Abstract
Objective
To compare mortality and length of hospital stay between patients with ESBL-producing E. coli bloodstream infections (BSIs) and patients with non-ESBL E. coli BSIs. We also aimed at describing risk factors for ESBL-producing E. coli BSIs and time to effective antibiotic treatment for the two groups.
Methods
A retrospective case-control study among adults admitted between 2014 and 2021 to a Norwegian University Hospital.
Results
A total of 468 E. coli BSI episodes from 441 patients were included (234 BSIs each in the ESBL- and non-ESBL group). Among the ESBL-producing E. coli BSIs, 10.9% (25/230) deaths occurred within 30 days compared to 9.0% (21/234) in the non-ESBL group. The adjusted 30-day mortality OR was 1.6 (95% CI 0.7–3.7, p = 0.248). Effective antibiotic treatment was administered within 24 hours to 55.2% (129/234) in the ESBL-group compared to 86.8% (203/234) in the non-ESBL group. Among BSIs of urinary tract origin (n = 317), the median length of hospital stay increased by two days in the ESBL group (six versus four days, p < 0.001). No significant difference in the length of hospital stay was found for other sources of infection (n = 151), with a median of seven versus six days (p = 0.550) in the ESBL- and non-ESBL groups, respectively.
Conclusion
There was no statistically significant difference in 30-day mortality in ESBL-producing E. coli compared to non-ESBL E. coli BSI, despite a delay in the administration of an effective antibiotic in the former group. ESBL-production was associated with an increased length of stay in BSIs of urinary tract origin.
Introduction
Escherichia coli is one of the most common pathogens causing bloodstream infections (BSIs) [Citation1]. E. coli BSIs may be hospital acquired or healthcare associated, but are also commonly of community-acquired origin [Citation2–4]. The incidence of E. coli BSIs has increased in the last decades, as well as the prevalence of third generation cephalosporin resistance caused by Extended-Spectrum Beta-Lactamases (ESBL) [Citation5–9]. Third generation cephalosporin resistant E. coli are estimated to be one of the leading causes of deaths associated with antimicrobial resistance [Citation10]. Furthermore, the spread of ESBL is associated with increased resistance to other important antibiotics (e.g. aminoglycosides, fluoroquinolones and trimethoprim-sulfamethoxazole) as several antibiotic resistance genes often assemble on the same mobile genetic elements that transfer between Gram-negative bacteria [Citation11].
The association between ESBL-producing Enterobacterales and outcomes including mortality, length of stay and effect of inappropriate empiric therapy have been examined with contradicting results. Two of the earliest meta-analyses found an increased risk of mortality associated with third generation resistant Enterobacterales [Citation12,13], but pointed out that results might have been influenced by different study designs and populations, and lack of, or incorrectly adjusted, effect estimates. Further, several of the included studies had few participants, and they included other pathogens than E. coli. Still, more recent meta-analyses support the initial findings of a higher mortality among third generation cephalosporin resistant Enterobacterales versus cephalosporin susceptible isolates, although again, estimates might be influenced by heterogenous study designs and a lack of adjusting for confounders [Citation14–17]. In contrast, other studies examining E. coli BSIs show no impact of ESBL-production on mortality [Citation18–21], although none of these are meta-analyses and they differ in design, study populations and use of empirical antibiotics. Besides study size, design and population, other factors that might influence study outcomes of ESBL-production on mortality are the prevalence of local antimicrobial resistance, national guidelines on empirical antibiotic treatment and sepsis management, hospital resources, definitions of sepsis, health care seeking behaviour and access to health care and pathogens examined.
To inform the local antibiotic stewardship program in our region with a low prevalence of ESBL, we conducted a study with the primary aim of comparing 30-day mortality and length of hospital stay in patients with ESBL-producing E. coli (ESBL-EC) BSIs to patients with non-ESBL E. coli (non-ESBL-EC) BSIs. The secondary aim was to describe risk factors for ESBL-producing E. coli BSIs and time to effective antibiotic treatment for the two groups.
Material and methods
This was a retrospective case-control study conducted at Akershus University Hospital, Norway, an emergency care hospital with 1,000 beds and a catchment area of 594,000 inhabitants (approx. 10% of the Norwegian population). The national prevalence of ESBL-production in E. coli blood culture isolates varied from 5.8% in 2014 to 7.1% in 2019 [Citation22], with a higher prevalence of 6% in 2014, 11% in 2019 and 6% in 2021 at Akershus University Hospital (annual antibiotic resistance reports published locally by the hospital laboratory). The main empiric antibiotic therapy followed national guidelines and did not change during the study period.
Adults (≥18 years) admitted to the hospital with growth of class A ESBL-producing E. coli [Citation23] in at least one blood culture bottle sampled between 1st January 2014 to 31st December 2021 were included as cases, with a minimum of 8 weeks between each included BSI episode. Controls were selected randomly among adults with non-ESBL-producing E. coli BSIs after matching by year of BSI and place of blood culture requisition (Emergency department or non-Emergency department).
