Abstract
Introduction
Cardiac surgery is required in up to half of the patients with infective endocarditis (IE). Positive valve cultures have been associated with higher in-hospital mortality. The aims were to identify risk factors for positive valve cultures and its relation to outcome.
Methods
Patients subjected to heart valve cultures due to surgery for IE in Skåne University Hospital, Lund, between 2012 and 2021 were identified through microbiology records. Risk factors for positive valve cultures and information on mortality and relapse were retrieved through medical records. Univariable and multivariable logistic regressions were performed.
Results
A total of 345 episodes with IE in 337 patients subjected to cardiac surgery were included and valve cultures were positive in 78 (23%) episodes. In multivariable logistic regression, preoperative fever (adjusted odds ratio (AOR) 2.6, 95% confidence interval (CI) 1.2–5.6, p = 0.02), prosthetic heart valve (AOR 3.3, CI 1.4–7.9, p = 0.01), a single affected valve (AOR 4.8, CI 1.2–20, p = 0.03), blood culture findings of S. aureus, enterococci, or coagulase negative staphylococci compared to viridans streptococci (AOR 20–48, p < 0.001), and a shorter duration of antibiotic treatment (p < 0.001), were associated to positive valve culture. One-year mortality was 13% and a relapse was identified in 2.5% of episodes. No association between positive valve cultures and one-year mortality or relapse was identified.
Conclusions
Positive valve cultures were associated to short preoperative antibiotic treatment, IE caused by staphylococci, preoperative fever and prosthetic valve but not to relapse or mortality.
Introduction
Infective endocarditis (IE) is a severe disease requiring cardiac surgery in up to 50% of all cases [Citation1]. The three main indications for surgery in the active phase of IE are heart failure, uncontrolled infection, and high risk of embolism [Citation2]. The optimal timing of surgery is unknown, but early surgical intervention has been indicated to be associated with lower risks of mortality [Citation3,Citation4]. Early surgical intervention also reduces the risk of embolic events and its complications [Citation3]. However, early surgical intervention leads to a shorter duration of preoperative antibiotic treatment which is associated with positive valve cultures [Citation5,Citation6]. When the timing of surgery is considered, a risk-benefit analysis for the individual patient should be performed by a multidisciplinary team [Citation2].
Patients with positive valve cultures after surgery have been suggested to have higher in-hospital mortality rate. Consequently, guidelines argue that a positive valve culture should lead to a new full course of treatment with antibiotics [Citation2,Citation7]. Prior studies have found shorter duration of preoperative antibiotic treatment and IE caused by Staphylococcus and Enterococcus species to be associated with positive valve cultures [Citation5,Citation6]. Large vegetations have also been associated with positive valve cultures [Citation8]. Relapse is rare in IE subjected to cardiac surgery, occurring in about 1% of the episodes. Previous studies suggest that the duration of postoperative antibiotic treatment does not affect the risk of relapse and positive valve cultures to be unhelpful in predicting relapse in IE [Citation9,Citation10].
To our knowledge, the association between positive valve cultures and other indicators for high activity of infection such as preoperative fever, number of embolic events and cardiac abscesses have not been investigated. The purpose of this study was to investigate whether such associations exist in our cohort and to evaluate the role of valve cultures as a prognostic factor in IE in relation to one-year mortality and relapse of IE.
Methods
Study population
Possible candidates for the study were found through searches of microbiological analyses performed by the Clinical Microbiology Laboratory at Region Skåne Medical Services, Lund. We included analyses performed between 2012 and 2021 at the request of the Department of Cardiothoracic surgery at Skåne University Hospital, Lund. Only patients subjected to valve surgery and with microbiological analyses of valve tissue were eligible.
Children below the age of 18, patients subjected to valve surgery without suspicion of IE, patients who underwent a full course of antibiotic therapy for IE prior to surgery, episodes defined as reject IE according to ESC 2015 criteria [Citation2] and patients with initial surgery at a different center were excluded. A similar study population, but with different exclusion criteria, has previously been presented where the potential usefulness of 16S rDNA gene PCR in IE surgery was described [Citation11].
The study was approved by the Swedish Ethical Review Authority (2021-05713).
Purpose and definitions
The primary purpose of this study was to investigate risk factors to predict valve culture positivity in patients undergoing surgery during active antimicrobial treatment for IE. The secondary purpose was to determine if valve culture positivity was predictive for poor outcomes defined as relapse in bacteremia or death and to describe risk factors for one-year mortality.
