Abstract
Introduction
Epidemiological data on extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales are most often based on microbiological laboratory isolates and do not consider important clinical data such as infection or colonisation, treatment, and outcome. This study aimed to assess prevalence of ESBL-producing Enterobacterales as the cause of infection in patients with suspected sepsis in the emergency department based on clinical data. It also examined the number of patients with suspected sepsis who had ESBL-producing pathogens, comparing estimates that were based on laboratory data versus a combination of laboratory and clinical data.
Methods
Patients with suspected sepsis in the emergency department at Skåne University Hospital, Lund, Sweden were included consecutively. Data were collected retrospectively from medical records.
Results
Of the 764 included patients, 223 patients had growth of Enterobacterales in any specimen (i.e. colonisation or infection according to laboratory data), while 191 patients had Enterobacterales detected in the blood or in the suspected focus of infection (i.e. an infection according to clinical and laboratory data). Eighteen patients had ESBL-producing Enterobacterales in any clinical specimen, 11 of whom had an infection with ESBL-producing Enterobacterales, resulting in a prevalence of infections with ESBL-producing Enterobacterales in infected patients with suspected sepsis of 1.8%. The number of patients with ESBL-producing Enterobacterales was not significantly different when infection was defined using laboratory data alone versus a combination of laboratory and clinical data [18/223 (8.1%) vs 11/191 (5.8%), p = 0.36].
Conclusions
The prevalence of ESBL-producing Enterobacterales infections among patients with suspected sepsis is low in an acute care setting in Sweden.
Introduction
A total of 5869 cases of extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales in clinical isolates were reported in Sweden 2020. This number was 18% lower than the previous year, which could presumably be attributed to measures to prevent the spread of COVID-19 during the pandemic resulting in less infections with ESBL-producing Enterobacterales as well but also less sampling. Before 2020, the prevalence of ESBL-producing Enterobacterales increased steadily [Citation1]. According to the Public Health Agency of Sweden and the National Veterinary Institute, Escherichia coli (E. coli) was the most common ESBL-producing Enterobacterales in Sweden with 6.2% of E.coli isolated from human urine samples and 7.6% of E. coli isolated from human blood samples being ESBL-producing in 2019 [Citation2].
Sepsis caused by ESBL-producing Enterobacterales is associated with a higher mortality compared with sepsis caused by non-ESBL-producing Enterobacterales, although the effect is lessened when the data are adjusted for inadequate empirical therapy, suggesting that the resistance to antibiotics is the main mediator of this increased mortality [Citation3]. Unnecessary broad antimicrobial therapy poses several risks which include increasing antimicrobial resistance, Clostridium difficile infections, kidney injury, and allergic reactions [Citation4]. Local information about resistance patterns is needed to guide the initial choice of antimicrobial therapy [Citation5]. Because ESBL findings in bacterial cultures are subject to mandatory reporting in Sweden, good epidemiological data on their prevalence in specimens exist. However, these reports seldom include important clinical data, as they report isolates from clinical samples from blood, cerebrospinal fluid, and urine and do not consider important aspects of the difference between infection and colonisation [Citation2]. The World Health Organisation (WHO) acknowledges this gap and calls for high quality data on sepsis and antimicrobial resistance epidemiology, and emphasises clinical chart review as the gold standard [Citation6].
This study aimed to use clinical data to assess the prevalence of ESBL-producing Enterobacterales infections in patients with suspected sepsis in the emergency department. Further, this study determined the number of patients with suspected sepsis in the emergency department who harboured ESBL-producing pathogens in estimates based on laboratory data compared with a combination of laboratory and clinical data. Finally, we examined whether ICD codes can be used to capture ESBL-producing Enterobacterales infections. Because information on the prevalence of ESBL-producing pathogens has implications for the widespread use of antibiotics, we also mapped initial antimicrobial therapies.