Data were extracted from patient’s electronic medical records and the laboratory data systems of the hospital. All data apply to the time of blood culture sampling, with the following exceptions: the length of stay was calculated as time from admission to discharge, and readmissions were defined as a new hospital admission within 30 days after discharge alive.
Clinical data
The 30-day mortality, length of stay and readmissions were recorded for all causes, i.e. not only related to BSIs. The source of infection was extracted from the discharge notes, and if lacking or uncertain, determined by examining the clinical records during the hospital stay. Several sources of infections were possible in any given BSI. Pyelonephritis, kidney abscesses and prostate gland infections were registered as urinary tract infections. Charlson Comorbidity Index was calculated based on comorbidities at the time of blood culture sampling, according to updated ICD-10 codes [Citation24]. The quick Sepsis related Organ Failure Assessment (qSOFA) [Citation25] was calculated based on the worst variable collected up to 24 h before obtaining the blood culture and used as a proxy to measure the severity of infection. Septic shock was defined as patients with a documented serum lactate >2 mmol/L and vasopressor treatment [Citation25] and was assessed up to 24 h before obtaining the blood culture. Neutropenia was defined as a neutrophil count < 0.5 × 109/L.
The infection was defined as hospital acquired (HA) if the blood culture was drawn more than 48 h after admission. A healthcare-associated (HCA) infection was recorded if the blood culture was sampled 48 h or less after admission in patients with one or more of the following criteria: hospital stay for two or more days in the previous 90 days, transfer from a healthcare institution, day surgery or an invasive procedure in the previous 30 days, regular dialysis or intravenous chemotherapy. A community acquired (CA) infection was registered if the blood culture was sampled less than 48 h after admission in patients without criteria of a HCA infection [Citation26].
Hospital admission abroad within the last 12 months were only recorded for non-Nordic countries, as these patients require contact precautions upon admission according to national guidelines for control of multidrug-resistant microbes, and were therefore registered in the patient file. Antibiotic therapy was recorded for the previous six months from the patient’s hospital medical journal and information upon admission, regardless of length of treatment, with the exception of pre-operative antibiotic prophylaxis.
The time to first effective dose of antibiotic was defined as the time between the blood culture was obtained until the first antibiotic towards which the microbe showed in vitro susceptibility was administered. Oral administrations of amoxicillin-clavulanate, cefalexin, pivmecillinam, trimethoprim and nitrofurantoin were not counted as effective therapy because breakpoints according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) only apply to uncomplicated urinary tract infections [Citation27].
Microbiological data
Blood cultures were incubated in the BACTEC automated blood culture system (Becton Dickinson, USA) and pathogens were identified by MALDI-TOF mass spectometry (Bruker Daltonics, Germany). Antimicrobial susceptibility testing was performed using disc diffusion (BBL Sensi-Disc, Becton Dickinson, USA and Oxoid susceptibility discs, Thermo Fisher Scientific, USA) in accordance with EUCAST recommendations [Citation28], or in cases of ambiguous results, with gradient tests (Etest, BioMérieux, France and MTS, Liofilchem, Italy) according to the manufacturer’s instructions. Susceptibility results were categorised using the EUCAST breakpoint tables at the time of reporting [Citation27]. The presence of class A ESBL-production was confirmed in isolates with resistance to at least one third generation cephalosporin by a positive synergy disc test between amoxicillin-clavulanate and one or more third or fourth generation cephalosporins. A polymicrobial blood culture was recorded if there was growth of other pathogens except E. coli. The time between blood culture sampling until registration of growth (time to positivity) was measured in hours and only included monomicrobial blood cultures.
Statistics
Baseline data are reported as absolute numbers with proportions or medians with interquartile ranges (IQR) unless otherwise stated. Continuous variables were analysed using the Mann Whitney U test and categorical variables with the Chi square or Fisher exact test as appropriate. We used logistic regression to assess the association of ESBL status with 30-day mortality and adjusted for age, sex, Charlson Comorbidity Index, type of infection (health associated, hospital or community acquired), urinary source of infection and septic shock. A Kaplan-Meier survival plot was generated to illustrate the unadjusted association between ESBL status and 30-day mortality with comparison of groups by the log-rank test. A subgroup analysis of length of stay was performed for BSIs of urinary tract origin, as we expected the majority of cases to stem from a urinary tract origin and these may have shorter lengths of stay than BSIs of other origins [Citation29]. A box plot was created for the unadjusted association of ESBL with the length of hospital stay. Study data were collected using REDCap and analysed in Microsoft Excel (version 2016) and STATA (version 17). A two-sided p-value of <0.05 was considered significant. All eligible ESBL-producing E. coli BSIs isolated in our hospital during the study period were included. The included sample size supports a detection of ≥ 9% difference in 30 day mortality with 80% power and a confidence level of 95%.
Results
A total of 468 BSI episodes (234 BSIs each in the ESBL- and non-ESBL group) from 441 patients were included (210 with ESBL-EC and 231 with non-ESBL-EC, one patient was included once in both groups). The main demographic and clinical characteristics are presented in , with additional characteristics included in Appendix A.
Table 1. Demographic and clinical characteristics of patients with E. coli blood stream infections.
