Abstract
Background
Veterinarians are at risk for numerous zoonotic infections. In this paper, we summarise descriptions of zoonotic infections from a questionnaire study and a series of work-related zoonotic cases, aiming to add to the knowledge on occupational zoonotic risks of veterinarians.
Methods
We collected data on zoonotic infections contracted by veterinarians in Finland in two studies:1) using a questionnaire in 2009, and 2) inviting veterinarians who had encountered an occupational zoonosis to report it in structured interviews in 2019.
Results and conclusions
In the questionnaire study in 2009, of 306 veterinarians several reported zoonotic bacterial skin infections (12%), dermatophytosis (ringworm; 4.2%), virus infections (3.9%), bacterial gastroenteritis (3.3%), other bacterial zoonoses (2.3%), and parasitic infections/infestations (2.3%). In the 2019 interviews, 16 occupational zoonosis cases were reported. Of them, seven were selected to the case series. The selected cases included Capnocytophaga canimorsus sepsis following a dog bite, cryptosporidiosis after a contact with calves, cutaneous listeriosis following calving assistance, Salmonella gastroenteritis contracted at laboratory, Trichophyton dermatophytosis after equine contact, Bacillus anthracis exposure at necropsy, and exposure to rabies through a horse bite. In four of the seven cases, the veterinarian disagreed or strongly disagreed with having had good knowledge of the zoonosis before the incident. The results from the questionnaire study and the case series illustrate the variety of zoonotic pathogens that veterinarians may encounter. There is a need to improve the occupational health of veterinarians and to increase awareness in the occupational health sector. We encourage addressing this need using a One Health approach.
Introduction
Veterinarians are commonly exposed to zoonotic pathogens at work [Citation1–5]. Globally, the zoonoses most frequently reported in veterinarians and veterinary students include campylobacteriosis, salmonellosis, brucellosis, cryptosporidiosis, bite infections, and ringworm [Citation1,Citation3,Citation6–10].
The wide variety of zoonotic risks is a challenge for occupational health care of veterinarians.
In Finland, the pathogens identified as main occupational zoonotic risks for veterinarians comprise Campylobacter spp., Capnocytophaga canimorsus, enterohaemorrhagic Escherichia coli, Listeria monocytogenes, Pasteurella spp., Salmonella spp., methicillin-resistant Staphylococcus aureus, Cryptosporidium spp., Toxoplasma gondii, and lyssaviruses (including rabies) [Citation11]. The country is officially free from several zoonotic animal diseases such as classical rabies and bovine brucellosis [Citation12], and the occurrence of Salmonella in livestock and Campylobacter in broilers are low [Citation13]. Some studies have focused on specific zoonotic infections of veterinarians in the country [Citation14–17].
Many zoonotic infections and zoonoses could be avoided by preventive measures. In Finland, over 90% of veterinarians have reported exposure to zoonotic pathogens in their work, 15% having contracted a zoonosis, and over 80% animal bites [Citation4]. Adherence to infection prevention and control practices needs more attention [Citation4,Citation16]. Self-evaluated knowledge of zoonoses and their prevention was fully agreed as good by 8.2% of veterinarians in 2009, and by 10.3% of veterinarians in another study in 2016 [Citation4]. An investigation of knowledge of emerging zoonotic parasites among medical doctors and veterinarians found room for improvement but also cross-sectoral knowledge [Citation18].
Overall, a major gap is that information about zoonotic risks of veterinarians remains limited and scattered. This is the case also in Finland. To contribute to filling in this gap, in this paper, we summarise results from two separate studies: i) descriptions of zoonoses from a questionnaire study and ii) description of a series of work-related zoonotic cases.
Materials and methods
Ethical considerations
The studies were approved by the Ethic Committee of Helsinki University Hospital (questionnaire study 303/13/03/00/2009; case series HUS/1015/2019). All participants provided written informed consent to participate in the study and to publish the case details. The participants of the case series study are informed of the content of the article.
Setting and study design
Approximately 3000 veterinarians are authorised to work in Finland (the Registry of Veterinarians, Finnish Food Authority). Here, we summarise previously unpublished data of selected questions from a cross-sectional questionnaire study conducted in 2009 [Citation4], and descriptions of a selection of cases from a retrospective observational case series study conducted in 2019. The selected cases are described based on information collected by semi-structured interviews and from available medical records. The diagnostic approaches, treatment decisions and follow-up were unaffected by this work.