Materials and methods
Data were collected retrospectively from the medical records of consecutive patients admitted to the emergency department at Skåne University Hospital, a tertiary hospital in Lund, Sweden. Criteria for inclusion were an age of at least 18 years and meeting the Sepsis Alert criteria, described below, which is used routinely in the emergency department at Skåne University Hospital for patients with suspected sepsis. The criteria for a Sepsis Alert are:
The highest triage level according to the Rapid Emergency Triage and Treatment System (RETTS), which entails fulfilling at least one of the following:
A respiratory rate higher than 30 breaths/min, or lower than 8 breaths/min
An oxygen saturation of less than 90%
A regular heart rate over 130 beats/min, or an irregular heart rate over 150 beats/min,
A systolic blood pressure under 90 mmHg
Active seizure, or the patient being unconscious (defined as a Glasgow coma score of 9 or lower)
and
Fever of at least 38 °C, OR a history of fever or chills [Citation7].
All patients meeting the criteria for a Sepsis Alert, are cared for immediately in the emergency room in the presence of an emergency physician, infection consultant, nurse, and assistant nurse. All Sepsis Alert patients are also subjected to blood sampling for biochemical and microbiological analyses, other microbiological sampling, control of vital parameters, clinical examination, and consideration of antimicrobial therapy.
Patients were included prospectively between the 13th of April 2017 to the 11th of February 2018 and again from 1st of October 2019 to the 31st of December 2019. Informed consents were achieved through an opt out procedure. Patients were notified per mail within 8 weeks following the ED visit and had the opportunity to opt out of inclusion in the study by sending back a prefilled form in a pre-franked envelope. Ethical approval for the study was obtained from the regional ethics board (file numbers 2014/741 and 2016/271). Clinical data, including demographic characteristics, comorbidities, severity of disease, evidence of infection and sepsis, infection foci, antimicrobial treatment, microbiological results, and survival, were gathered retrospectively by reviewing the clinical charts between the 1st of June 2020 to the 5th of February 2021. After data collection, all data were anonymized.
Bacterial isolates were identified at Clinical Microbiology (Laboratory Medicine Skåne, Lund, Sweden) per standard clinical practice. Susceptibility and resistance of Enterobacterales to beta-lactam antibiotics were characterised phenotypically according to clinical practice for ESBL-A and ESBL-M. Enterobacterales isolates resistant to cefotaxime or ceftazidime are tested with combination disc diffusion test with discs containing cefpodoxime, clavulanic acid and cloxacillin [Citation8]. Infections were deemed to be caused by Enterobacterales if Enterobacterales grew either in blood cultures or cultures taken from the suspected site of infection according to the caring physician. Sepsis was defined using a Sepsis-3 definition which was modified for respiratory function in order to be applicable outside the intensive care unit (ICU), supplement I (supplementary material) [Citation9]. Organ dysfunction was defined as an increase in the total sequential organ failure assessment (SOFA) score of at least two. To assess respiratory function in the SOFA score, partial pressure of oxygen in arterial blood (PaO2) was preferably used. When not available, the oxygen saturation by pulse oximetry (SpO2) was used for values below 96% and PaO2 was calculated using the Severinghaus equation [Citation10]. Septic shock was defined according to the sepsis-3 definitions as having a lactate of at least 2 mmol/L and requiring vasopressors to keep the mean arterial pressure above 65 mmHg, despite adequate fluid resuscitation [Citation9]. We regarded missing values in the SOFA as within the range of values from adjacent days, since this is in accordance with clinical management and a common approach in retrospective sepsis studies. Any patient with an infection who did not meet sepsis or septic shock criteria as defined in this study was classified as having an infection without organ dysfunction. Initial antimicrobial therapy was classified according to the first dose that was received. If combinations of antimicrobial therapy were administered, all individual therapies were recorded. The adequacy of an antimicrobial therapy was assessed in infected patients, based on their antibiograms if available; otherwise, it was determined empirically [Citation11]. Risk factors for ESBL-producing Enterobacterales infection were colonisation with ESBL-producing Enterobacterales prior to admission and admission to a hospital abroad in the past 6 months [Citation12,Citation13]. Risk factors for severe disease were risk of septic shock (the need for vasopressor or >2000 mL intravenous fluid for mean arterial pressure (MAP) >70), neutropenia (neutrophil count <1.0 × 109/L), and immunocompromization (immunodeficiency or glucocorticoid use equivalent to 20 mg prednisolone or more per day, alkylating agents, cyclosporine, mycophenolate, sirolimus, tacrolimus use within the past 3 months, and receipt of immunosuppressive monoclonal antibodies within the past 6 months) [Citation9,Citation14].