Mortality
Forty-six deaths (46/468, 9.9%) occurred within 30 days after obtaining a positive E. coli blood culture (). Among these, 14 deaths (30.4%) occurred within 26 h. In the ESBL group, 25 deaths occurred among 230 BSIs (10.9%; data missing for four BSIs) compared to 21/234 (9%) in the non-ESBL group during the first 30 days (unadjusted OR 1.2, 95%CI 0.7 − 2.3, p = 0.495). After adjustment for sex, age, type of infection (healthcare or hospital versus community acquired), urinary tract as source of infection and septic shock before obtaining the blood culture, the 30-day mortality OR was 1.6 (95%CI 0.7 − 3.7, p = 0.248). Thirty-day survival according to ESBL status is presented in . Variables associated with 30-day mortality are presented in Appendix B.
Figure 1. Thirty-day survival in ESBL-producing E. coli compared to non-ESBL-producing E. coli blood stream infections.
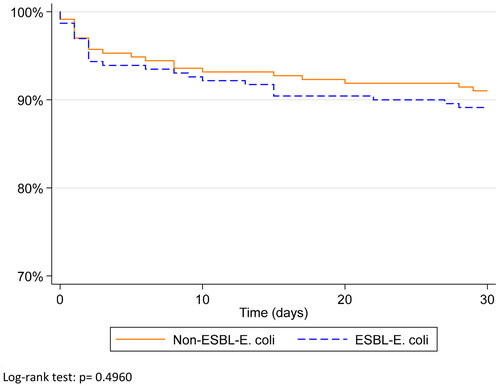
Table 2. All-cause mortality for patients with ESBL-producing E. coli compared to non-ESBL-producing E. coli bloodstream infections.
Time to effective therapy
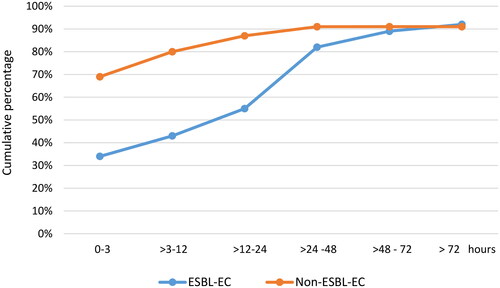
Effective antibiotic treatment was administered within 24 h to 55.2% (129/234) in the ESBL-group compared to 86.8% (203/234) in the non-ESBL group (). A history of ESBL-colonisation-/infection reduced the time to administration of the first effective antibiotic, but it was still delayed compared to the non-ESBL group (Appendix C). There were missing data for 26 (5.6%) BSIs in which effective antibiotics were administered, but in which we could not ascertain the time to administration of the first effective antibiotic (9 (3.8%) among the ESBL-group and 17 (7.3%) among the non-ESBL group (p = 0.099)). Only two patients did not receive any antibiotics at all (one in each group). In the 45 BSIs in whom death occured within 30 days an effective antibiotic had been administered within 3 h and 24 h of obtaining a blood culture in 26 (57.8%) and 35 (77.8%) BSIs, respectively (missing data for one deceased). Antibiotics administered in relation to ordering blood cultures are summarised in Appendix D.
Length of hospital stay
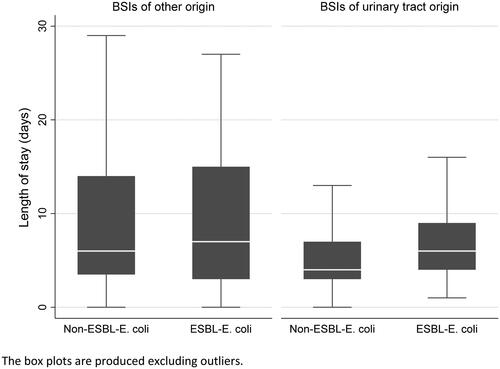
The overall unadjusted median length of stay was seven (IQR 4-10) days in the ESBL-group compared to five (IQR 3-8) days in the non-ESBL group (p = 0.001). In a subanalysis among BSIs of urinary tract origin (n = 317), the median length of stay increased by two days in the ESBL group, with a median of six (IQR 4-9) versus four (IQR 3-7) days in the non-ESBL group (p < 0.001). No significant difference was observed in the patients with other sources of infection (n = 151), with a median length of stay of seven (IQR 3-15) days in the ESBL-group compared to six (IQR 3.5-14) days in the non-ESBL-group (p = 0.550) ().
Risk factors for ESBL-producing E. coli BSIs
Variables univariatly associated with ESBL-producing BSIs were healthcare-associated infections, admissions from other healthcare institutions and hospitals, BSIs of urinary tract origin, indwelling urinary catheters, antibiotic therapy in the previous six months, hospital admission outside a Nordic country in the previous twelve months and a history of infection or colonisation with an ESBL-producing microbe (). A previous history of ESBL colonisation/- infection was recorded in 93/234 (39.7%) ESBL-EC BSIs compared to only 2/234 (0.9%) non-ESBL-EC BSIs. Among the ESBL-EC BSIs with a documented history of ESBL-producing microbes, the median time between the first and the most recent detection of an ESBL-producing microbe (in any samples) and the current BSI was 197 days and 61 days, respectively.