Questionnaire study
An extensive questionnaire study was conducted among veterinarians attending the Annual Veterinary Congress in Helsinki, Finland, 2009. Selected other results from the study have been published [Citation4,Citation14,Citation15,Citation17]. The participants (N = 306) comprised 15% of the authorised veterinarians in Finland, were born between 1930 and 1986, and most were female [Citation4].
An digital skip-pattern questionnaire, accessible in both Finnish and Swedish, was employed to gather information on age, gender, and self-reported health, use of personal protective equipment and work-related animal contacts. Additionally, the questions covered living environment, travel history, outdoor activities during leisure time, pet ownership, dietary habits, and kitchen hygiene practices. The questionnaire comprised both multiple-choice questions and open-ended questions, allowing responding in writing. Prior to the study, the questionnaire underwent technical testing and a pilot phase involving nine individuals, including four veterinarians, to gather feedback and enhance the clarity of the questions.
In this paper, we summarise previously unpublished parts of the questionnaire data: the detailed answers of the veterinarians who reported having been identified as a carrier of resistant bacteria, having had a zoonosis or having had slowly-healing vesicular or other type of skin lesions considered to be of infectious origin. We focused on the reported suspected or confirmed diagnoses.
Case series study
The cases were identified by posting a request on a closed national-level social media (Facebook) discussion forum of veterinarians and end-phase veterinary students, encouraging veterinarians who had contracted an occupational zoonosis to contact the researchers electronically (Eduix Ltd, version 3, https://e-lomake.fi/en). The request was posted on May 18, 2019, and followed by two reminders. The recruiting reached approximately 2000 veterinarians and veterinary students. Snowball approach [Citation19] was used: the veterinarians were encouraged to forward the message to colleagues, and thus the total number of reached veterinarians remained unknown.
Based on the initial contacts, three authors (PJ, AMKV, PMK) shortlisted suitable cases for structured interviews. Eligible for inclusion were all who contacted the research team with a description of a work-related zoonosis contracted as veterinarian. Exclusion criteria were non-zoonotic infections, probably non-occupational infections, and unconfirmed diagnoses with scarce information. The final selection was determined by the predefined criteria and a goal to encompass various zoonoses. We did not limit the selection based on when the cases had happened. We selected cases caused by different types of pathogens and in different types of veterinary work, and only included one case for each pathogen.
The structured interviews were personal and conducted by one author (AMKV). The questions covered the diagnosis and how it was reached and confirmed; the presumed or confirmed source, transmission route and time of the infection; possible secondary infections; symptoms and their duration; the time until recovery; and treatment and possible sequelae. Some participants shared medical records and laboratory results.
We asked the participants to self-evaluate their knowledge of the particular zoonotic infection before they acquired it, and their general knowledge of zoonoses then and at the time of the interview. Moreover, we asked whether they had after the episode changed their precautions against the particular infectious agent and zoonotic pathogens in general.
Results
Zoonoses reported in the questionnaire study
The questionnaire was answered by 306 veterinarians in total. None (0%) had been identified as a carrier of multidrug-resistant bacteria (). Data provided by 105 veterinarians (34% of all the 306 participants) were analysed further in this study, because they had reported a history of zoonosis (n = 46; 15%; [Citation4]), slowly-healing vesicular skin lesions (n = 15; 4.9%; [Citation4]), and/or other types of skin lesions suggestive of infectious origin (n = 74; 24%) ().
Table 1. Self-reported zoonotic infections, zoonoses and skin lesions suggestive of zoonotic origin based on the responses of all the veterinarians (N = 306) in the questionnaire study 2009.
The most commonly reported zoonoses among the veterinarians were various bacterial skin infections (n = 36, 12% of all the 306 veterinarians; ). Commonly, these were described as an undiagnosed papular-pustular-vesicular dermatitis (n = 23, 7.5%) in arms associated with cattle contact, in particular calving assistance (n = 22, 7.2%). Five (1.6%) veterinarians reported a confirmed or suspected cutaneous listeriosis. Moreover, six (2.0%) reported a confirmed or suspected Erysipelothrix rhusiopathiae skin infection, presumably originating from swine farms, laboratory, slaughterhouse, or necropsy. A single case (0.3%) of an abscess caused by methicillin-resistant S. aureus was reported.
Ten (3.3%) of all the 306 veterinarians described a history of a zoonotic bacterial gastroenteritis (). Seven (2.3%) reported salmonellosis, and the work-related sources mentioned included laboratory exposure and illegally imported animals. Furthermore, single cases (0.3% each) of enterohaemorrhagic E. coli (EHEC) from a bull feedlot, campylobacteriosis, and Yersinia enterocolitica gastroenteritis were reported.
