Abstract
Background
Peritonitis is a major cause of morbidity in peritoneal dialysis (PD) and an independent risk factor for elevated all-cause mortality. The aims of this study were to report the incidence, trend, aetiology, and antimicrobial susceptibility of PD-associated peritonitis and catheter-related infections in South Sweden between 2011–2020.
Methods
This population-based observational cohort study included all patients with PD between the years 2011–2020 in the county of Skåne. Data was accessed through the Swedish Renal Registry and the Department of Clinical Microbiology in Lund. Definitions issued by the International Society for Peritoneal Dialysis were implemented to assess PD-associated infections.
Results
Medical records of 675 paediatric and adult PD patients were eligible for inclusion. Of those, 208 (31%) were female and the median age was 67 years (range 0-91). The overall rate of PD-peritonitis was 0.38 episodes per year at risk. Out of 484 episodes of peritonitis, 61% (n = 295) were caused by Gram-positive bacteria. There were 289 occurrences of exit site infections, of which most (n = 152, 53%) were Gram-positive. Tunnel infections occurred in 16 episodes and were caused by S. aureus or P. aeruginosa. Among all isolates, 37 were of MRSE, four of ESBL-producing E. coli, and one of MRSA.
Conclusion
The crude rate of PD-peritonitis was stable during the study period. Gram-positive bacteria dominated the microbial aetiology, and antibiotic resistance was limited. It is important to monitor the aetiology, incidence, and resistance rates in PD-associated infections, to base empirical antibiotic regimens and facilitate prevention.
Introduction
The Global Burden of Disease Study group recognises chronic kidney disorders (CKD) as one of the leading contributors to global mortality and morbidity [Citation1–3]. Peritoneal dialysis (PD) has been adopted for clinical use since the 1950s, the first published trials dating back to as early as 1923 [Citation4,Citation5]. With proper implementation and management, PD entails multiple advantages over haemodialysis, such as fewer dietary restrictions and enhanced freedom and patient autonomy, since exchanges can be performed by the patient outside of health care facilities. One major drawback of PD is the risk of infectious complications, which can engage at three main sites: the peritoneum (peritonitis), the subcutaneous catheter pathway (tunnel infections), and the dermis surrounding the implanted catheter (exit site infections, ESIs). Infections arising from the tunnel or exit site are referred to as catheter-related infections (CRIs).
Infectious complications were, historically, the main hurdle to overcome before PD could be deemed an appropriate treatment alternative in uraemia [Citation4,Citation6]. To this day, peritonitis is a major cause of mortality in PD, and is an independent risk factor for elevated all-cause mortality [Citation7–9]. Adverse consequences include hospitalisation and catheter loss. In turn, CRIs are known to occasionally propagate along the catheter pathway and engage the peritoneal lining, resulting in subsequent peritonitis and associated complications [Citation10,Citation11].
The diagnostic gold standards for both peritonitis and CRIs are issued by the International Society for Peritoneal Dialysis (ISPD). Additionally, the ISPD provides guidelines suggesting optimal units of epidemiological measures, reporting frequency, as well as quality of care and outcome targets. Moreover, the guidelines include recommendations on evidence-based management, prevention, and mitigation [Citation12–14]. Non-compliance with the definitions proposed by the ISPD in the scientific community is an acknowledged issue, leading to inconsistencies across studies in the manner peritonitis is defined – either by explicitly introducing modifications to the ISPD criteria, or by not establishing a clear definition at all [Citation13,Citation15].
Most episodes of PD-associated peritonitis are caused by Gram-positive organisms, known to form biofilm on foreign bodies, including catheters. Peritonitis due to S. epidermidis, Streptococci, S. aureus and Enterococci spp. are reported to have the highest incidence, followed by the Gram-negative E. coli, Klebsiella spp. and Pseudomonas spp. Fungal peritonitis is relatively rare in PD, but is also burdened with a high mortality and morbidity compared to bacterial infections [Citation16].
Since there is an association between CRIs and subsequent occurrence of peritonitis, prompt management of exit site infections (ESIs) is recommended to prevent peritoneal engagement [Citation10,Citation11]. S. aureus and P. aeruginosa are two ESI pathogens associated with a greater incidence of subsequent involvement of the tunnel or peritoneum. It is especially important to ensure adequate ESI treatment and recovery in those instances, considering the risk of further complications [Citation17]. Additionally, peritonitis with more occult organisms (whether atypical or resistant to the preferred antimicrobial therapy) may pose an elevated risk for complications, as sufficient treatment can be delayed [Citation18].