Microbiological findings
Among 468 BSIs, 422 (90.2%) were monomicrobial. There was no significant difference in the prevalence of polymicrobial infections between the ESBL and non-ESBL group (p = 0.280). E. coli was isolated from urinary tract samples in 242/317 (76.3%) BSIs diagnosed as originating in the urinary tract.
The median time to blood culture positivity for monomicrobial BSIs with recorded laboratory data (n = 408) was 11.8 h (IQR 10.6 − 13.8 h, range 4.4 − 105.1 h), with no significant difference between the ESBL- and non-ESBL groups (p = 0.522).
Antimicrobial resistance results are shown in Appendix E. Co-resistance with antibiotics outside the beta-lactam group was higher among the ESBL-EC isolates, especially for ciprofloxacin, gentamicin and trimethoprim-sulfamethoxazole.
Discussion
The main finding of this single centre retrospective case-control study was the lack of a significant difference in 30-day mortality between ESBL-EC and non-ESBL-EC BSIs. This occurred even though treatment with an effective antibiotic often was delayed in the ESBL-EC BSIs. Furthermore, ESBL-production increased the length of hospital stay in E. coli BSIs of urinary tract origin.
The observed 30-day mortality of 9.9% was among the lower rates compared to previous studies [Citation19,20,Citation30–34], including a review of E. coli BSIs in adults in high-income countries that reported a pooled case-fatality rate of 12.4% [Citation2]. Mortality rates might vary depending on numerous factors, including whether studies are restricted to community- or hospital acquired infections, certain patient groups as well as the infectious origins of BSIs. In our study, a large proportion of the BSIs were of urinary tract origin and community acquired, both factors shown to be associated with a reduced mortality [Citation19,Citation20,Citation34–36].
After adjusting for confounders, there was an increased 30-day mortality OR of 1.6 in the ESBL-group, but this did not reach statistical significance. This finding is in accordance with other studies which did not observe any impact of ESBL-production on mortality in E. coli BSIs [Citation18–21]. However, several meta-analyses have found an increased risk of mortality associated with third generation resistant Enterobacterales, reporting pooled, mostly unadjusted OR of 1.5 − 2, albeit with important limitations [Citation13–16]. As our study was powered according to the earliest meta-analyses with an estimated risk ratio of approximately two [Citation12,Citation37], and the 30-day mortality in the ESBL-EC BSIs was low compared to other estimates, a type two error cannot be ruled out.
The lack of association between ESBL-production and mortality despite the delayed administration of effective antibiotics in a significant proportion of the ESBL-EC BSIs may be explained by several factors. First, most deaths within 30 days occurred in patients who did receive effective antibiotics early. Second, almost one third of the 30-day mortality occurred within 26 h after the blood cultures had been obtained, similar to findings in an English national study of E. coli BSIs [Citation20]. This suggests that some deaths might not be preventable even with effective antibiotic treatment [Citation38]. Third, a high proportion of the BSIs in this study were of urinary tract origin. Since several antibiotics accumulate in the urinary tract, some antibiotics declared resistant in vitro might actually have been effective in vivo and active treatment achieved earlier than recorded. Finally, the study registered all-cause mortality, so not all deaths were necessarily caused by E. coli infection. Similarly, other studies have reported a lack of association between initially delayed effective antibiotic treatment and mortality in antimicrobial resistant E. coli [Citation19,Citation29,Citation39–41]. Nevertheless, this is still a controversial topic and likely impacted by a number of confounding factors, as other studies have found a clear association between delayed antibiotic therapy and risk of death [Citation42,Citation43]. The Surviving Sepsis Campaign has concluded that antibiotic treatment should be administered within one hour in adults with septic shock or a high likelihood of sepsis, but may be delayed in order to ascertain the diagnosis in adults with possible sepsis without shock (preferably no more than three hours) [Citation44].
The median length of stay increased by two days in ESBL-EC BSIs originating from the urinary tract compared to non-ESBL-EC BSIs. This is consistent with other studies which have demonstrated an increased length of stay in patients with bacteraemia caused by third generation cephalosporin-resistant E. coli [Citation14,Citation15,Citation19,Citation29,Citation45]. Patients with BSIs of urinary tract origin had a shorter length of stay compared to BSIs of other origins, presumably because these patients often are discharged with oral antibiotics after only a few days of intravenous therapy. The delayed treatment with an effective parenteral antibiotic thus prolongs the stay in the ESBL-EC group more than the non-ESBL-EC. In addition, ESBL-producing microbes are often co-resistant to oral antibiotics, sometimes leaving parenteral therapy the only treatment option. Considering the rising antimicrobial resistance, alternative solutions for parenteral administration of antibiotics outside hospitals such as in outpatient clinics, home nursing care or nursing homes are important to mitigate the effect of ESBL-production on the length of hospital stay.
