Abstract
Background
Mortality rates peaked early in the COVID-19 pandemic and then declined. Possible explanations are pharmacological and non-pharmacological treatments, vaccines and changing demographics. We sought to evaluate temporal trends in clinical characteristics and survival of patients hospitalised with COVID-19 during the first two years of the pandemic in Denmark.
Methods
In this observational study, we included all adults with COVID-19 consecutively admitted to three hospitals in Copenhagen, Denmark, from March 2020 through March 2022. The primary outcome was overall survival up to day 90 from admission. We used multivariable Cox proportional hazards models to estimate the association of survival within five consecutive time-periods, based on admission date, adjusted for baseline characteristics, vaccination status, remdesivir and dexamethasone treatment.
Results
In 1630 included patients, the median age [IQR] was 68 [52, 79] years, 56.6% were men and 86.2% had comorbidity. Clinical characteristics changed over time. The crude 90-day mortality rate peaked in March–June 2020 with 28.9%, decreased from July 2020 to 17.5%, and increased again in January-March 2022 to 28.6%. Lower hazard ratios for death were observed in individuals admitted from July 2020 and persisted after adjusting for baseline characteristics. Adjusting for vaccination, remdesivir treatment and dexamethasone treatment attenuated the association in patients requiring low-flow oxygen.
Conclusions
Our study suggests lower hazard rates for mortality in patients hospitalised with COVID-19 from July 2020 compared to March-June 2020, mainly driven by lower mortality in patients with a need of oxygen at baseline.
Keywords:
Introduction
On March 1, 2020, the first case of COVID-19 was admitted to a Danish hospital (https://experience.arcgis.com/experience/aa41b29149f24e20a4007a0c4e13db1d/page/). Since, nearly 70,000 hospital admissions and 8,000 deaths have occurred in Denmark (https://experience.arcgis.com/experience/aa41b29149f24e20a4007a0c4e13db1d/page/, https://experience.arcgis.com/experience/aa41b29149f24e20a4007a0c4e13db1d/page/Nationalt). Rapidly, novel and repurposed drugs were added to the COVID-19 treatment guideline (https://infmed.dk/guidelines#covid-19_retningslinje_(2023v25).pdf). In May 2020, remdesivir was recommended as standard of care in hypoxemic patients with COVID-19 (https://infmed.dk/guidelines#covid-19_retningslinje_(2023v25).pdf) [Citation1], and in June 2020 dexamethasone was also recommended (https://infmed.dk/guidelines#covid-19_retningslinje_(2023v25).pdf) [Citation2]. In February 2021, the anti-interleukin-6 receptor monoclonal antibody, tocilizumab, was included in the guideline (https://infmed.dk/guidelines#covid-19_retningslinje_(2023v25).pdf) [Citation3,Citation4]. Furthermore, anticoagulation has been recommended to all hospitalised patients since April 2020 (https://infmed.dk/guidelines#covid-19_retningslinje_(2023v25).pdf). The vaccine program in Denmark began on December 27, 2020, and by January 2022, more than 80% of the population had completed the initial COVID-19 vaccination protocol of two vaccine doses [Citation5]. More than 80% of administered vaccines were BNT162b2 [Citation5].
Early in the pandemic, short-term mortality rates were reported to peak during the first couple of months and then decline [Citation6–12]. In addition to the rapid development in the use of pharmacological treatments in COVID-19, improvement in non-pharmacological approaches such as oxygenation and the use of prone positioning may explain the improved outcomes [Citation13–16]. Old age and pre-existing comorbidity are well-described risk-factors for adverse outcomes in COVID-19. Hence, changing the demographics of hospitalised patients during different surges could also explain decreasing mortality rates [Citation7].
Data on trends in characteristics and outcomes during more than two years of the pandemic is limited. In this three-centre retrospective cohort study, we sought to describe the characteristics and mortality of patients admitted with COVID-19 during the first two years of the pandemic and to evaluate temporal trends in survival and clinical characteristics. Furthermore, we sought to investigate whether vaccination status or treatment during hospitalisation explained variability in mortality over time.