Other bacterial infections were reported by seven (2.3%) veterinarians (). These included borreliosis, Q fever (acquired abroad), tularaemia, and listeriosis.
Twelve (3.9%) of all the veterinarians reported symptomatic virus infections of zoonotic origin (). Nephropatia epidemica caused by Puumala hantavirus had been diagnosed in six (2.0%), influenza from swine was reported by two (0.7%) and vesicular skin lesions presumably caused by a zoonotic virus by four (1.3%) participants.
Infection or infestation with parasites was reported by seven (2.3%) of the veterinarians (). Toxoplasma gondii infection or toxoplasmosis had been diagnosed in three (1.0%) during pregnancy. In two veterinarians, (0.7%), scabies had been diagnosed or suspected. Furthermore, single cases (0.3% each) of cryptosporidiosis from calves and flea bites from dog contact were reported.
Dermatophytosis (ringworm) was reported by 13 (4.2%) veterinarians (). Microsporum canis and Tricophyton verrucosum had both been diagnosed in three different (1.0%) veterinarians. All M. canis cases had reportedly originated from cats, and two T. verrucosum cases from cattle.
Case series
Altogether 16 veterinarians responded to the request. Of them, 13 were enrolled via the electronic form, and three from further contacts. They reported infections caused by Shigella, Campylobacter, C. canimorsus, Cryptosporidium (n = 8), Listeria (n = 2), Salmonella Infantis, and Trichophyton. Additionally, exposure to Bacillus anthracis and rabies virus were reported. One veterinarian reported contracting two pathogens.
Seven cases were included in the case series: C. canimorsus sepsis, cryptosporidiosis, cutaneous listeriosis, Salmonella gastroenteritis, and Trichophyton dermatophytosis, as well as exposure incidents to B. anthracis and to classical rabies.
Case 1. Capnocytophaga canimorsus sepsis
A previously healthy small animal veterinary practitioner had accidentally been exposed to corrosive substance, resulting in skin abrasions in the right forearm. On the following day, a street dog imported from Thailand bit the right palm piercing the skin. Three days later, the veterinarian was admitted to emergency room with high fever (40 °C), chills, vomiting and diarrhoea, facial and upper body petechiae, metabolic acidosis, hypotension, and renal insufficiency. C-reactive protein was 444. The patient was immediately transferred to intensive care unit, and diagnosed with sepsis, disseminated intravascular coagulopathy, myocardial infarction and renal failure with renal infarction. After four days, the laboratory informed about a slowly growing gram-negative rods in blood culture, confirmed next day as C. canimorsus. At that time, the abrasions had developed to necrotic wounds. The medical report mentioned exposure to canine saliva as the cause of the sepsis, and the presumed portals of entry were both the skin abrasions and the bite. The patient received antibiotics (vancomycin, ceftriaxone, levofloxacine, metronidazole, meropenem plus fluconazole). After 18 days of hospitalisation, the patient was discharged, and treatment continued with oral amoxicillin plus clavulanic acid. The veterinarian was partially fit for work after 1.5 months, reaching normal condition after three months. The lesions in the forearm healed within a year, leaving loss of muscles and scar tissue.
Case 2. Cryptosporidiosis
A mixed practice veterinarian had severe cryptosporidiosis. The symptoms started one week after disbudding calves over three days at a calf rearing barn. The calves had recently arrived to the farm and some of them had diarrhoea. Cause of the diarrhoea in the calves was not diagnosed; samples taken after the appearance of human cases were negative for Cryptosporidium. As for the veterinarian, a zoonotic faecal-oral transmission associated with the disbudding visits was suspected, because during the incubation period, no other likely exposures were recognised. The symptoms included watery diarrhoea, abdominal cramps, severe headache, and lack of energy. Cryptosporidiosis was confirmed from faecal sample by staining and microscopy. Tests for selected bacterial and viral pathogens, including norovirus, proved negative. Three-day nitazoxanide treatment was initiated two weeks after symptom onset, and the veterinarian recovered fully in three weeks. Several people on the farm reportedly also developed diarrhoea, and the patient’s partner became a suspected secondary case.