To be able to monitor and timely promote preventive measures, it is important to report population-based incidences and trends of infectious complications of PD. Data from the past decades have indicated a steady decline in global peritonitis incidence, a trend assigned to evolving evidence-based management and therapeutic innovations, such as the introduction of technological solutions like the Y-set and twin bag systems [Citation19–22]. However, there is a lack of well-designed, population-based studies reporting the incidence and trend of peritonitis incidence in different settings, and the main risk factors of peritoneal peritonitis remain elusive. Consistency in monitoring and reporting of PD-peritonitis incidence and aetiologies is of importance, to be able to implement swift and appropriate measures, and to facilitate the implementation of proper empirical antimicrobial therapy.
To gain more knowledge of PD-peritonitis in our setting, the aims of this study were to report the incidence, trend, aetiology, and antimicrobial susceptibilities in PD-associated infections in South Sweden between 2011–2020.
Methods
Study design, setting and definitions
This population-based observational cohort study included all patients with peritoneal dialysis between the years 2011–2020 in the county of Skåne, south Sweden. There are ten hospitals in Skåne, of which five provide peritoneal dialysis. The county has a population of nearly 1.4 million people, and all PD patients in the region obtain a basic understanding of PD physiology and practical knowledge of proper exchange techniques through a training programme upon PD commencement. There are also patients that receive various degrees of assistance with their PD, either from a qualified medical professional or from a relative that has completed the training programme along with the patient. All patients that had been registered in the Swedish Renal Registry and treated with PD during the study period in Skåne were screened for inclusion. Exclusion criteria were missing medical records, completion of PD prior to 2011, PD commencement following 2020, and PD treatment received at centres beyond the geographic scope. PD-associated infectious complications documented in the medical records were assessed in accordance with the ISPD definitions and diagnostic criteria, applying the 2022 updated guidelines for peritonitis, and the 2017 updated guidelines for CRIs [Citation12,Citation13] ().
Table 1. Description of implemented terminology, as adapted from the International Society for peritoneal dialysis. [Citation12,Citation13].
Data sources
Data (personal identification numbers) was accessed through the Swedish Renal Registry (SRR), a nationwide database and quality registry for nephrology in Sweden since 1991. The Department of Clinical Microbiology in Lund is responsible for all microbiological diagnostics in the region covering both public and private clinics, including hospitals, out-patient clinics, and primary care. Medical records were reviewed using the software Melior (Melior, Siemens Healthcare Services, Upplands Väsby, Sweden). Laboratory Registers at the Department of Clinical Microbiology, Region Skåne, were accessed through Laboratory Information Management System ww-lab (Autonik, Nyköping, Sweden).
Data variables
All medical records were reviewed according to a predefined study protocol, including data on age, sex, symptoms, vital signs, biomarkers including c-reactive protein and white blood-cell count, comorbidities, immunosuppression, radiological imaging, microbiological aetiology, antimicrobial therapy. Comorbidities were assessed using the Charlson Comorbidity Index (CCI), the total score modified with no allocation of points for age or history of CKD [Citation23].
Microbiology
During the period investigated in this study, blood culturing was done using BacT/Alert 3D (Biomerieux) until December 2012, and BACTEC FX (Becton Dickinson) from that point onwards, using a 5-day incubation standard and both anaerobic and aerobic bottles used in pairs being the standard for blood cultures. Sterile biopsies and fluids other than blood were primarily cultured with direct streaking on agar plates (blood agar in CO2, fastidious anaerobe agar under anaerobic conditions), combined with tryptic soy broth, as well as other selective or non-selective media at the discretion of the clinical microbiologist responsible. Standard incubation time for sterile non-blood samples was seven days. Antibiotic susceptibility testing was performed with disc diffusion using the EUCAST breakpoints as the main method, with gradient tests as a backup and complement.
Statistical analysis
Numerical data is presented with medians, means and interquartile range (IQR), and qualitative data as proportions (%). Incidence of peritonitis is presented as episodes per patient year. Time at risk refers to the interval between the first PD exchange performed with the intention of continuing long-term dialysis therapy, and the final day of PD. Episodes occurring with an idle catheter, prior to the day of PD commencement, are reported as pre-PD peritonitis and do not contribute to incidence measures, as these episodes are outside of the time at risk [Citation13,Citation24]. Incidences of CRIs are sex- and age-standardized according to the population of Skåne in 2020 and reported as number of episodes per unit of time, without consideration of patient years [Citation12]. All statistical analyses were performed using Prism version 7 (GraphPad).