Methods
Study cohort
This observational study included all Danish residents (18 years or older) with confirmed SARS-CoV-2 infection consecutively admitted to three connected university-affiliated hospitals (Hvidovre Hospital, Amager Hospital and Glostrup Hospital) in Copenhagen, Denmark, from March 2020 through March 2022. The catchment area of the hospitals located in the southern part of the greater Copenhagen area includes 600,000 inhabitants. The hospitals receive unselected patients. In general, less severe cases were managed at all locations while severe cases requiring intensive care treatment were treated at Hvidovre Hospital. Dedicated COVID-19 wards were established in March of 2020 and maintained over the summer. From mid-2020, cases of COVID-19 were treated in medical wards and intensive care units. Cases were confirmed by reverse transcriptase polymerase chain reaction on an oropharyngeal swab or lower respiratory tract specimen 10 days prior to or during the first 72 h of hospitalisation.
Variables
Patient information was obtained through manual review of electronic medical records and included demographic variables, comorbidities, radiographic infiltration on chest X-ray, baseline respiratory support, date of administration of SARS-CoV-2 vaccine(s) and pharmacologic COVID-19 treatment (remdesivir, dexamethasone, tocilizumab, and anticoagulation) during hospitalisation. All-cause readmissions within 30 days of discharge and all-cause mortality up to day 90 d after admission were ascertained for all study participants.
Chest X-ray was obtained within 24 h of admission, and baseline respiratory support (no oxygen, oxygen through a low-flow device, oxygen through a high-flow device or invasive mechanical ventilation) was recorded. Patients admitted seven days or more after the first dose of the COVID-19 vaccine were categorised as partially vaccinated, and patients admitted seven days or more after the second dose were categorised as fully vaccinated [Citation17]. Vaccination status was grouped in five categories: not vaccinated, partially vaccinated, fully vaccinated (second dose less than six months prior to admission date), fully vaccinated (second dose more than six months prior to admission date), and fully vaccinated including a booster vaccine at least seven days prior to admission date.
Patients were categorised in five time-periods based on admission date: time-period 1 (March–June 2020), time-period 2 (July–December 2020), time-period 3 (January–June 2021), time-period 4 (July–December 2021) and time-period 5 (January-March 2022). Coincidentally, time-period 1 and 2 were predominantly the index variant of SARS-CoV-2, time-period 3 predominantly the alpha variant, time-period 4 predominantly the delta variant, and time-period 5 predominantly the omicron variant [Citation18].
Statistical analyses
The primary outcome of interest was overall survival up to day 90 from admission. All observations were censored at death or day 90. Baseline characteristics are presented as numbers with percentages or medians with interquartile range (IQR). Comparisons of baseline variables between time-periods were performed using χ2–test, Fisher’s-exact test or Mann–Whitney U test, as appropriate. Kaplan–Meier plots were used to visualise and compare unadjusted survival rates between time-periods.
We used multivariable Cox proportional hazards models to estimate the association between time-period and all-cause mortality. Estimates are presented as crude and adjusted hazard ratios (aHRs) with 95% confidence intervals (CI). We calculated scaled Schoenfeld residuals to test the proportional hazards assumption systematically for each variable in the multivariable models. If any regression variable failed the proportional hazards test, we used stratification of this variable to account for non-proportional hazards.
We created a core model (model 1) adjusting for age group (<50 years, 50–65 years, 66–80 years or >80 years), sex (male or female), presence of arterial hypertension (yes/no), diabetes mellitus (yes/no), cardiovascular disease (yes/no), chronic obstructive pulmonary disease (yes/no), cancer (yes/no) or other (yes/no), presence of radiographic infiltration on chest X-ray and time-period (1–5). We then expanded the multivariable Cox model to include vaccination status (model 2), vaccination status and remdesivir treatment (model 3), and vaccination status and dexamethasone treatment (model 4), to evaluate whether vaccination status, remdesivir or dexamethasone treatment affected survival rates. To address immortal time bias, i.e. the period of time during which the outcome event cannot occur, remdesivir and dexamethasone treatment were considered as time-dependent variables. The analyses were stratified by subgroups based on baseline respiratory support. As a sensitivity analysis, we restricted the subgroup requiring high-flow oxygen or invasive mechanical ventilation at baseline to include only patients requiring high-flow oxygen.
p values < .05 were considered to indicate statistical significance. Data analyses were performed using R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Approval
This study was approved by the Danish Board of Health (record no. 31–1522–84 and 31–1521–309) and the Capital Regional Data Protection Centre (record no. P-2020–492). By Danish legislation, this type of study is exempt from ethical committee approval.
Results
Study population characteristics
Overall, 1630 individuals were admitted with COVID-19 to the three hospitals from March 2020 through March 2022. Demographics and clinical characteristics of the five time-periods are presented in . Supplementary Table S1(a–c) presents clinical characteristics by subgroups of baseline respiratory support.