Compared to non-ESBL-EC BSIs, patients with ESBL-EC BSIs were more often admitted from other healthcare institutions and hospitals, had healthcare-associated infections, previous antibiotic exposure, hospital admission abroad, an indwelling urinary catheter or BSIs of a urinary tract origin. These have been identified as risk factors of infections with ESBL-producing pathogens in other studies [Citation29,Citation30,Citation46–48]. Notably, 39.7% of the ESBL-EC BSIs had a recent history of ESBL-colonisation/-infection compared to almost none in the non-ESBL-EC BSIs. This supports data from a Swedish population-based study which found that colonisation is a substantial risk factor for subsequent BSIs with ESBL-producing Enterobacterales, and that this risk declines rapidly during the first year after detection [Citation49].
The median time between blood culture sampling until registration of growth in this study was 11.8 h. Depending upon laboratory opening hours, current diagnostics may provide susceptibility results of E. coli within 18-24 h after blood culture sampling in a substantial proportion of BSIs [Citation50]. Developing rapid, culture-independent diagnostics could further optimise patient treatment and prevent unnecessary use of broad-spectrum antibiotics [Citation51].
There are several limitations to this study. First, the retrospective design means that information bias was most likely present, especially since some variables were easier to collect from the most recent period when the electronic medication records had been introduced at our hospital. Second, there has been a national campaign to secure rapid treatment of sepsis during the end of the study period. In an effort to reduce these biases alike in the ESBL- and non-ESBL-groups, the controls were matched by year of blood culture sampling. Third, both mortality and length of stay were recorded for all causes, meaning that not all associations were necessarily caused by E. coli blood stream infections. Fourth, only a small proportion of patients presented with septic shock at the time of obtaining the blood culture, reflecting the strict criteria we used for recording septic shock in the study and was thus likely underreported. Fifth, further analysis of mortality and the source of infection was hampered by the large number of deaths occurring with an unknown source of infection. The proportion of unkown origin of infections was similar in the ESBL-EC and non-ESBL-EC BSIs. Sixth, information on limitations of life-sustaining treatment was not collected systematically, thus any influence on the results cannot be assessed. Finally, as a single centre study from a university hospital, our data may not be transferable to other settings, especially considering the many factors involved in diagnosing and treating sepsis that may affect outcomes.
Conclusion
Even though effective antibiotic administration often was delayed in the ESBL-EC BSIs, the difference in 30-day mortality between the ESBL-EC and non-ESBL-EC BSIs did not reach a level of statistical significance. In a setting with a low ESBL-prevalence, our findings support the continued use of empiric carbapenem-sparing antibotic regimens in patients with E. coli bloodstream infections, although individual assessments of risk factors of invasive ESBL-production as well as the severity of illness must be taken into account when choosing the empiric regimen.
Ethical approval
Ethical approval was granted by the Norwegian Regional Committee for Medical and Health Research Ethics (REK reference 2019/918) with a waiver of informed consent and from the Akershus University Hospital’s Data Protection Official (19/07915).
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- World Health Organization. Global antimicrobial resistance and use surveillance system (GLASS) report 2021. Geneva. Licence: CC BY-NC-SA 3.0 IGO.
- Bonten M, Johnson JR, van den Biggelaar AHJ, et al. Epidemiology of Escherichia coli bacteremia: a systematic literature review. Clin Infect Dis. 2021;72(7):1211–1219. doi:10.1093/cid/ciaa210.
- Laupland KB, Gregson DB, Church DL, et al. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin Microbiol Infect. 2008;14(11):1041–1047. doi:10.1111/j.1469-0691.2008.02089.x.
- Vihta K-D, Stoesser N, Llewelyn MJ, et al. Trends over time in Escherichia coli bloodstream infections, urinary tract infections, and antibiotic susceptibilities in oxfordshire, UK, 1998–2016: a study of electronic health records. Lancet Infect Dis. 2018;18(10):1138–1149. doi:10.1016/s1473-3099(18)30353-0.
- UK Health Security Agency. Quarterly epidemiology commentary: mandatory MRSA, MSSA and gram-negative bacteraemia and C. difficile infection in England (up to October to December 2021). London: UK Health Security Agency; April 2022.
- Diekema DJ, Hsueh PR, Mendes RE, et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother. 2019;63(7):e00355-19. doi:10.1128/AAC.00355-19.
- van der Mee-Marquet NL, Blanc DS, Gbaguidi-Haore H, et al. Marked increase in incidence for bloodstream infections due to Escherichia coli, a side effect of previous antibiotic therapy in the elderly. Front Microbiol. 2015;6:646. doi:10.3389/fmicb.2015.00646.
- Kontula KSK, Skogberg K, Ollgren J, et al. Population-based study of bloodstream infection incidence and mortality rates, Finland, 2004-2018. Emerg Infect Dis. 2021;27(10):2560–2569. doi:10.3201/eid2710.204826.
- Gladstone RA, McNally A, Pöntinen AK, et al. Emergence and dissemination of antimicrobial resistance in Escherichia coli causing bloodstream infections in Norway in 2002–17: a nationwide, longitudinal, microbial population genomic study. Lancet Microbe. 2021;2(7):e331–e341. doi:10.1016/s2666-5247(21)00031-8.
- Murray CJL, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi:10.1016/s0140-6736(21)02724-0.
- Mathers AJ, Peirano G, Pitout JD. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant enterobacteriaceae. Clin Microbiol Rev. 2015;28(3):565–591. doi:10.1128/CMR.00116-14.
- Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother. 2007;60(5):913–920. doi:10.1093/jac/dkm318.
- Rottier WC, Ammerlaan HS, Bonten MJ. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum beta-lactamase-producing enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother. 2012;67(6):1311–1320. doi:10.1093/jac/dks065.
- MacKinnon MC, Sargeant JM, Pearl DL, et al. Evaluation of the health and healthcare system burden due to antimicrobial-resistant Escherichia coli infections in humans: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2020;9(1):200. doi:10.1186/s13756-020-00863-x.
- Shamsrizi P, Gladstone BP, Carrara E, et al. Variation of effect estimates in the analysis of mortality and length of hospital stay in patients with infections caused by bacteria-producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. BMJ Open. 2020;10(1):e030266. doi:10.1136/bmjopen-2019-030266.
- Ling W, Furuya-Kanamori L, Ezure Y, et al. Adverse clinical outcomes associated with infections by Enterobacterales producing ESBL (ESBL-E): a systematic review and meta-analysis. JAC Antimicrob Resist. 2021;3(2):dlab068. doi:10.1093/jacamr/dlab068.
- Jiang AM, Liu N, Zhao R, et al. Clinical outcomes and prognostic factors in bloodstream infections due to extended-spectrum beta-lactamase-producing enterobacteriaceae among patients with malignancy: a meta-analysis. Ann Clin Microbiol Antimicrob. 2020;19(1):53. doi:10.1186/s12941-020-00395-7.
- de Lastours V, Laouenan C, Royer G, et al. Mortality in Escherichia coli bloodstream infections: antibiotic resistance still does not make it. J Antimicrob Chemother. 2020;75(8):2334–2343. doi:10.1093/jac/dkaa161.
- Richelsen R, Smit J, Schonheyder HC, et al. Outcome of community-onset ESBL-producing Escherichia coli and Klebsiella pneumoniae bacteraemia and urinary tract infection: a population-based cohort study in Denmark. J Antimicrob Chemother. 2020;75(12):3656–3664. doi:10.1093/jac/dkaa361.
- Abernethy JK, Johnson AP, Guy R, et al. Thirty day all-cause mortality in patients with Escherichia coli bacteraemia in England. Clin Microbiol Infect. 2015;21(3):251 e1-8–251.e8. doi:10.1016/j.cmi.2015.01.001.
- Liang T, Xu C, Cheng Q, et al. Epidemiology, risk factors, and clinical outcomes of bloodstream infection due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in hematologic malignancy: a retrospective study from Central South China. Microb Drug Resist. 2021;27(6):800–808. doi:10.1089/mdr.2020.0033.
- NORM/NORM-VET. 2019. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. Tromsø/Oslo 2020. ISSN:1502-2307 (print)/1890-9965 (electronic). Available from: www.antibiotikaresistens.no.
- Giske CG, Sundsfjord AS, Kahlmeter G, et al. Redefining extended-spectrum β-lactamases: balancing science and clinical need. J Antimicrob Chemother. 2009;63(1):1–4. doi:10.1093/jac/dkn444.
- Thygesen SK, Christiansen CF, Christensen S, et al. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of patients. BMC Med Res Methodol. 2011;11(1):83. doi:10.1186/1471-2288-11-83.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287.
- Friedman ND, Kaye KS, Stout JE, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137(10):791–797. doi:10.7326/0003-4819-137-10-200211190-00007.
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 9.0. 2019. Available from: http://www.eucast.org.
- The European Committee on Antimicrobial Susceptibility Testing. EUCAST disk diffusion method for antimicrobial susceptibility testing. Manual version 9.0. 2021. Available from: www.eucast.org.
- Lin WP, Huang YS, Wang JT, et al. Prevalence of and risk factor for community-onset third-generation cephalosporin-resistant Escherichia coli bacteremia at a medical center in Taiwan. BMC Infect Dis. 2019;19(1):245. doi:10.1186/s12879-019-3880-z.
- Xiao T, Wu Z, Shi Q, et al. A retrospective analysis of risk factors and outcomes in patients with extended-spectrum beta-lactamase-producing Escherichia coli bloodstream infections. J Glob Antimicrob Resist. 2019;17:147–156. doi:10.1016/j.jgar.2018.12.014.
- Jauneikaite E, Honeyford K, Blandy O, et al. Bacterial genotypic and patient risk factors for adverse outcomes in Escherichia coli bloodstream infections: a prospective molecular epidemiological study. J Antimicrob Chemother. 2022;77(6):1753–1761. doi:10.1093/jac/dkac071.
- Leistner R, Sakellariou C, Gurntke S, et al. Mortality and molecular epidemiology associated with extended-spectrum beta-lactamase production in Escherichia coli from bloodstream infection. Infect Drug Resist. 2014;7:57–62. doi:10.2147/IDR.S56984.
- Ha YE, Kang CI, Cha MK, et al. Epidemiology and clinical outcomes of bloodstream infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in patients with cancer. Int J Antimicrob Agents. 2013;42(5):403–409. doi:10.1016/j.ijantimicag.2013.07.018.