Results
Patient characteristics and details of chronic kidney disease
A total of 709 patients were identified through the SRR, and 675 were finally included for analysis (). Of the 675 patients, 31% (n = 208) were female, and the median age was 67 years (range 0-91) (). The median modified CCI was 1 (range 0-9). Fourteen patients (2%) were <18 years of age at the time of PD commencement. The mean BMI was 26.9 (range 15.1-44.5), and 57% (n = 385) of patients were overweight or obese (BMI above 25) at time of PD commencement. One third of the patients (n = 225) had automated peritoneal dialysis (APD) as part of their dialysis regimen whereas 60% (n = 405) were reported to only use manual exchanges (CAPD). For 7% of patients (n = 45), no information regarding type of PD was found. The median duration from catheter insertion to dialysis commencement was 32 days (range 0–2000). Furthermore, 54% of the patients (n = 366) had an idle catheter for more than four weeks, and 4% (n = 24) carried an idle catheter for one whole calendar year or longer. The primary causes of renal failure in the study population were diabetes mellitus (n = 193, 29%), glomerulonephritis (n = 130, 19%), hypertension (n = 119, 18%), and polycystic kidney disease (n = 56, 8%) ().
Figure 1. Flowchart of steps of inclusion.
aPatients that received PD at centres outside of the county of Skåne, Sweden.
bPatients that received PD before 1 January 2011 or after 31 December 2020.
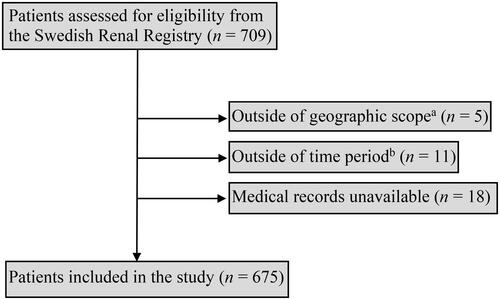
Table 2. Baseline characteristics of study population at PD commencement.
Table 3. Aetiology of end-stage renal disease necessitating renal replacement therapy.
Peritonitis episodes and recurrences
During the study time, 40% (n = 273) of PD patients suffered from a total of 484 episodes of PD-associated peritonitis. Of the affected patients, 175 (64%) experienced one peritonitis episode, 46 patients (17%) experienced two episodes, 26 patients (10%) had three episodes and 14 patients (5%) had four episodes. Twelve patients (4%) were affected by at least five episodes each during the study time.
Out of the 400 patients that commenced and completed their entire PD treatment within the decade-long surveillance window, 174 (44%) were affected by at least one episode of peritonitis, and remaining 56% of patients experienced no peritonitis complicating their time on PD. On average, 80% of patients remained peritonitis-free each calendar year.
The median time to the first peritonitis episode was 11.6 months (IQR 13.5). Only four occurrences of pre-PD peritonitis and seven episodes of catheter-insertion peritonitis were found, affecting 1.3% of catheter insertions between 2011–2020. There were 21 episodes of peritonitis recurrences (4% of all reported episodes), and 26 (5%) relapse peritonitis episodes.
Peritonitis incidence
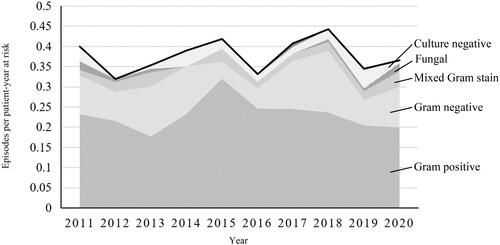
The overall PD-peritonitis rate was 0.38 episodes per year at risk, with the incidence trend remaining stable across the decade (). Incidence was highest for patients of age 0-10 years, at 0.66 episodes per year at risk for the five patients that were 10 years old or younger (). For adults, the incidence was highest between ages 71-80 years old, at 0.42 episodes per year at risk. Four patients received PD between the age of 91-100 years old and had the lowest age-specific peritonitis incidence of 0.14 episodes per year at risk. The mean age- and sex- adjusted rate of PD-associated peritonitis in the entire county was 3.83 episodes per 100,000 person years (95% CI 2.75-4.91) ().
Figure 2. A. Distribution of peritonitis in relation to age groups.B. Peritonitis incidence among age groups.
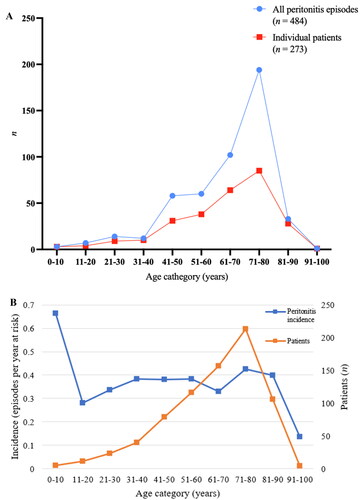
Table 4. Annual sex- and age-standardised rates of PD-peritonitis.