For these purposes, a retrospective review of medical records was performed. A predetermined protocol of variables was used to collect data about microbiological analyses (blood cultures, valve cultures and 16S-rDNA analysis), demographics, predisposing factors, comorbidities, data on presentation, treatment, work-up and outcomes. Using the ESC 2015 modified criteria [Citation2], all episodes were defined as definite, possible, or rejected IE. An updated version of Charlson comorbidity score was used to describe comorbidities [Citation12]. Valvular heart disease was defined as any form of congenital valve disease, moderate or severe valve stenosis or valve insufficiency known prior to the included episode. Persistent fever at the time of surgery was defined as temperature measurement above 38 °C within 48 h of the surgery. CRP at the time of surgery was defined as the CRP-measurement closest to the surgery, but not exceeding 48 h before the surgery. CRP-ratio was calculated as CRP at the time of surgery divided by the highest CRP recorded before the surgery during the same admission. CRP-ratio was used as an indication for remaining inflammatory activity at the time of surgery. Vegetation size was defined as the longest measurement of the largest visualized vegetation on echocardiography. Preoperative antibiotic duration was defined as the duration of treatment with an antibiotic effective against the causative agent. The number of embolic events were divided into groups due to high numbers of embolic events often not being described as a fixed number in the medical records. Surgical intervention was defined as any form of cardiothoracic surgery such as valve surgery, re-exploration, or surgical debridement, within the same hospital stay or within 30 days. Surgical intervention was further divided into reoperation and re-exploration. Reoperation was defined as another valve surgery related to the present episode within the same hospital stay or within 30 days. Re-exploration due to complications such as bleeding, tamponade, or myocardial complications where valve surgery was not performed was defined as re-exploration. Relapse was defined as bacteremia with the same causative agent as the previous after the end of antibiotic treatment for IE but within one year or relapse of IE within a year even if the agent was unknown. An episode of IE within a year but with a different causative agent was not considered as a relapse. Follow-up data about relapse and mortality was gathered for 365 days for all patients unless death occurred earlier. Events after day 365 were not recorded. If data was not available for the full 365 days, it was defined as missing.
Microbiological analysis of heart valves
Valve cultures are routinely performed in patients subjected to valve surgery due to IE at our center. All valve cultures included in the study were performed as part of the clinical routine at the Clinical Microbiology Laboratory at Region Skåne Medical Services, Lund. Tissue culture methods and 16S-rDNA gene PCR and sequencing at the Clinical Microbiology Laboratory at Region Skåne Medical Services, Lund were performed as described earlier [Citation11]. Growth of bacteria in the valve cultures was considered as positive findings if they were not regarded as contaminants. Valve cultures were defined as contaminated if a typical IE agent (Staphylococcus aureus, viridans streptococci, Streptococcus bovis, HACEK group or enterococci) was found in blood cultures with nontypical different agent in valve cultures, or if concordant findings of a causative agent in blood cultures and 16S-analysis did not match a positive finding in the valve cultures. If 16S analysis was not performed in a given episode, this was regarded as a negative 16S analysis.
Statistical analysis
All statistical analyses were performed in IBM SPSS Version 29.0 Armonk (NY). For analysis of patient characteristics and follow-up data, Mann–Whitney U-test was used in non-normally distributed continuous variables. Chi-square test and Fisher’s exact test were used for categorical variables, when appropriate. To examine factors associated with positive valve cultures a forward stepwise multivariable logistic regression was performed with the variables marked in . Episodes with missing data in any of the included variables were excluded from the regression analysis. Univariable logistic regression was performed to be able to compare crude odds ratio (COR) and adjusted odds ratios (AOR). No collinearity between the independent variables was found. Mortality was assessed with Kaplan-Meier curves and Cox regression. A stepwise forward approach was used in Cox regression with positive valve cultures being forced into the analysis. Kaplan-Meier curves were divided into positive and negative valve cultures and log-rank test was used for comparison. In regression analysis, no more than one variable per 10 outcomes were used in the final models. A p-value below 0.05 was considered to be statistically significant.
Table 1. Characteristics of episodes with negative and positive valve culture.
Blood culture findings were divided into six groups for the multivariable logistic regression. The reference group for the logistic regression was set to viridans streptococci combined with S. bovis, to be able to compare the findings with previous studies which used a similar reference group [Citation6]. The other groups were S. aureus, enterococci, Coagulase-negative staphylococci (CoNS), other agents and blood culture-negative endocarditis (BCNE). For the logistic regression, the duration of antibiotic treatment was divided into 5 groups of 3 days from 0-2 with the last group being 11 days or more.