Case 3. Cutaneous listeriosis
A livestock practitioner with athopy developed an episode of maculo-papulo-pustulo-vesicular dermatitis in both arms after manually assisting calving. The dermatitis started with prickly and burning sensation a few hours after calving assistance. Starting the following day, needle-prick-sized maculae became visible and grew to papules and vesicles, eventually disrupting with mainly purulent, occasionally serous discharge. Within few days, the papules and vesicles merged into an erythematous, oedematous mat of skin inflammation. The veterinarian developed fever and visited primary health care, informing about the possibility of a zoonosis. Irritation, allergic reaction, and infection were considered as differential diagnoses. The clinical signs worsened following the use of a corticosteroid ointment, whereas oral antihistamine had no effect. A sample taken for bacterial culture revealed a mixed infection with Listeria spp., streptococci and staphylococci. Listeria spp. was the only finding considered clinically relevant. A seven-day peroral penicillin course was started, resulting in recovery within two days. Several similar dermatitis episodes recovering with antibiotic treatment had since recurred after assisting the delivery of dead calves including prolonged contact with obstetric fluids.
Case 4. Salmonella gastroenteritis
A veterinarian working in a diagnostic laboratory was infected with Salmonella enterica subsp. enterica serovar Infantis. The veterinarian’s task was to serotype Salmonella isolates cultured from animal carcases or faeces, by slide agglutination test. The reaction of the suspended bacteria was observed on the slide at eye-height. The symptoms included fever, shivering, and diarrhoea. The diagnosis of Salmonella gastroenteritis was confirmed from a faecal sample in the same laboratory. The patient recovered without treatment, and follow-up samples turned negative after approximately one month.
Case 5. Trichophyton dermatophytosis (ringworm)
A veterinarian contracted Trichophyton dermatophytosis likely from a 1-year-old foal with visible skin lesions consistent with fungal infection. The foal lived at a stable where several horses had had fungal skin infection. The foal was treated with washing and administrating topical antifungal – the procedure suspected to have exposed the veterinarian. After approximately one week, the veterinarian noticed a 3–4 cm wide scaly circular lesion on the dorsal side of the arm. The diagnosis was based on clinical appearance and confirmed by culture. A 2-week antifungal treatment resulted in full recovery.
Case 6. Bacillus anthracis exposure
A veterinarian participated in a post-mortem examination of a bovine that had died after gastrointestinal clinical signs. Suspicion of anthrax exposure arose during laboratory analyses of samples taken in another context from the same herd and B. anthracis was identified by culture, bioassay, and PCR [Citation20,Citation21]. The exposed veterinarian was treated with post-prophylactic antibiotics for 40 days and remained asymptomatic.
Case 7. Exposure to rabies
A livestock practitioner with a history of autoimmune condition got severely bitten by a horse. The horse had been imported to Finland from Estonia and showed neurological clinical signs [Citation22]. When the veterinarian injected analgesics, the horse bit the veterinarian’s elbow with trismus. The veterinarian’s coat and skin were torn, and the arm felt numb. Having treated the horse, the veterinarian washed the wound with water and soap, disinfected it, and on the following day, contacted a veterinary officer. The horse was euthanized and its head sent for examination for rabies. Rabies was preliminarily diagnosed from the horse’s brain two days after the bite, and verified three days later. The veterinarian had been vaccinated against rabies more than five years earlier. Post-exposure prophylaxis (PEP) for rabies was initiated in less than 24 h after exposure, and continued as a five dose regimen, on days 3, 7, 14, and 28. Inactivated rabies vaccine and immunoglobulin (RIG) were administered [Citation22]. The veterinarian reported receiving the vaccine injections and RIG intramuscularly in the unaffected arm. After the third injection, about one week after the exposure, the veterinarian felt myalgia, trembling, salivation, difficulty swallowing, and fever, and started a sick leave. Few months later, the wound was still open and required debridement, and left chronic nerve damage and scar tissue. The veterinarian felt the experience was traumatic.
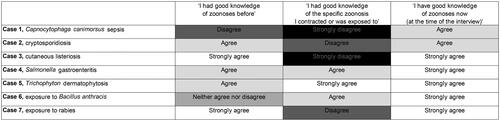
Self-evaluated knowledge of zoonoses of the cases
summarises the self-reported level of agreement to the zoonosis-related knowledge statements among the veterinarians included in the case series study. Four of the seven veterinarians disagreed or strongly disagreed with the statement of having had good knowledge of the specific zoonosis. All agreed or strongly agreed with having good knowledge of zoonoses at the time of the interview. Several reported an improvement in their protective practices afterwards with respect to the specific zoonosis.
Discussion
This two-faceted study summarised information on zoonotic pathogens and zoonoses and veterinarians, based on a questionnaire study and a case series. Together the results illustrate that veterinarians may become occupationally exposed to a wide variety of zoonotic pathogens in northern Europe.