Occurrence and incidence of CRIs
In total, 289 exit-site infections were identified in 168 (25%) of the included PD patients, an average crude rate of 28.9 ESI episodes per year. Of the affected patients, 99 (59%) had a single episode of ESI reported, 39 patients (23%) had two episodes reported, 17 (10%) experienced three occurrences of ESI, and the remaining 14 PD patients (8%) had at least four episodes of ESI each.
In total, 16 cases of PD-related tunnel infection were reported in 13 patients, i.e. an annual average of 1.6 tunnel infections. For two patients, multiple episodes of tunnel involvement were reported (two and three, respectively), remaining 11 patients experienced one tunnel infection.
Guideline compliance
During the study period, a total of 577 episodes of diagnosed and managed peritonitis episodes were documented in the medical records, of which 484 (84%) fulfilled the diagnostic criteria of the ISPD guidelines; 93 (16%) episodes did not meet the criteria. The main reason of unfulfilled criteria was the presence of typical symptoms of PD-peritonitis (abdominal pain, cloudy effluent) combined with negative culture and either negative or no WBC count on effluent (52 episodes, 56%). In 24 (26%) cases, peritonitis was diagnosed due to positive cultures in absence of a positive WBC count and typical symptoms. The remaining five (5%) episodes were diagnosed due to a positive WBC count, but lacked typical symptoms and were negative on culture.
Out of the 473 reported cases of ESI, 289 (61%) were congruent with the diagnostic recommendations, i.e. exhibited presence of purulent discharge. The main rationale for non-compliance with the ISPD diagnostic criteria was erythema of the exit site without discharge (applies to 96 episodes, 52%). In 25 cases (14%), the ESI diagnosis was set due to positive exit site culture during an ongoing peritonitis episode, without adequate symptoms from the exit site. For the remaining 63 episodes (34%), there was no, or insufficient, mention of clinical presentation provided in the medical records.
Microbiological aetiologies of PD-peritonitis
Out of 484 episodes of PD-associated peritonitis, 61% (n = 295) were caused by Gram-positive bacteria only, 25% (n = 120) were Gram negative, and in 6% (n = 30) of cultures bacteria from both groups on the Gram stain were present. Culture-negative peritonitis constituted 6% (n = 29) of all episodes during the study period.
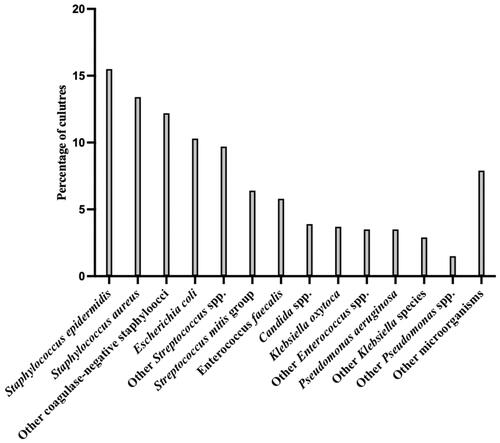
The most frequently found genus was Staphylococcus (n = 199, 41%), of which S. epidermidis (n = 75, 16%) and S. aureus (n = 65, 13%) were most common (). Streptococci were the second most common (n = 77, 16%), of which S. mitis (n = 30, 6%) and S. salivarius (n = 20, 4%) were most frequent, while 2% (n = 10) consisted of β-haemolytic Streptococci. Additionally, Escherichia coli (n = 50, 10%), Klebsiella spp. (n = 32, 7%) and Enterococcus faecalis (n = 28, 6%) were also often occurring on cultures. Candida spp. was found in 4% (n = 19) of peritonitis episodes, with C. albicans cultured in 2% (n = 12) of cases. A single episode of peritonitis caused by M. tuberculosis was identified (Supplementary Table 1).
E. coli, Enterococci, and Pseudomonas spp. were relatively less common in early PD-associated peritonitis, i.e. within the first six months of PD commencement, than in later episodes. Similarly, Staphylococci were more frequently found in late peritonitis (44% of episodes) than in early episodes (41%), especially Coagulase-negative Staphylococci (25% and 31% of cultures in early and late PD-associated peritonitis, respectively). S. aureus, however, was slightly more frequent in early PD-associated peritonitis (16%), compared to late episodes (13%). Bacteroides and Proteus spp. were only found in late PD-associated peritonitis in this study (3% and 2% respectively). Culture-negative peritonitis was more frequent during the first six months of PD (14%) than later (4%). As were Klebsiella spp., which were found in 9% of early, and 6% of late episodes ().