Results
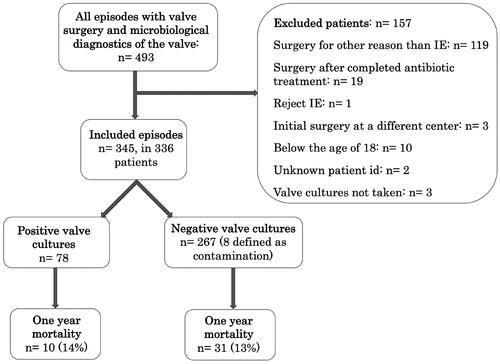
493 patients were eligible for the study and after exclusion of 157 patients, the final study population consisted of 336 patients with 345 episodes of IE (see for a study flow chart). Characteristics of the whole study population is presented in .
Microbiological findings in blood and valve cultures
Valve cultures were performed in all 345 episodes and blood cultures in 341 episodes. Eight valve cultures were defined as contaminated according to our criteria. Microbiological findings in these episodes can be seen in . Blood cultures and valve cultures identified a possible causative agent in 322 (94%) and 78 (23%) episodes, respectively. Viridans streptococci (28%), S. aureus (26%) and enterococci (11%) were the most common agents in blood cultures, whereas S. aureus (37%), enterococci (17%) and CoNS (15%) were the most common bacteria in valve cultures. A full description of the microbiological findings is presented in .
Table 2. Microbiological findings in blood and valve cultures.
Factors associated with positive valve cultures in univariable analysis
Characteristics of the infection and differences between episodes with positive and negative valve cultures in univariable analysis are presented in . Episodes of IE with positive valve cultures had a larger vegetation size on average, were more likely to have preoperative fever, had a lower median duration of antibiotic treatment, and were more likely to be caused by S. aureus, enterococci or CoNS.
Follow-up blood cultures were only performed in 145 episodes. Persistent bacteremia was significantly associated with positive valve culture in univariable analysis. In this subset of patients, the causative agent was not significantly associated with the outcome positive valve culture ().
Table 3. Episodes with follow-up blood cultures.
Factors associated with positive valve cultures in multivariable analysis
The results of multivariable logistic regression are shown in . Persistent bacteremia was not considered for inclusion since there was too much missing data. Five variables were included in the final model (duration of preoperative antibiotic treatment, preoperative fever, the presence of a prosthetic valve, the number of affected valves, and blood culture findings). In the model, 316 (92%) episodes with complete data across all the variables marked in were used. Missing data on vegetation sizes and CRP-ratios made up all of the 29 missing data entries. All five variables included in the final logistic model remained significantly associated with a positive valve culture (). Number of positive cases in each group of duration of antibiotic treatment can be seen in . The analysis was performed both with duration of antibiotic treatment in groups and as a continuous variable. presents the analysis with duration of antibiotic treatment as a continuous variable. The same variables remained associated with positive valve cultures with slightly more narrow confidence intervals.
Table 4. Multivariable analysis for positive valve cultures.
Follow-up data
Follow-up data in the whole study population as well as data divided into negative and positive valve cultures are presented in . Patients received a median duration of postoperative intravenous antibiotic treatment of 28 days with an interquartile range of 22–32. Information about relapse was only available in 276 and one-year mortality in 315 episodes due to that some patients received postoperative care at hospitals outside of the Region of Skåne. Further surgical interventions were performed in 40 (11%) episodes, and it was performed more frequently in NVC (13%) compared to PVC (6.4%), but the difference was not statistically significant. One-year mortality was seen in 41 (13%) episodes. One-year mortality was 14% and 13% respectively in patients with PVC and NVC, p = 0.72. Kaplan–Meier curves with one-year follow-up can be seen in . A total of seven (2.5%) episodes with relapse were found. Five (2.4%) episodes with NVC had a relapse and two (3.1%) with PVC. A description of relapses is presented in . Relapse of IE was only confirmed in three cases (1.1%). No significant association between the result of valve cultures and outcomes was found.
Figure 2. Kaplan–Meier curves presenting survival in patients with positive and negative valve cultures until one year after surgery.
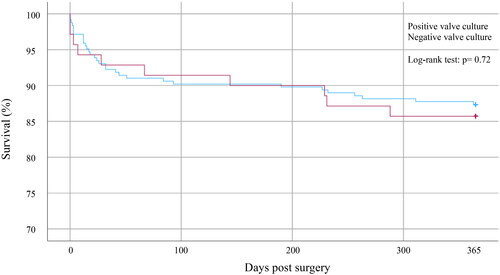
Table 5. Outcomes in episodes with negative and positive valve cultures.