Categorical variables are presented as frequencies and percentages, and continuous variables as medians. The groups were compared with Pearson’s χ2 or Fisher’s exact tests for proportions if an expected number was below 5, as appropriate, while the independent samples median test was used to compare medians. The data were analysed in SPSS version 27.00 statistical software.
Results
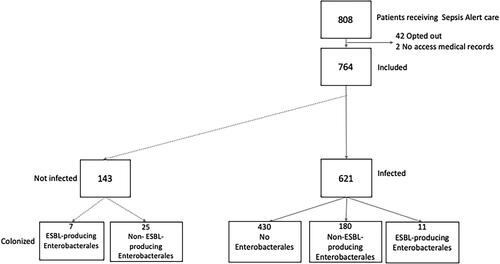
There were 808 patients treated as Sepsis Alerts in the emergency department at Skåne University Hospital in Lund, Sweden during the study period. Forty-two patients opted out of inclusion and 2 patients had inaccessible medical records. Of the 764 remaining patients, 621 had an infection (). When combining laboratory and clinical data, we found a prevalence of 1.8% of ESBL-producing Enterobacterales infections in infected patients with suspected sepsis in the emergency department: of the 191 patients with Enterobacterales infection, 11 (5.8%) had an infection caused by ESBL-producing Enterobacterales, which was not significantly different when compared with the number identified using laboratory data alone [18 (8.1%) with ESBL-producing Enterobacterales out of 223 with Enterobacterales in any specimen] (p = 0.36).
Out of the 180 patients with infections caused by non-ESBL-producing Enterobacterales, 27 (15.0%) had an infection without organ dysfunction, 153 (85%) had sepsis, and 11 (5.8%) had septic shock. Of the 11 patients with infections caused by ESBL-producing Enterobacterales, 1 (9.1%) had an infection without organ dysfunction and 10 (90.9%) had sepsis. shows the demographic and clinical characteristics for patients with ESBL-producing and non-ESBL-producing Enterobacterales and patients without Enterobacterales.
Table 1. Patient characteristics.
Two of the ESBL-producing bacteria were Klebsiella pneumoniae (K. pneumoniae) species and the rest were E. coli, whereas 118 of the non-ESBL-producing Enterobacterales were E. coli, 38 were K. pneumoniae and 10 were Enterobacter species.
Fourteen of all the patients included and seven of the patients with an infection caused by ESBL-producing Enterobacterales were known to be colonised with ESBL-producing Enterobacterales prior to admission. There were no carbapenemase-producing Enterobacterales found. The results of microbiological testing are presented in .
Table 2. Results of microbiological testing.
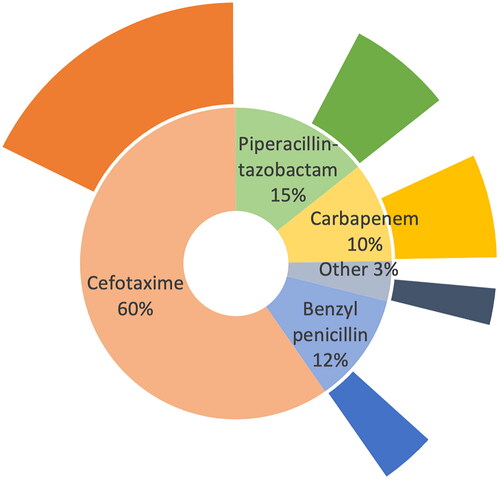
Median time from arrival in the emergency department to antibiotic administration was 27 min (interquartile range (IQR) 20–40). Initial antimicrobial therapy was most often cefotaxime, followed by piperacillin-tazobactam, benzyl penicillin, and carbapenems (, ). Of the patients with identified pathogens from the infection foci, 267 (80%) received effective antimicrobial therapy. All patients with infections caused by ESBL-producing Enterobacterales received intravenous antibiotics in the emergency department. Six patients with infections caused by ESBL-producing Enterobacterales received cefotaxime (including one patient who received metronidazole plus cefotaxime), two patients received piperacillin-tazobactam (including one patient who received levofloxacin plus piperacillin-tazobactam), and three patients received carbapenem. Three of the patients with infections caused by ESBL-producing Enterobacterales who received cefotaxime were known to be colonised with ESBL-producing Enterobacterales before arriving at the emergency department. shows the proportions of all patients with initial antimicrobial treatment in the various antimicrobial groups.