Table 5. Microbiology findings related to early PD-peritonitis (within the first six months after PD commencement) or late-onset peritonitis, i.e. six months after PD commencement.
Microbiological aetiologies of PD-associated ESI
Out of 289 episodes of PD-associated exit site infections, the aetiology was Gram-positive bacteria in 53% (n = 152) of the episodes, Gram negatives in 34% (n = 97), and in 5% (n = 13) both Gram-positive and Gram-negative bacteria were found. Further, 6% (n = 16) of all ESIs were culture negative.
The most common ESI pathogens were Staphylococcus spp. (n = 150, 52%), dominated by S. aureus (n = 142, 49%), and coagulase-negative staphylococci (CoNS) found in 3% (n = 10). Other important pathogens were P. aeruginosa (n = 39, 13%) and Klebsiella spp. (n = 22, 8%), with species found K. pneumoniae (n = 9, 3%), K. oxytoca (n = 5, 2%), and K. aerogenes (n = 3, 1%) (Supplementary Table 2). A single culture of C. parapsilosis was identified. In contrast to peritonitis and tunnel infections, for some exit site cultures the isolates were not always reported at the species level, but as “skin flora” or “mixed flora” instead – those unspecified cultures constituted 3% (n = 10) of ESIs. Associated peritonitis occurred in 2% (n = 5) of ongoing ESI episodes.
Microbiological aetiologies of PD-associated tunnel infections
A total of 16 episodes of tunnel infections were found in the study, with S. aureus and P. aeruginosa identified in 63% (n = 10) and 38% (n = 6) of the cultures, respectively. In two episodes of tunnel infections – one with S. aureus, and one with P. aeruginosa – associated peritonitis occurred simultaneously.
Blood cultures
Blood cultures were collected in 243 episodes of ongoing peritonitis, and 8% (n = 20) were reported positive. Median time to detection (TTD) was 18.4 h for positive aerobic bottles and 10.6 h for positive anaerobic bottles. Out of the 20 positive blood cultures, CoNS were found in seven (35%). Ten peritonitis episodes exhibited concordant blood and dialysate pathogens on cultures, of which four consisted of E. coli (). Blood cultures were collected in eight episodes of CRI, all of which were negative.
Table 6. Peritonitis episodes with positive blood cultures.
Antimicrobial susceptibility testing
A single case of methicillin resistant Staphylococcus aureus (MRSA) was reported in an episode of ESI, but no cases were found in patients with peritonitis. In total, 37 strains of methicillin resistant Staphylococcus epidermidis (MRSE) were identified, one in ESI and the remaining in strains associated with peritonitis. Four cases of ESBL-producing E. coli were identified, of which three were identified in peritonitis and one in an ESI. One case of peritonitis was caused by an isolate of E. coli with AmpC-production.
None of the S. aureus (n = 62) and CoNS isolates (n = 100) tested for vancomycin susceptibility exhibited resistance ( and Citation8).
Table 7. Antimicrobiological resistance in peritonitis.
Table 8. Antimicrobial resistance in exit site infections.
Ceftazidime resistance was detected in 8% (n = 14) of the 52 tested E. coli, 14% of Enterobacter spp. (14 tested), and 29% of Citrobacter spp. (14 tested). All examined Klebsiella, Pseudomonas, and Serratia spp. were ceftazidime susceptible.
For gentamycin, resistance was detected in 33% of 102 tested CoNS, 2% of S. aureus (63 tested), 8% of E. coli (52 tested), and 7% of Citrobacter spp. (14 tested). None of the tested Klebsiella, Proteus, Enterobacter, Serratia, nor Morganella spp. were gentamycin resistant.
For cefotaxime, 8% of E. coli (53 tested), 19% of Enterobacter spp. (16 tested), 2% of Klebsiella spp. (50 tested), and 4% of Citrobacter spp. (14 tested) were resistant.
Discussion
This population-based observational cohort study aimed to report the incidence, trend and aetiology of PD-peritonitis and catheter-associated infections in South Sweden between 2011–2020. We found a mean crude rate of 0.38 PD-peritonitis episodes per year at risk, and the mean time to the first peritonitis episode was close to fourteen months. The rate of PD-peritonitis was stable during the study period and Gram-positive bacteria dominated the microbiological aetiology of peritonitis and CRIs, Staphylococci in particular.
For adult patients, the age-specific incidence started low, with 0.28 episodes per year at risk between ages 21-30 years, remained stable for patients aged 31–70 years at an average of 0.36 episodes per year at risk, and peaked to 0.42 episodes per year at risk in patients of ages 71–90. The age-specific incidence was highest and lowest at the far extremes. Those groups were, however, very small, with only five patients in the 0–10 years of age group, and four in the 91-100 years group.