- Lefort A, Panhard X, Clermont O, et al. Host factors and portal of entry outweigh bacterial determinants to predict the severity of Escherichia coli bacteremia. J Clin Microbiol. 2011;49(3):777–783. doi:10.1128/JCM.01902-10.
- Sakellariou C, Gurntke S, Steinmetz I, et al. Sepsis caused by extended-spectrum beta-lactamase (ESBL)-positive K. pneumoniae and E. coli: comparison of severity of sepsis, delay of anti-infective therapy and ESBL genotype. PLoS One. 2016;11(7):e0158039. doi:10.1371/journal.pone.0158039.
- Palacios-Baena ZR, Gutierrez-Gutierrez B, De Cueto M, et al. Development and validation of the INCREMENT-ESBL predictive score for mortality in patients with bloodstream infections due to extended-spectrum-beta-lactamase-producing enterobacteriaceae. J Antimicrob Chemother. 2017;72(3):906–913. doi:10.1093/jac/dkw513.
- World Health Organization. Antimicrobial resistance: global report on surveillance. 2014. France. Available from: https://www.who.int/publications/i/item/9789241564748.
- Rhee C, Jones TM, Hamad Y, et al. Prevalence, underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA Netw Open. 2019;2(2):e187571. doi:10.1001/jamanetworkopen.2018.7571.
- Tocut M, Zohar I, Schwartz O, et al. Short- and long-term mortality in patients with urosepsis caused by Escherichia coli susceptible and resistant to 3rd generation cephalosporins. BMC Infect Dis. 2022;22(1):571. doi:10.1186/s12879-022-07538-5.
- Martinez-Nadal G, Puerta-Alcalde P, Gudiol C, et al. Inappropriate empirical antibiotic treatment in high-risk neutropenic patients with bacteremia in the era of multidrug resistance. Clin Infect Dis. 2020;70(6):1068–1074. doi:10.1093/cid/ciz319.
- Joo EJ, Park DA, Lee NR, et al. Impact of appropriateness of empiric therapy on outcomes in community-onset bacteremia by extended-spectrum-beta-lactamase producing Escherichia coli and klebisella pneumoniae definitively treated with carbapenems. Eur J Clin Microbiol Infect Dis. 2017;36(11):2093–2100. doi:10.1007/s10096-017-3031-7.
- Tacconelli E, Cataldo MA, Mutters NT, et al. Role of place of acquisition and inappropriate empirical antibiotic therapy on the outcome of extended-spectrum beta-lactamase-producing enterobacteriaceae infections. Int J Antimicrob Agents. 2019;54(1):49–54. doi:10.1016/j.ijantimicag.2019.04.007.
- Lodise TP, Zhao Q, Fahrbach K, et al. A systematic review of the association between delayed appropriate therapy and mortality among patients hospitalized with infections due to Klebsiella pneumoniae or Escherichia coli: how long is too long? BMC Infect Dis. 2018;18(1):625. doi:10.1186/s12879-018-3524-8.
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi:10.1007/s00134-021-06506-y.
- Naylor NR, Pouwels KB, Hope R, et al. The health and cost burden of antibiotic resistant and susceptible Escherichia coli bacteraemia in the english hospital setting: a national retrospective cohort study. PLoS One. 2019;14(9):e0221944. doi:10.1371/journal.pone.0221944.
- Richelsen R, Smit J, Laxsen Anru P, et al. Risk factors of community-onset extended-spectrum beta-lactamase Escherichia coli and Klebsiella pneumoniae bacteraemia: an 11-year population-based case-control-control study in Denmark. Clin Microbiol Infect. 2021;27(6):871–877. doi:10.1016/j.cmi.2020.08.004.
- Biehl LM, Schmidt-Hieber M, Liss B, et al. Colonization and infection with extended spectrum beta-lactamase producing Enterobacteriaceae in high-risk patients – review of the literature from a clinical perspective. Crit Rev Microbiol. 2016;42(1):1–16. doi:10.3109/1040841X.2013.875515.
- Rodriguez-Bano J, Picon E, Gijon P, et al. Community-onset bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: risk factors and prognosis. Clin Infect Dis. 2010;50(1):40–48. doi:10.1086/649537.
- Isendahl J, Giske CG, Hammar U, et al. Temporal dynamics and risk factors for bloodstream infection with extended-spectrum beta-lactamase-producing bacteria in previously-colonized individuals: national population-based cohort study. Clin Infect Dis. 2019;68(4):641–649. doi:10.1093/cid/ciy539.
- Akerlund A, Jonasson E, Matuschek E, et al. EUCAST rapid antimicrobial susceptibility testing (RAST) in blood cultures: validation in 55 european laboratories. J Antimicrob Chemother. 2020;75(11):3230–3238. doi:10.1093/jac/dkaa333.
- Rello J, van Engelen TSR, Alp E, et al. Towards precision medicine in sepsis: a position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin Microbiol Infect. 2018;24(12):1264–1272. doi:10.1016/j.cmi.2018.03.011.