Figure 2. Proportion of patients with initial antimicrobial treatment in the various antimicrobial groups. The outer circle of the chart reflects the proportion of patients within each initial antimicrobial category fulfilling risk factors for resistant pathogen or severe disease, n = 764.
Only 3 out of the 621 patients with an International Classification of disease (ICD) code for infection had an ICD code for ESBL (0.5%) which is significantly lower than the 11 out of 621 (1.8%) patients with a clinical diagnosis of infection caused by ESBL-producing bacteria (p = <0.01).
Discussion
The aim of this study was to assess the prevalence of ESBL-producing Enterobacterales as the cause of infection in the emergency department based on clinical data. Out of all the infected suspected sepsis cases included in this study only 1.8% were caused by ESBL-producing Enterobacterales. We found that 8.1% of Enterobacterales found in any specimen were ESBL-producing (based on laboratory data alone) compared to 5.8% with infections caused by ESBL-producing Enterobacterales (based on both clinical and laboratory data). The difference in prevalence of infection with ESBL-producing Enterobacterales calculated based on laboratory data and calculated based on clinical and laboratory data was not statistically significant.
A lot is known about the epidemiology of ESBL-producing Enterobacterales in laboratory clinical isolates, but differences between infection and colonisation, treatment, and outcome are rarely considered—what little has been reported has been limited to bloodstream infections [Citation2,Citation15]. Several initiatives for surveillance of antimicrobial resistance data have been launched, such as the Central Asian and Eastern European Surveillance of Antimicrobial Resistance (CAESAR), European Antimicrobial Resistance Surveillance Network (EARS-Net), the Latin American Network for Antimicrobial Resistance Surveillance (ReLAVRA), and the Global Antimicrobial Resistance and Use Surveillance System (GLASS). These surveillance systems provide vast comparable data and contribute to the surveillance of antimicrobial resistance and emerging antimicrobial resistance, antimicrobial consumption, and attributable mortality. Yet, they are based principally on data on microbial isolates in clinical samples [Citation16,Citation17]. Routinely generated data are important for surveillance but need to be validated and nuanced in clinical studies, as was done in the present study [Citation6]. The 2019 data from EARS-Net demonstrate a prevalence of ESBL of 7.8% in invasive isolates of E. coli and 8.3% in invasive isolates of K. pneumoniae in Sweden; the higher rates compared to in our study might partly be due to the coverage of other departments other than the emergency department by EARS-Net [Citation17]. A Danish study screened all patients in emergency departments and found a prevalence of colonisation by ESBL-producing pathogens of 4.5%. Their higher prevalence, compared with the rates of ESBL-producing Enterobacterales in any specimen in the current study could be attributed to their screening including samples as rectal swabs, capturing colonisation that did not appear in clinical samples [Citation18].
ICD codes are intended for morbidity reporting and are extensively used for research internationally, but they identified only 0.5% of infected patients as having ESBL-producing bacteria, while the clinical data used in this study found that 1.8% of infected patients had ESBL-producing bacteria [Citation19]. This discrepancy suggests that ICD codes in administrative hospital discharge data cannot be used for reliable estimates of ESBL epidemiology.