Our results agree with previously published results, as the overall incidence of PD-peritonitis based on registries across the globe has been estimated to 0.3 episodes per patient year [Citation25]. However, PD-incidence rates vary across the globe ranging from 0.75 episodes per year at risk in the African region, 0.38 in the United Kingdom, and 0.26 in the United States [Citation26–29]. If these discrepancies are attributed to differences in risk factors, such as patient demographics or clinical characteristics, or infection prevention and control measures, or differences in definitions, monitoring, or reporting, is yet to be determined. Although the patients in our cohort were older (median age 67 years) compared to these studies, it is precarious to compare crude incidence rates between different populations as the populations are heterogeneous, with different comorbidities and aetiology of chronic kidney disease. Also, different studies use different patient populations, definitions and outcomes, and the lack of sex- and age-standardization also hampers such comparison.
Education of patients and staff on the diagnosis and prompt management of peritonitis and CRIs may be vital in the outcome, and symptom awareness influence the crude incidence of peritonitis. In our study, a minority of cases (16%) were managed as PD-peritonitis even though they failed to comply with the current ISPD definitions and diagnostic criteria. Although absence of clinical information in medical journals does not automatically mean that clinical symptoms were absent, overestimating infectious complications to peritoneal dialysis leads to overuse of antimicrobial treatment and further resistance. Compared to other studies, the rate of antimicrobial resistant bacteria, such as MRSA and ESBL-producing bacteria, was however low in our study indicating that our setting is low-endemic for these bacteria.
The overall microbiological aetiology in our study was dominated by Gram-positive bacteria, in agreement with previous studies. Over the past decade, inconsistent results of analyses have pointed to Gram-positive peritonitis constituting between 37% and 62% of cases in the North America and Europe regions – with estimates from Spain and UK of 56% and 38%, respectively [Citation28–31]. Similarly, the rate of Gram-negative peritonitis ranges between 13% to 31% across previous reports, in line with our results (24.8% Gram-negatives) [Citation16,Citation29,Citation32,Citation33].
The incidence rate of peritonitis was stable during the study period, which is consistent with previous studies [Citation34–36]. However, to the best of our knowledge, recent estimates of PD-related infectious complications are rare in the literature, highlighting the need for continued monitoring and reports.
Our results show that, for PD-related peritonitis in our setting, the current empirical treatment of vancomycin and tobramycin is appropriate and provides coverage of nearly all pathogens cultured. This may be preferable to regimens that rely on ceftazidime. It is important to be vigilant of the results of both blood and peritoneal fluid cultures, narrowing down the antimicrobial treatment if possible.
The diagnostic yield of blood cultures was low, as only 8% (n = 20) of blood cultures collected in confirmed peritonitis cases were positive. Also, seven of these positive blood cultures identified CoNS, which could be attributed to true infection, or dermal contamination at the time of sample collection. Considering that only one of those peritonitis episodes was caused by CoNS (considering the results of peritoneal fluid cultures), and the remaining six episodes exhibited discordant pathogens on cultures, the true positivity rate of blood cultures was close to 6%. Still, obtaining blood cultures should be maintained in the workup of suspected cases of infectious complications to peritoneal dialysis, as this could give important information of aetiology and antimicrobial resistance. Also, if the primary suspicion of PD peritonitis is mistaken, blood cultures could reveal important pathogens related to a different infection.
The majority of peritonitis episodes with concordant blood and peritoneal fluid cultures were caused by E. coli. Conversely, 8% of all E. coli peritonitis exhibited bacteraemia with the same species. Two additional concordant cultures were positive for Gram-negative enteric organisms in blood (K. oxytoca and E. cloacae respectively). A feasible explanation could be that those instances were caused by enteric peritonitis, which the ISPD defined as peritonitis arising from an intestinal process as source, rather than the PD access itself. However, data on other co-occurring infections or diagnoses was not collected for this study, and as such it is not possible to confirm or further explore whether enteric peritonitis is more likely to cause bacteraemia.
Strengths and limitations
This study benefits from its population-based design and large cohort of patients included in the analysis. Moreover, the coverage and reporting degree of the SRR is nearly complete. The ISPD-issued diagnostic criteria were applied to each reported episode of peritonitis or catheter-related infection to assess for clinical practice compliance with international standards. We present detailed data on pathogen distribution, antibiotic susceptibility, and incidence of PD-associated infections. Limitations of the study include the retrospective study design, with risk of selection bias, as well as reporting bias, relying on registry data and medical records. We intend to further evaluate the outcome and risk factors for PD-peritonitis in our cohort in a future study. Continued reports of the aetiology, resistance rates and incidence of infectious complications to peritoneal dialysis are important, to base empirical antibiotic treatment and possible prevention of further episodes.