Factors associated with one-year mortality
Differences in characteristics of the infection between patients who lived through the first year and those with one-year mortality are presented in . In univariable analysis, age, Charlson score, further surgical intervention and IE caused by S. aureus or CoNS were all associated with one year mortality. Five variables were included in the final model (age, Charlson score, blood culture findings, positive valve cultures, and further surgical intervention). All variables, except for positive valve cultures, were significantly associated with one-year mortality in multivariable analysis ().
Table 6. Characteristics of patients with one-year mortality and final mortality model.
Discussion
In the present study, blood cultures and valve cultures identified a possible causative agent in 94% and 23%, respectively. We found that preoperative fever; duration of preoperative antibiotic treatment; the presence of a prosthetic valve; the number of affected valves; and blood culture findings of S. aureus, enterococci, CoNS to be independently associated with positive valve culture. There was no association between valve culture results and outcomes. Age, comorbidities, further surgical intervention, and blood culture findings of S. aureus and CoNS were associated with one-year mortality.
Gisler et al. [Citation6] previously described Staphylococcus species and Enterococcus species to be associated with a higher probability of positive valve cultures when compared to viridans streptococci. Similar results were found in this study with S. aureus, CoNS, and enterococci all being associated with positive valve cultures when compared to viridans streptococci and S. bovis as the reference. Our decision to divide Staphylococcus species into S. aureus and CoNS, due to the difference in pathogenicity of the bacteria, did not seem to make a difference since both were associated with positive valve cultures compared to the reference group. One hypothesis prior to this study was that the association between causative agents and positive valve culture could be explained by the fact that patients with enterococci and S. aureus have a more rapid progression of the disease, which would lead to earlier surgery and shorter preoperative antibiotic treatment.
Persistent bacteremia was significantly associated with the outcome PVC and the causative agent in this subset of patients had no significant association with PVC. This result must be interpreted with caution since follow-up blood cultures were only performed in a minority of episodes. These episodes were most likely selected on a clinical basis and therefore not missing at random. Due to the high number of missing data and likelihood of bias this variable was not included in multivariable analysis.
In accordance with Gisler et al. [Citation6] and Mekontso Dessap et al. [Citation5], a shorter duration of preoperative antibiotic treatment was independently associated with positive valve culture. The chosen statistical method of analyzing duration of preoperative antibiotic treatment as an ordinal variable wrongly assumes all data in the same group to be the same while a duration of zero or two and three or five days of treatment might make a big difference for the outcome of the valve culture. Using the variable as a continuous variable would not necessarily be preferred since it wrongly assumes that the odds decrease equally as much per day of increased duration of treatment as Gisler et al. showed the early days of antibiotic treatment to be more impactful. Since both statistical methods have flaws, we decided to also present the analysis with antibiotic treatment as a continuous variable in the appendix.
Fillâtre et al. [Citation8] found vegetation size larger than 10 mm to be independently associated with positive valve cultures. In our univariable analysis of association between vegetation size as a continuous variable and a positive culture, the same association was found. However, the results could not be reproduced in multivariable analysis in the current study nor in the study by Fillâtre et al.
In contrast to the present study, Gisler et al. found prosthetic valves not to be independently associated with positive valve cultures. Surprisingly, neither other signs of high activity of the infection such as aortic root abscess formation nor a high number of embolic events were independently associated with positive valve cultures. Prior to the study, a high CRP-ratio was speculated to be an indication of high remaining activity of the infection, but CRP-ratio was not independently associated with positive valve cultures. However, in our study, preoperative fever was independently associated with positive valve cultures.
Halavaara et al. [Citation13] reported a one-year mortality rate slightly higher than in our present study in patients who were subjected to heart valve surgery due to IE. A difference in mortality between patients with a positive and negative valve culture has previously been reported [Citation6,Citation7] but such a difference was not evident in our population. Our follow-up data must be interpreted with caution due to missing data. However, our findings question the use of PVC as a prognostic factor in IE. This indicates that whether the valve culture is predicted to be positive or not, should not be a deciding factor in the decision of surgical timing. These findings in combination with the rarity of relapse suggest that patients with positive valve culture get sufficient antibiotic treatment with current guidelines. In accordance with Morris et al. [Citation9] our study could not find an association between positive valve cultures and relapse of IE. Duration of postoperative antibiotic treatment at the study site differs between patients with PVC and NVC. The duration of postoperative treatment in PVC is 4-6 weeks and in NVC patients finish their initially planned antibiotic treatment but get at least 2 weeks of postoperative antibiotic treatment. This might influence the fact that the outcomes did not differ in PVC and NVC in our study, but it also suggests that patients with PVC get sufficient treatment when compared to NVC. Duration of postoperative antibiotic treatment was not included in the mortality analysis because a majority of the mortality was seen during postoperative antibiotic treatment. As a consequence, duration of postoperative antibiotic treatment was cut short in these cases and this would introduce a strong bias into the analysis.