Table 1. Clinical characteristics of patients hospitalised with COVID-19 from March 2020 through March 2022.
Time-period 1 (admitted March–June 2020) included 291 (17.9%) patients, time-period 2 (admitted July–December 2020) 365 (22.4%) patients, time-period 3 (admitted January–June 2021) 432 (26.5%) patients, time-period 4 (admitted July–December 2021) 346 (21.2%) patients and time-period 5 (admitted January–March 2022) 196 (12.0%) patients.
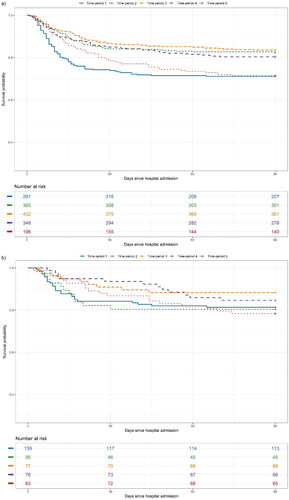
Patients in time-periods 1 and 5 were older than patients in time-periods 2, 3 and 4. More patients in time-period 1 had more comorbidity than those in the other four time-periods, and the distribution of the comorbidities differed between the time-periods except for diabetes mellitus. Patients in time-period 5 were less likely to have pulmonary infiltrates on X-ray at admission. Patients in time-periods 2, 3 and 4 more often required supplemental oxygen at admission with a higher proportion needing high-flow oxygen than in time-periods 1 and 5. The proportion of fully vaccinated patients increased with time to 75.0% in time-period 5. More than 70% of patients admitted after June 2020 received remdesivir and dexamethasone, although a decline in dexamethasone treatment was observed in time-period 5. The proportion of patients progressing to invasive mechanical ventilation was stable at 8–10% during 2020 and 2021 but decreased to 1.5% from January 2022. Mortality rates decreased from July 2020 but increased again in 2022. The readmission rate was stable over time at 14–18%.
Survival outcomes
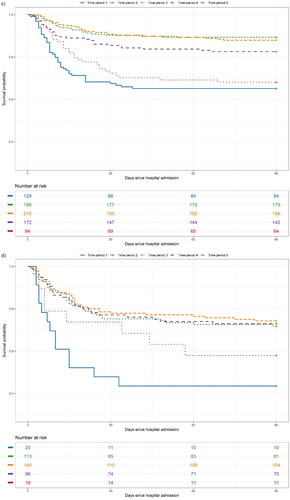
All 1630 individuals were included in the survival analyses. The Kaplan–Meier survival rates up to day 90 are shown overall and by subgroups of baseline respiratory support in . shows unadjusted and adjusted HR for mortality up to day 90 by subgroups of baseline oxygen requirements.
Figure 1. (a–d) Unadjusted survival probability up to day 90 in patients with COVID-19 in five time-periods hospitalised between March 2020 and March 2022, shown overall and by baseline oxygen requirements: (a) overall, (b) no oxygen, (c) low-flow oxygen and (d) high-flow oxygen or invasive mechanical ventilation.
Table 2. Unadjusted and adjusted hazard ratios for all-cause mortality to day 90 in four time periods compared to March–June 2020.
In individuals not requiring supplemental oxygen, there was no significant difference in HR for mortality between time-periods 2, 3, 4 and 5 and time-period 1 in the core adjusted model (model 1) or when adding vaccination status (model 2). When further adjusting for remdesivir treatment (model 3), a significantly lower HR was seen in time-period 3 compared to time-period 1 (aHR: 0.39, 95% CI: 0.16–0.94). When adding dexamethasone (model 4), HR was significantly lower in time-period 3 than in time-period 1 (aHR: 0.25, 95% CI: 0.10–0.65).
In individuals requiring low-flow oxygen, significantly lower HRs were seen in time-periods 2, 3 and 4 than in time-period 1 in the adjusted model 1 (aHR: 0.28, 95% CI: 0.16–0.47, aHR: 0.32, 95% CI: 0.20–0.53, and aHR: 0.51, 95% CI: 0.31–0.84, respectively). Adjusting for vaccination status (model 2) and further remdesivir (model 3) and dexamethasone (model 4), attenuated the association for time-period 4 in all models.