Conclusion
The crude rate of PD-peritonitis was stable during the study period and Gram-positive bacteria, in particular Staphylococcus species, dominated the microbiological aetiology of both peritonitis and catheter-related infections. Antimicrobial resistance was limited in this setting, although MRSE was found fairly frequently. It is important to monitor the aetiology and incidence of infectious complications to peritoneal dialysis, along antimicrobial resistance rates and patterns, to provide evidence-based empirical antibiotic regimens and mitigate or prevent subsequent complications.
Ethical approval
This study was granted ethical approval from the Ethical Review Board (DNR-2020-06524) and was conducted in compliance with the ethical principles adopted in the 2013 Declaration of Helsinki and the 2016 Declaration of Taipei.
Author’s contributions
MT, MS and OL conceived the study and performed data acquisition. Project administration was provided by OL. The methodology study design was finalised by MT, MS, GM and OL. Data curation, analysis and visualisation was performed by MT, TS and OL. The manuscript was initially drafted by MT and OL and critically revised by TS, MS and GM. All authors approved the final version of the manuscript.
Supplemental Material
Download MS Word (27.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Vos T, Abajobir AA, Abbafati C, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2.
- Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. The Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3.
- Cockwell P, Fisher L-A. The global burden of chronic kidney disease. Lancet. 2020;395(10225):662–664. doi: 10.1016/S0140-6736(19)32977-0.
- Stevens RE, Baskin S, Greene JA, et al. Peritoneal dialysis in the management of chronic renal failure. JAMA. 1964;190(13):1128–1130. doi: 10.1001/jama.1964.03070260040017.
- Palmer RA. As it was in the beginning: a history of peritoneal dialysis. Perit Dial Int. 1981;2(1):16–22. doi: 10.1177/089686088100200107.
- Feiman R, Mena Castro E, Gordillo-Paniagua G. [Chronic peritoneal dialysis in childhood]. Bol Med Hosp Infant Mex. 1981;38(3):415–424.
- Zhang Q, Ren H, Xie J, et al. Causes of death in peritoneal dialysis patients with different kidney diseases and comorbidities: a retrospective clinical analysis in a Chinese center. Int Urol Nephrol. 2014;46(6):1201–1207. doi: 10.1007/s11255-013-0561-5.
- Chen JHC, Johnson DW, Hawley C, et al. Association between causes of peritoneal dialysis technique failure and all-cause mortality. Sci Rep. 2018; 8(1):3980. doi: 10.1038/s41598-018-22335-4.
- Boudville N, Kemp A, Clayton P, et al. Recent peritonitis associates with mortality among patients treated with peritoneal dialysis. J Am Soc Nephrol. 2012;23(8):1398–1405. doi: 10.1681/ASN.2011121135.
- Lloyd A, Tangri N, Shafer LA, et al. The risk of peritonitis after an exit site infection: a time-matched, case-control study. Nephrol Dial Transplant. 2013; 28(7):1915–1921. doi: 10.1093/ndt/gft002.
- van Diepen AT, Tomlinson GA, Jassal SV. The association between exit site infection and subsequent peritonitis among peritoneal dialysis patients. Clin J Am Soc Nephrol. 2012; 7(8):1266–1271. doi: 10.2215/CJN.00980112.
- Szeto CC, Li PK, Johnson DW, et al. ISPD catheter-related infection recommendations: 2017 update. Perit Dial Int. 2017;37(2):141–154. doi: 10.3747/pdi.2016.00120.
- Li PK, Chow KM, Cho Y, et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022; 42(2):110–153. doi: 10.1177/08968608221080586.
- Wilkie M. The 2016 ISPD update on prevention and treatment of peritonitis-grading the evidence. Perit Dial Int. 2016; 36(5):469–470. doi: 10.3747/pdi.2016.00118.
- Sahlawi MA, Wilson G, Stallard B, et al. Peritoneal dialysis-associated peritonitis outcomes reported in trials and observational studies: a systematic review. Perit Dial Int. 2020; 40(2):132–140. doi: 10.1177/0896860819893810.
- Zelenitsky SA, Howarth J, Lagacé-Wiens P, et al. Microbiological trends and antimicrobial resistance in peritoneal dialysis-related peritonitis, 2005 to 2014. Perit Dial Int. 2017;37(2):170–176. doi: 10.3747/pdi.2016.00136.
- Holley JL, Bernardini J, Piraino B. Risk factors for tunnel infections in continuous peritoneal dialysis. Am J Kidney Dis. 1991; 18(3):344–348. doi: 10.1016/s0272-6386(12)80093-x.