The WHO has urged for the establishment of national processes to improve sepsis care. Sweden has addressed this call by developing a clinical pathway for sepsis that includes the implementation of sepsis alerts, with prompt initiation of antimicrobial therapy [Citation20]. Because the current study measured the prevalence of ESBL-producing Enterobacterales in patients who were included in these sepsis alerts, our results can be useful as guidance for treatment decisions. In Sweden, for community-acquired sepsis that is due to a suspected infection focus in either the urinary tract or the lungs – the two most common infection foci – empirical treatment includes cefotaxime [Citation21]. Cefotaxime resistance is present in Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus (MRSA), but these pathogens are uncommon in the emergency department—MRSA is much less frequent than ESBL-producing Enterobacterales, causing only 98 bacteraemic infections in Sweden in 2019, and Pseudomonas aeruginosa mainly causes nosocomial infections [Citation2,Citation22]. Accordingly, for suspected sepsis in Swedish emergency departments, initial treatment with broader spectrum antibiotics than cefotaxime is typically only indicated for ESBL-producing Enterobacterales, for certain infection foci that require broad-spectrum antibiotic therapy and in severe disease [Citation11]. This study implies that ESBL-producing Enterobacterales are uncommon as a cause of suspected sepsis in acute care in Sweden and that cefotaxime should be an adequate initial choice of treatment for most cases of suspected community-acquired sepsis in the emergency department, unless comorbidities or the focus of infection requires broad-spectrum antibiotic therapy or if there are risk factors for antimicrobial resistance.
Therefore, the initial use of carbapenems in this study is likely excessive [Citation11]. The risk of inadequate antimicrobial therapy needs to be weighed against the risk of unnecessarily broad antimicrobial therapy, which can promote antimicrobial resistance [Citation23]. Unnecessarily broad antimicrobial therapy is also associated with higher mortality, probably due to collateral damage [Citation24,Citation25]. Conversely, several patients with ESBL-producing Enterobacterales infections received inefficacious antimicrobial therapy, having been administered cefotaxime. Previous colonisation with third-generation cephalosporin-resistant Enterobacterales/ESBL-producing Enterobacterales, and hospitalisation abroad are risk factors for infection with third-generation cephalosporin-resistant and ESBL-producing Enterobacterales [Citation12,Citation13]. Finding risk factors that can be reliably used in the emergency department to predict infection with ESBL-producing Enterobacterales and using established risk factors or prediction scores for ESBL-producing Enterobacterales could improve accuracy in choosing the correct empiric antibiotic treatment and could be crucial in making better individualised decisions when selecting an antibiotic therapy.
The major weakness of this study is the small study size, which limits the comparison between laboratory and clinical epidemiological data on ESBL-producing Enterobacterales prevalence. A major strength of the study is the fact that we included a clinical chart review, something that is not typically included in similar studies perhaps due to its labour-intensiveness. A more efficient study design could be to combine data from the surveillance of antimicrobial resistance, like that of Swedres-Svarm, with a clinical chart review—i.e. performing a clinical chart review of the medical records of all patients with ESBL-producing bacteria in any clinical specimen. Using such clinical chart review to validate data on microbial isolates could yield more accurate estimates and in-depth knowledge of ESBL-producing Enterobacterales epidemiology.
Conclusion
Sepsis caused by ESBL-producing Enterobacterales is still rather rare in an acute care setting in Sweden. Although clinical data and laboratory isolates may yield varying estimates, the ESBL-producing Enterobacterales prevalence calculated using these two methods was not significantly different in this study. Due to their rarity, there is currently no need to routinely use broad-spectrum antibiotics with efficacy against ESBL-producing bacteria to treat suspected community-acquired sepsis, and an opportunity exists for more restrictive and targeted use of broad-spectrum antibiotics and antibiotic stewardship.
Supplemental Material
Download MS Word (15.2 KB)Acknowledgements
We thank Jane Fisher of AdvanSci Research Solutions and Sean Kim of Blue Pencil Science for editing this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- ESBL-producing Enterobacteriaceae. 2020 Sweden: public Health Agency; [cited 2021 Sep 20]. Available from: https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistik-a-o/sjukdomsstatistik/extended-spectrum-beta-lactamase-esbl/?p=93854#statistics-nav.
- Swedres-Svarm. Swedres-Svarm 2019. Sales of antibiotics and occurrence of resistance in Sweden. Solna/Uppsala. 2020.
- Rottier WC, Ammerlaan HSM, Bonten MJM. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum β-lactamase-producing enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother. 2012;67(6):1311–1320. doi:10.1093/jac/dks065.