In individuals requiring high-flow oxygen or invasive mechanical ventilation, aHRs in model 1 were significantly lower in time-periods 2, 3, 4 and 5 compared to time-period 1 (aHR: 0.15, 95% CI: 0.08–0.31, aHR: 0.16, 95% CI: 0.08–0.31, aHR: 0.30, 95% CI: 0.14–0.62, and aHR: 0.24, 95% CI: 0.09–0.66, respectively). Further adjusting for vaccination status (model 2), remdesivir (model 3) and dexamethasone (model 4) did not change effect estimates. In the sensitivity analysis repeating all four models in the subgroup only requiring high-flow oxygen, the association in time-period 4 was lowered when adjusting for dexamethasone (aHR: 0.30, 95% CI: 0.08, 1.12) (Supplementary Table S2).
Discussion
Here, we provide an assessment of temporal trends in demographics and mortality in adults hospitalised with COVID-19 in three hospitals in Denmark during the first two years of the pandemic. Patient composition changed during the different time periods and crude mortality rates decreased from July 2020, were stable through 2021 and increased again from January 2022. Several other studies support this finding, describing high mortality risk in the months of March and April 2020 followed by a decrease which then plateaued [Citation6–11,Citation19]. Currently, data on COVID-19-related mortality in 2022 compared to early 2020 has not been published
In patients requiring supplemental oxygen at admission, significantly lower HR for death were observed in time-periods 2, 3, and 4 and numerically in time-period 5 compared to time-period 1 in the unadjusted analyses. Although patients in time-period 1 were older and more comorbid, known risk-factors for mortality [Citation20–22], the improved survival remained after adjusting for these variables. When further adjusting for vaccination status, the association seemed to weaken in time-period 4 in patients requiring low-flow oxygen. We observed a rapid increase in the proportion of fully vaccinated individuals in this subgroup during mid-2021 (time-period 1–3: 0–3.7%, time-period 4: 46.5%, see Supplementary Table S1(b)) which is, at least partly, a possible explanation for the improved outcome in this group. This is supported by several studies, which show a protective effect of COVID-19 vaccines on mortality [Citation23–25]. When further adding remdesivir and dexamethasone, respectively, the adjusted HR was nearly 1 in time-period 4, indicating that better outcomes seen in this time-period were due to a combination of vaccination status and remdesivir and/or dexamethasone. This is in accordance with previous studies, reporting better outcomes in patients requiring low-flow oxygen receiving remdesivir and/or dexamethasone [Citation1,Citation2,Citation26–28]. According to our results, vaccination status and remdesivir or dexamethasone did not seem to play a role in the improved outcomes in individuals requiring high-flow oxygen/IMV at admission, as HRs were unaffected after adjusting for vaccination status, remdesivir and dexamethasone. The effect of remdesivir in COVID-19 seems to vary depending on oxygen requirements at baseline, with a more pronounced effect in patients requiring low-flow oxygen compared to high-flow [Citation29]. This is consistent with our results.
From January 2022, a different pattern of patient characteristic was observed which coincided with the dominance of the novel highly transmissible SARS-CoV-2 variant of concern, Omicron (B.1.1.529) (>90% of cases by mid-January 2022 [Citation18]), resulting in a record high number of case in Denmark [Citation18]. Fewer patients required oxygen at admission, had infiltrates on X-ray and fewer patients without baseline oxygen requirements progressed to use of supplemental oxygen and/or IMV during hospitalisation, indicating milder disease associated with B.1.1.529 than with previous strains of SARS-CoV-2. Several studies support this finding [Citation30–33]. Despite less severe disease, we observed higher mortality in time-period 5 than in the three previous time-periods, reflected in increasing HR which persisted after adjustment for risk factors in patients with no need of oxygen or need of low-flow oxygen at baseline. A major difference between the time-periods of this study is the median symptom duration: 1 d for time-period 5 compared to 6–7 d for time-periods 1–4 ( and Supplementary Table S1(a,b)), indicating that patients admitted from January 2022 were more often diagnosed with COVID-19 at or during admission in contrast to the four previous time-periods. Throughout the pandemic, it has been discussed how to quantify COVID-19 and mortality, whether a patient died of or with COVID-19. A Danish study has estimated that the proportion of coincidental deaths in SARS-CoV-2 infected individuals rose to around 40% in the Omicron period compared to 10–20% in previous periods [Citation34]. The difference in symptom duration between our time-periods indicates more ‘incidental’ infections in time-period 5, in agreement with the previous study [Citation34]. Incidental infections, and thus deaths, make it difficult to compare mortality between time-periods, especially time-period 5.