Previous findings of association between staphylococcal IE and mortality [Citation6,Citation7] were confirmed in our study in both univariable and multivariable analysis. Other factors such as age, comorbidities and further surgical intervention were also associated with one-year mortality in both univariable and multivariable analysis. Further surgical intervention leads to more risks and is often performed in patients with complicated infections. Previous findings of the association between timing of surgery and mortality could not be confirmed in our study [Citation3,Citation4]. Other factors indicating a severe infection such as embolic events, heart abscesses and large vegetation size were surprisingly not associated with one-year mortality.
The bacteria that were found in the valve cultures defined to be contaminated, were all agents where contamination is a possible explanation in the clinical setting. Cutibacterium acnes is however an agent seen both in IE and as a contamination [Citation14]. Several other bacteria that were defined as causes of IE in our cohort such as Rothia spp [Citation15]., Corynebacterium spp [Citation16]. and CoNS [Citation17] can also be found as contamination of blood cultures. The evaluation of a finding of such bacteria in blood cultures is therefore important and sometimes difficult to perform.
At the time of data gathering, this study used the ESC 2015 criteria [Citation2] to exclude patients defined as reject IE. Using the newly proposed 2023 Duke-ISCVID IE criteria [Citation18] would only have a minimal effect on the study population as the criteria for rejecting IE are similar to those used in this study. However, it could influence the valve cultures defined as contamination, due to the typical agents for IE being based on the ESC 2015 criteria. The two cases of C. acnes and one case of C. parapsilosis defined as contaminations, would be classified as a typical agent by 2023 Duke-ISCVID IE criteria in the setting of intracardiac prosthetic material. In one of the episodes where C. acnes was defined as contamination, a prosthetic valve was affected.
A strength of the study is the size of the study population. To our knowledge, it is the largest study population examining risk factors for positive valve cultures. All patients were subjected to surgery at the same hospital, serving a designated region. Patients were, however, included over a time period of 10 years and clinical management might therefore have differed between early and late episodes. The retrospective study design limits the possible conclusions drawn since no causality, only associations, can be demonstrated.
Conclusions
Our study confirms that short preoperative antibiotic treatment and IE caused by staphylococci are associated with positive valve cultures after IE-surgery and suggest that preoperative fever, prosthetic valve, and a single affected valve are also risk factors for having a positive valve culture. Our study could not confirm positive valve cultures to be associated with a higher mortality or a risk for relapse. Age, comorbidities, further surgical intervention, and IE caused by S. aureus or CoNS were all associated with a higher risk of one-year mortality.
Acknowledgments
We thank Dr Fredrik Kahn for input on statistical analyses, Dr Helena Lindberg for help with retrieving data on mortality, Mrs Emma Söderdahl for administrative help and Mrs Lena Hyllebusk for help with retrieving microbiological data. This research was supported by the Swedish governmental funds for clinical research to MR, by a postdoctoral grant from the Swedish Society for Medical Research and project grants from the Swedish Medical Association, the Thelma Zoégas foundation for medical research and the Mats Kleberg foundation to TS. A part of this article was presented at the ISCVID meeting at the University of Barcelona 18th–20th of June 2022 as an abstract.
Contributions
GJ contributed to the design of the study, gathered clinical data, performed statistical analyses, and drafted the manuscript. TS provided microbiological knowledge and contributed to the design of the study. PG reviewed echocardiograms where the size of vegetations were missing. SR provided cardiothoracic surgery knowledge and contributed to the design of the study. MR designed the study, provided tutoring, and revised the manuscript. All authors provided valuable critique on the manuscript and accepted the final version.
Disclosure statement
No potential conflict of interest was reported by the authors.
Appendix 1. Microbiological findings in episodes with valve cultures defined as contaminated.
Appendix 2. Number of PVC in each group of duration of antibiotic treatment.
Appendix 3. Univariable and stepwise forward multivariable logistic regression with duration of preoperative antibiotic treatment as a continuous variable.