- Tamma PD, Avdic E, Li DX, et al. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308–1315. doi:10.1001/jamainternmed.2017.1938.
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi:10.1007/s00134-021-06506-y.
- WHO. Global report on the epidemiology and burden of sepsis: current evidence, identifying gaps and future directions. Geneva: World Health Organization. 2020.
- Sepsis Sweden. Region Skåne; [cited 2019 Sep 19]. Available from: https://vardgivare.skane.se/vardriktlinjer/infektioner/ako/sepsis/#ICD-10-SE.
- NordicAST. v_9_0_brytningspunkttabell_nordicast_se_final190325_locked. Nordic Committee on Antimicrobial Susceptibility Testing. 2019.
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). Jama. 2016;315(8):801–810. doi:10.1001/jama.2016.0287.
- Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol Respir Environ Exerc Physiol. 1979;46(3):599–602. doi:10.1152/jappl.1979.46.3.599.
- Infektionsläkarföreningen S. Vårdprogrammet för sepsis och septisk chock. Svenska infektionsläkarföreningen. 2022.
- Holmgren A, Ljung A, Bremell D. An easy-to-use scoring system for predicting bacteraemia with third-generation cephalosporin-resistant enterobacterales in a low-resistance setting. Infect Dis. 2020;52(4):242–248. doi:10.1080/23744235.2019.1705389.
- Fröding I, Valik JK, Bolinder L, et al. Prediction of bloodstream infection caused by extended-spectrum β-lactamase-producing enterobacterales in patients with suspected community-onset sepsis. Int J Antimicrob Agents. 2019;53(6):820–829. doi:10.1016/j.ijantimicag.2019.02.008.
- Poutsiaka DD, Davidson LE, Kahn KL, et al. Risk factors for death after sepsis in patients immunosuppressed before the onset of sepsis. Scand J Infect Dis. 2009;41(6–7):469–479. doi:10.1080/00365540902962756.
- Institute PHAoSaNV. A report on swedish antibiotic sales and resistance in human medicine (swedres) and swedish veterinary antibiotic resistance monitoring (svarm). Solna/Uppsala; 2021.
- WHO. Global antimicrobial resistance surveillance system (GLASS) report: early implementation 2017-2018. Geneva: WHO. 2018.
- ECDC ECfDPaC. Antimicrobial resistance in the EU/EEA(EARS-Net). Annual epidemiological report 2019. Stockholm: ECDC. 2020.
- Skjøt-Arkil H, Mogensen CB, Lassen AT, et al. Carrier prevalence and risk factors for colonisation of multiresistant bacteria in danish emergency departments: a cross-sectional survey. BMJ Open. 2019;9(6):e029000. doi:10.1136/bmjopen-2019-029000.
- Jetté N, Quan H, Hemmelgarn B, et al. The development, evolution, and modifications of ICD-10: challenges to the international comparability of morbidity data. Med Care. 2010;48(12):1105–1110. doi:10.1097/MLR.0b013e3181ef9d3e.
- Strålin K, Linder A, Brink M, et al. Design of a national patient-centred clinical pathway for sepsis in Sweden. Infect Dis. 2023;55(10):716–724. doi:10.1080/23744235.2023.2234033.
- Infektionsläkarföreningen. Vårdprogram för sepsis och septisk chock. 2022.
- Moore NM, Flaws ML. Focus: pseudomonas aeruginosa. Epidemiology and pathogenesis of Pseudomonas aeruginosa infections. Clin Lab Sci. 2011;24(1):43–46. doi:10.29074/ascls.24.1.43.
- Holmes AH, Moore LS, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387(10014):176–187. doi:10.1016/S0140-6736(15)00473-0.
- Rhee C, Kadri SS, Dekker JP, et al. Prevalence of antibiotic-resistant pathogens in Culture-Proven sepsis and outcomes associated With inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open. 2020;3(4):e202899. doi:10.1001/jamanetworkopen.2020.2899.
- Webb BJ, Sorensen J, Jephson A, et al. Broad-spectrum antibiotic use and poor outcomes in community-onset pneumonia: a cohort study. Eur Respir J. 2019;54(1):1900057. doi:10.1183/13993003.00057-2019.