This study has limitations. First, as this is an observational study, we cannot confirm any causality of the associations observed. Second, although adjusting for several variables in our models, residual confounding may remain. Potential explanatory factors for lower mortality such as clinician experience, changing thresholds for hospital admission during different time periods and changing patient behaviour (e.g. when presenting to the hospital) could affect our estimates but are difficult to measure. Additionally, data did not include do-not-resuscitate status, which could affect the outcome. Also, since not all positive tests were genotyped in Denmark during this study period, we have no information on the SARS-CoV-2 variant on an individual level, although the change in dominant strain approximately followed the periods into which our cohorts were divided: Alpha (B.1.1.7) was detected in more than 50% of sequenced samples by week 7 of 2021 and more than 90% by week 10 of 2021. Delta (B.1.617.2) were detected in more than 50% of samples by week 26 of 2021 and more than 90% by week 28 of 2021. Omicron (B.1.1.529) was detected in more than 50% of samples by week 51 of 2021 and more than 90% by week 1 of 2022 [Citation18]. Third, our mortality rates reflect patients from a limited number of hospitals in Denmark. Nevertheless, our cohort is large, and we benefit from standardised registration and complete follow-up. Finally, the analysis included hospitalised individuals only and was, thus, unable to evaluate the possible effects of vaccination and pre-hospital treatments on the risk of hospitalisation and subsequent risk of death.
In conclusion, we found lower hazard rates of mortality in patients hospitalised with COVID-19 from July 2020 compared to the first pandemic months of March-June 2020, mainly driven by lower mortality in patients with a need of oxygen at baseline, which could not be explained by patient characteristics. Vaccination status, treatment with remdesivir and/or dexamethasone appeared to partly explain the reduced mortality in patients requiring low-flow oxygen at admission but only dexamethasone seemed to have an effect in patients requiring high-flow oxygen or invasive mechanical ventilation at admission.
Supplemental Material
Download MS Word (54.7 KB)Disclosure statement
T. B. reports unrestricted grants from Novo Nordisk Foundation, Simonsen Foundation, Lundbeck Foundation, Kai Foundation and Erik and Susanna Olesen’s Charitable Fund, unrestricted grants and personal fees for serving as an advisory board member from GlaxoSmithKline and Merck, Sharp and Dohme, unrestricted grants and personal fees for teaching/serving as an advisory board member from Pfizer and Gilead, personal fees for serving as an advisory board member from Janssen, personal fees for teaching/serving as an advisory board member from AstraZeneca, personal fees for teaching from Boehringer Ingelheim, Abbvie and Bavarian Nordic, and personal fees for serving as a board member from Pentabase, outside the submitted work.
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid-19 – Final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764.
- Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436.
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0.
- Gordon AC, Mouncey PR, Al-Beidh F, et al. Interleukin-6 receptor antagonists in critically ill patients with covid-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433.
- Mathieu E, Ritchie H, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). 2020. Published online at OurWorldInData.org
- Moon RC, Mackey RH, Cao Z, et al. Is coronavirus disease 2019 (COVID-19) less deadly now? Trends in in-hospital mortality among hospitalized COVID-19 patients in the United States. Clin Infect Dis. 2022;74(12):2238–2242. doi: 10.1093/cid/ciab830.
- Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2021;16(2):90–92. doi: 10.12788/jhm.3552.
- Asch DA, Sheils NE, Islam MN, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 During the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471–478. doi: 10.1001/jamainternmed.2020.8193.
- Bennett TD, Moffitt RA, Hajagos JG, et al. Clinical characterization and prediction of clinical severity of SARS-CoV-2 infection among US adults using data from the US national COVID cohort collaborative. JAMA Netw Open. 2021;4(7):e2116901. doi: 10.1001/jamanetworkopen.2021.16901.
- Jones S, Mason N, Palser T, et al. Trends in risk-adjusted 28-day mortality rates for patients hospitalized with COVID-19 in England. J Hosp Med. 2021;16(5):290–293. doi: 10.12788/jhm.3599.
- Roth GA, Emmons-Bell S, Alger HM, et al. Trends in patient characteristics and COVID-19 in-hospital mortality in the United States during the COVID-19 pandemic. JAMA Netw Open. 2021;4(5):e218828. doi: 10.1001/jamanetworkopen.2021.8828.
- Benfield T, Bodilsen J, Brieghel C, et al. Improved survival among hospitalized patients with COVID-19 treated with remdesivir and dexamethasone. A nationwide population-based cohort study. Clin Infect Dis. 2021;73(11):2031–2036. doi: 10.1093/cid/ciab536.
- Ghelichkhani P, Esmaeili M. Prone position in management of COVID-19 patients; a commentary. Arch Acad Emerg Med. 2020;8:e48.