Appendix 4. Cases defined as relapses with case description and information about antibiotic treatment.
References
- Tornos P, Iung B, Permanyer-Miralda G, et al. Infective endocarditis in Europe: lessons from the euro heart survey. Heart. 2005;91(5):571–575. doi: 10.1136/hrt.2003.032128.
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European society of cardiology (ESC)endorsed by: European association for Cardio-Thoracic surgery (EACTS), the European association of nuclear medicine (EANM). Eur Heart J. 2015;36(44):3075–3128. doi: 10.1093/eurheartj/ehv319.
- Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366(26):2466–2473. doi: 10.1056/NEJMoa1112843.
- Mahesh Anantha N, Toufik Mahfood H, Andre CK, et al. Early versus late surgical intervention or medical management for infective endocarditis: a systematic review and meta-analysis. Heart. 2016;102(12):950.
- Mekontso Dessap A, Zahar J-R, Voiriot G, et al. Influence of preoperative antibiotherapy on valve culture results and outcome of endocarditis requiring surgery. J Infect. 2009;59(1):42–48. doi: 10.1016/j.jinf.2009.04.009.
- Gisler V, Dürr S, Irincheeva I, et al. Duration of pre-operative antibiotic treatment and culture results in patients with infective endocarditis. J Am Coll Cardiol. 2020;76(1):31–40. doi: 10.1016/j.jacc.2020.04.075.
- García-Granja PE, López J, Vilacosta I, et al. Impact of valve culture in the prognosis of active left-sided infective endocarditis. Clin Infect Dis. 2018;68(6):1017–1023. doi: 10.1093/cid/ciy684.
- Fillâtre P, Gacouin A, Revest M, et al. Determinants and consequences of positive valve culture when cardiac surgery is performed during the acute phase of infective endocarditis. Eur J Clin Microbiol Infect Dis. 2020;39(4):629–635. doi: 10.1007/s10096-019-03764-z.
- Morris AJ, Drinković D, Pottumarthy S, et al. Bacteriological outcome after valve surgery for active infective endocarditis: implications for duration of treatment after surgery. Clin Infect Dis. 2005;41(2):187–194. doi: 10.1086/430908.
- Rao VP, Wu J, Gillott R, et al. Impact of the duration of antibiotic therapy on relapse and survival following surgery for active infective endocarditis. Eur J Cardiothorac Surg. 2019;55(4):760–765. doi: 10.1093/ejcts/ezy325.
- Johansson G, Sunnerhagen T, Ragnarsson S, et al. Clinical significance of a 16S-rDNA analysis of heart valves in patients with infective endocarditis: a retrospective study. Microbiol Spectr. 2023;11(3):e0113623. doi: 10.1128/spectrum.01136-23.
- Quan H, Li B, Couris CM, et al. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433.
- Halavaara M, Martelius T, Järvinen A, et al. Impact of pre-operative antimicrobial treatment on microbiological findings from endocardial specimens in infective endocarditis. Eur J Clin Microbiol Infect Dis. 2019;38(3):497–503. doi: 10.1007/s10096-018-03451-5.
- Boman J, Nilson B, Sunnerhagen T, et al. True infection or contamination in patients with positive cutibacterium blood cultures—a retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2022;41(7):1029–1037. doi: 10.1007/s10096-022-04458-9.
- Odeberg G, Bläckberg A, Sunnerhagen T. Infection or contamination with rothia, kocuria, arthrobacter and pseudoglutamicibacter-a retrospective observational study of non-Micrococcus micrococcaceae in the clinic. J Clin Microbiol. 2023;61(4):e0148422. doi: 10.1128/jcm.01484-22.
- Rasmussen M, Mohlin AW, Nilson B. From contamination to infective endocarditis-a population-based retrospective study of corynebacterium isolated from blood cultures. Eur J Clin Microbiol Infect Dis. 2020;39(1):113–119. doi: 10.1007/s10096-019-03698-6.
- Finkelstein R, Fusman R, Oren I, et al. Clinical and epidemiologic significance of coagulase-negative staphylococci bacteremia in a tertiary care university israeli hospital. Am J Infect Control. 2002;30(1):21–25. doi: 10.1067/mic.2002.118406.
- Fowler VG, Durack DT, Selton-Suty C, et al. The 2023 Duke-International society for cardiovascular infectious diseases criteria for infective endocarditis: updating the modified duke criteria. Clin Infect Dis. 2023;77(4):518–526. doi: 10.1093/cid/ciad271.