Appendix A:
Additional clinical data of patients with E. coli blood stream infections
Appendix B:
Variables associated with 30-day mortality
Appendix C:
Time to administration of the first in vitro susceptible antibiotic
Appendix D:
Antibiotics administered in relation to ordering blood cultures
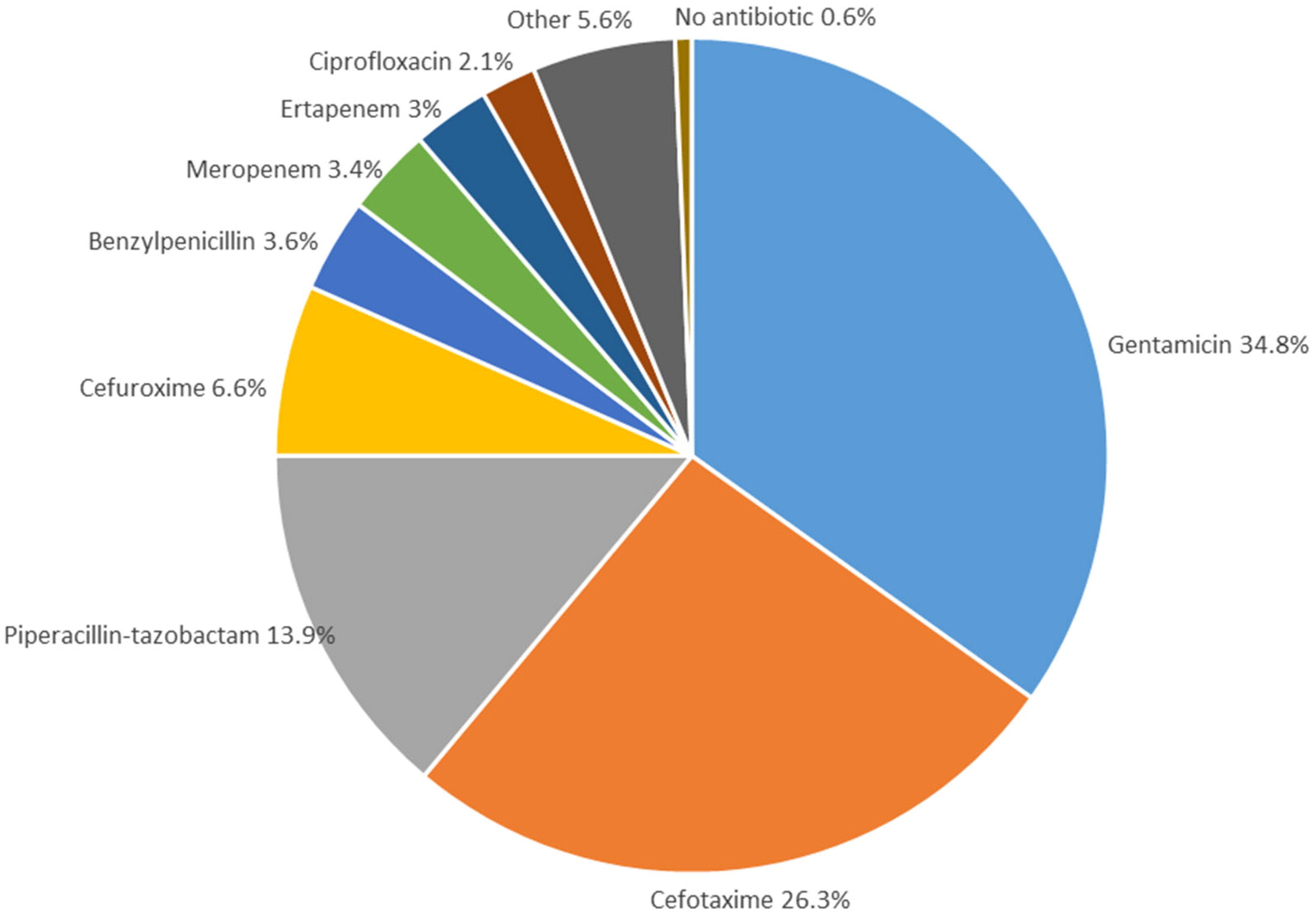 The figure shows the first antibiotic treatment prescribed in the hospital in relation to ordering blood cultures, irrespective of in vitro susceptibility results. For patients in which blood cultures were ordered by the Emergency department, the antibiotic is the first administered upon arrival at the hospital. For inpatients, the antibiotic prescribed in relation to obtaining the blood culture is recorded (e.g. if an antibiotic treatment was changed to a new treatment). For BSIs treated with combination therapy (e.g. aminoglycosides and penicillins), only the antibiotic with the broadest gram-negative coverage is included in the figure.
The figure shows the first antibiotic treatment prescribed in the hospital in relation to ordering blood cultures, irrespective of in vitro susceptibility results. For patients in which blood cultures were ordered by the Emergency department, the antibiotic is the first administered upon arrival at the hospital. For inpatients, the antibiotic prescribed in relation to obtaining the blood culture is recorded (e.g. if an antibiotic treatment was changed to a new treatment). For BSIs treated with combination therapy (e.g. aminoglycosides and penicillins), only the antibiotic with the broadest gram-negative coverage is included in the figure.