/ajrccm.161.3.9902005.
- Shedrawy J, Deogan C, Öhd JN, et al. Cost-effectiveness of the latent tuberculosis screening program for migrants in Stockholm region. Eur J Health Econ. 2021;22(3):445–454. doi: 10.1007/s10198-021-01265-5.
- Zammarchi L, Casadei G, Strohmeyer M, et al. A scoping review of cost-effectiveness of screening and treatment for latent tubercolosis infection in migrants from high-incidence countries. BMC Health Serv Res. 2015;15:412. doi: 10.1186/s12913-015-1045-3.
- Rendell NL, Batjargal N, Jadambaa N, et al. Risk of tuberculosis during pregnancy in Mongolia, a high incidence setting with low HIV prevalence. Int J Tuberc Lung Dis. 2016;20(12):1615–1620. doi: 10.5588/ijtld.16.0314.
- Sobhy S, Babiker Z, Zamora J, et al. Maternal and perinatal mortality and morbidity associated with tuberculosis during pregnancy and the postpartum period: a systematic review and meta-analysis. BJOG. 2017;124(5):727–733. doi: 10.1111/1471-0528.14408.
- Malhamé I, Cormier M, Sugarman J, et al. Latent tuberculosis in pregnancy: a systematic review. PLOS One. 2016;11(5):e0154825. doi: 10.1371/journal.pone.0154825.
- Borgdorff MW, Sebek M, Geskus RB, et al. The incubation period distribution of tuberculosis estimated with a molecular epidemiological approach. Int J Epidemiol. 2011;40(4):964–970. doi: 10.1093/ije/dyr058.
- WHO. Global tuberculosis report 2022. Geneva: WHO; 2022. Available from: https://www.who.int/publications/i/item/9789240061729.
- Dobler CC, Luu Q, Marks GB. What patient factors predict physicians’ decision not to treat latent tuberculosis infection in tuberculosis contacts? PLOS One. 2013;8(9):e76552. doi: 10.1371/journal.pone.0076552.
- Seedat F, Hargreaves S, Nellums LB, et al. How effective are approaches to migrant screening for infectious diseases in Europe? A systematic review. Lancet Infect Dis. 2018;18(9):e259–e271. doi: 10.1016/S1473-3099(18)30117-8.
- Rustage K, Lobe J, Hayward SE, et al. Initiation and completion of treatment for latent tuberculosis infection in migrants globally: a systematic review and meta-analysis. Lancet Infect Dis. 2021;21(12):1701–1712.
- Sterling TR, Njie G, Zenner D, et al. Guidelines for the treatment of latent tuberculosis infection: recommendations from the national tuberculosis controllers association and CDC, 2020. MMWR Recomm Rep. 2020;69(1):1–11. doi: 10.15585/mmwr.rr6901a1.
- Page KR, Sifakis F, Montes de Oca R, et al. Improved adherence and less toxicity with rifampin vs isoniazid for treatment of latent tuberculosis: a retrospective study. Arch Intern Med. 2006;166(17):1863–1870. doi: 10.1001/archinte.166.17.1863.