- COVID-19 Treat Guidelines. Oxygenation and ventilation for adults. 2023 [cited 2023 Jan 17]. https://www.covid19treatmentguidelines.nih.gov/management/critical-care-for-adults/oxygenation-and-ventilation-for-adults/
- Mellado-Artigas R, Ferreyro BL, Angriman F, et al. High-flow nasal oxygen in patients with COVID-19-associated acute respiratory failure. Crit Care. 2021;25(1):58. doi: 10.1186/s13054-021-03469-w.
- Ehrmann S, Li J, Ibarra-Estrada M, et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021;9(12):1387–1395. doi: 10.1016/S2213-2600(21)00356-8.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577.
- Danish Covid-19 Genome Consortium. Genomic overview of SARS-CoV-2 in Denmark. 2022. https://www.covid19genomics.dk/statistics.
- Fiore MC, Smith SS, Adsit RT, et al. The first 20 months of the COVID-19 pandemic: mortality, intubation and ICU rates among 104,590 patients hospitalized at 21 United States health systems. PLOS One. 2022;17(9):e0274571. doi: 10.1371/journal.pone.0274571.
- Alharbi A-HM, Rabbani SI, Halim Mohamed AA, et al. Analysis of potential risk factors associated with COVID-19 and hospitalization. Front Public Health. 2022;10:921953. doi: 10.3389/fpubh.2022.921953.
- Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York city: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2.
- Rosenthal N, Cao Z, Gundrum J, et al. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. 2020;3(12):e2029058. doi: 10.1001/jamanetworkopen.2020.29058.
- Tenforde MW, Self WH, Adams K, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326(20):2043–2054. doi: 10.1001/jama.2021.19499.
- Naouri D, Vuagnat A, Beduneau G, et al. Trends in clinical characteristics and outcomes of all critically ill COVID-19 adult patients hospitalized in France between march 2020 and june 2021: a national database study. Ann Intensive Care. 2023;13(1):2. doi: 10.1186/s13613-022-01097-3.
- Moffa MA, Shively NR, Walsh TL. Characteristics of postvaccination coronavirus disease 2019 hospitalizations prior to booster vaccines. Open Forum Infect Dis. 2022;9(4):ofac014. doi: 10.1093/ofid/ofac014.
- Mozaffari E, Chandak A, Zhang Z, et al. Remdesivir treatment in hospitalized patients with COVID-19: a comparative analysis of in-hospital all-cause mortality in a large multi-center observational cohort. Clin Infect Dis. 2022;75(1):e450–e458. doi: 10.1093/cid/ciab875.
- Marx K, Gončarova K, Fedders D, et al. Clinical outcomes of hospitalized COVID-19 patients treated with remdesivir: a retrospective analysis of a large tertiary care center in Germany. Infection. 2023;51(1):97–108. doi: 10.1007/s15010-022-01841-8.
- Diaz GA, Christensen AB, Pusch T, et al. Remdesivir and mortality in patients With coronavirus disease 2019. Clin Infect Dis. 2022;74(10):1812–1820. doi: 10.1093/cid/ciab698.
- Beckerman R, Gori A, Jeyakumar S, et al. Remdesivir for the treatment of patients hospitalized with COVID-19 receiving supplemental oxygen: a targeted literature review and meta-analysis. Sci Rep. 2022;12(1):9622. doi: 10.1038/s41598-022-13680-6.
- Petrone D, Mateo-Urdiales A, Sacco C, et al. Reduction of the risk of severe COVID-19 due to omicron compared to Delta variant in Italy (November 2021 – February 2022). Int J Infect Dis. 2023;129:135–141. doi: 10.1016/j.ijid.2023.01.027.
- Ward IL, Bermingham C, Ayoubkhani D, et al. Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with Delta (B.1.617.2): retrospective cohort study. The BMJ. 2022;378:e070695. doi: 10.1136/bmj-2022-070695.
- Maslo C, Friedland R, Toubkin M, et al. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 omicron wave compared with previous waves. JAMA. 2022;327(6):583–584. doi: 10.1001/jama.2021.24868.
- Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437–446. doi: 10.1016/S0140-6736(22)00017-4.
- Friis NU, Martin-Bertelsen T, Pedersen RK, et al. COVID-19 mortality attenuated during widespread omicron transmission, Denmark, 2020 to 2022. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2023;28(3):2200547. doi: 10.2807/1560-7917.ES.2023.28.3.2200547.