- Cho Y, Struijk DG. Peritoneal dialysis–related peritonitis: atypical and resistant organisms. Semin Nephrol. 2017; 37(1):66–76. doi: 10.1016/j.semnephrol.2016.10.008.
- Szeto CC. Peritonitis rates of the past thirty years: from improvement to stagnation. Perit Dial Int. 2014;34(2):151–153. doi: 10.3747/pdi.2014.00007.
- Szeto CC, Li PK. Peritoneal dialysis-associated peritonitis. Clin J Am Soc Nephrol. 2019;14(7):1100–1105. doi: 10.2215/CJN.14631218.
- Daly C, Cody JD, Khan I, et al. Double bag or Y-set versus standard transfer systems for continuous ambulatory peritoneal dialysis in end-stage kidney disease. Cochrane Database Syst Rev. 2014;2014(8):CD003078. doi: 10.1002/14651858.CD003078.pub2.
- Daly CD, Campbell MK, MacLeod AM, et al. Do the Y‐set and double‐bag systems reduce the incidence of CAPD peritonitis? A systematic review of randomized controlled trials. Nephrol Dial Transplant. 2001;16(2):341–347. doi: 10.1093/ndt/16.2.341.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8.
- Li PK, Szeto CC, Piraino B, et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36(5):481–508. doi: 10.3747/pdi.2016.00078.
- Marshall MR. A systematic review of peritoneal dialysis-related peritonitis rates over time from national or regional population-based registries and databases. Perit Dial Int. 2021; 42(1):39–47. doi: 10.1177/0896860821996096.
- Okpechi IG, Ekrikpo U, Moloi MW, et al. Prevalence of peritonitis and mortality in patients with ESKD treated with chronic peritoneal dialysis in Africa: a systematic review. BMJ Open. 2020;10(12):e039970. doi: 10.1136/bmjopen-2020-039970.
- Bello AK, Okpechi IG, Osman MA, et al. Epidemiology of peritoneal dialysis outcomes. Nat Rev Nephrol. 2022; 18(12):779–793. doi: 10.1038/s41581-022-00623-7.
- Perl J, Fuller DS, Bieber BA, et al. Peritoneal dialysis-related infection rates and outcomes: results from the peritoneal dialysis outcomes and practice patterns study (PDOPPS). Am J Kidney Dis. 2020;76(1):42–53. doi: 10.1053/j.ajkd.2019.09.016.
- de la Espada Piña V, Ganga PLQ, Junquero JMG, et al. Two decades of analysis of peritonitis in peritoneal dialysis in andalusia: epidemiological, clinical, microbiological and progression aspects. Nefrologia. 2021;41(4):417–425. doi: 10.1016/j.nefroe.2021.10.004.
- Mujais S. Microbiology and outcomes of peritonitis in North America. Kidney Int Suppl. 2006;70(103):S55–S62. doi: 10.1038/sj.ki.5001916.
- Wang HH, Huang CH, Kuo MC, et al. Microbiology of peritoneal dialysis-related infection and factors of refractory peritoneal dialysis related peritonitis: a ten-year single-center study in Taiwan. J Microbiol Immunol Infect. 2019;52(5):752–759. doi: 10.1016/j.jmii.2018.10.013.
- dos Santos ACML, Hernandes RT, Montelli AC, et al. Clinical and microbiological factors predicting outcomes of nonfermenting gram-negative bacilli peritonitis in peritoneal dialysis. Sci Rep. 2021;11(1):12248. doi: 10.1038/s41598-021-91410-0.
- Kitterer D, Latus J, Pöhlmann C, et al. Microbiological surveillance of peritoneal dialysis associated peritonitis: antimicrobial susceptibility profiles of a referral center in Germany over 32 years. PLOS One. 2015;10(9):e0135969. doi: 10.1371/journal.pone.0135969.
- Zeng Y, Jiang L, Lu Y, et al. Peritoneal dialysis-related peritonitis caused by gram-negative organisms: ten-years experience in a single center. Ren Fail. 2021;43(1):993–1003. doi: 10.1080/0886022X.2021.1939050.
- van Esch S, Krediet RT, Struijk DG. 32 years’ experience of peritoneal dialysis-related peritonitis in a university hospital. Perit Dial Int. 2014; 34(2):162–170. doi: 10.3747/pdi.2013.00275.
- Zeng Y, Jiang X, Feng S, et al. The influence of seasonal factors on the incidence of peritoneal dialysis-associated peritonitis. Ren Fail. 2020;42(1):807–817. doi: 10.1080/0886022X.2020.1804401.