In the questionnaire study in 2009, a variety of zoonotic infections were reported (). The cases included in the case series study covered five of the ten pathogens identified as the main infectious health risks to veterinarians in Finland [Citation11], and four other pathogens from the list were described in the replies of the questionnaire study. Most of the seven pathogens covered in the case series are endemic in Finland ().
Table 2. Occurrence and disease status in Finland of the seven zoonotic pathogens selected to the case series study.
It should be emphasised that we did not aim to evaluate how common or important the different zoonotic infections were, and the results should not be considered representative of the frequency of the infections. While the two approaches used in this work both have their limitations, their findings add to the limited and scattered knowledge on zoonotic infections of veterinarians. Importantly, this paper can guide design of future studies. An optimal study type for investigating zoonotic infections of veterinarians could be a prospective cohort study. Moreover, several zoonotic pathogens, including emerging pathogens and variants, would merit targeted research focus as occupational hazards.
The two studies were separate and their ethical permissions did not allow combining the data at individual-level. The questionnaire study took place at a professional event, the recruitment for the case series study reached primarily veterinarians using social media, and participation was voluntary. Severe cases may be overrepresented, and recall bias was likely. The level of available evidence to confirm the infections as zoonotic and linked to the presumed exposures varied. We had direct contact only to veterinarians, and not to their occupational health care providers. The questionnaire study was conducted in 2009 and the cases reported in the 2019 had occurred between the 1980s and 2010s. For some of the pathogens, diagnostic possibilities to confirm the infection may have improved or became more widely available. For some of the pathogens, the exposure risks may not reflect the current situation well, and some respective guidelines and procedures have been improved. New potential hazards in different work environments of veterinarians include highly pathogenic avian influenza A(H5N1) virus infecting birds and mammals, including animals kept for their fur [Citation34].
Potentially life-threatening pathogens C. canimorsus and rabies virus should be remembered with bite injuries [Citation35,Citation36]. It is crucial to emphasise the risk for rabies even in regions officially free of the disease. It is important to wash bite injuries with soap for 15 min and subsequently disinfect. Additionally, in category III rabies exposures, WHO recommends PEP vaccinations on days 0 and 3, without RIG, for immunised individuals. With five PEP vaccine doses and RIG given in the unaffected arm of the previously immunised veterinarian, the clinical signs reported indicate likely an adverse reaction [Citation37] instead of effect of rabies virus. Deviations from the PEP protocol have also been reported elsewhere [Citation38].
Cryptosporidium parvum has recently become more common in Finland, in both cattle and humans () [Citation24,Citation26]. Accordingly, several cryptosporidiosis cases were available for inclusion to the case series in 2019, while only single case was reported in the questionnaire study in 2009. Same typing methods can be used for human and animal samples to confirm, rule out and trace zoonotic transmission [Citation10].
Some zoonotic risks may be more common than recognised. Despite its world-wide spread [Citation3,Citation7–9,Citation39], ringworm has received little attention in Finland. Published case reports of cutaneous listeriosis are rare (this study, [Citation40]), but the condition may be frequent among veterinarians. The case of the laboratory-acquired Salmonella infection highlights the variety of environments veterinary work involves.
The case of B. anthracis exposure is an example of a re-emerging zoonosis: the spores may survive in soil for decades. While during the 1960s several anthrax cases were detected annually in Finland, they later became rare [Citation41]. Consequently, awareness of anthrax is challenging to maintain, especially regarding its unspecific manifestations [Citation30].
Five of the seven veterinarians strongly agreed with having good knowledge of zoonoses at the time of the interview (). This proportion is higher than the percentages reporting to fully agree with similar statement in two questionnaire studies [Citation4]. This difference may reflect the importance of personal experiences or illustrate participation bias. How much veterinarians themselves should know about zoonoses in relation to their own occupational health is a pertinent question: indeed, mapping also the knowledge of occupational health providers would be relevant.
Our results highlight that the occupational health of veterinarians is connected with the One Health concept – veterinarians work at the interface of humans and animals, and can encounter zoonotic infections. The results presented can be useful to increase awareness about zoonotic risks in the occupational health sector and to improve occupational health of veterinarians.
Acknowledgments
We warmly thank the veterinarians who participated, and Dr. Sari Pakkanen, University of Helsinki, for administrative support in the preparation of the case series study.
Disclosure statement
PMK is currently affiliated to MSD Animal Health. The study was initiated before the affiliation change, and MSD Animal Health has had no influence on the content of this work and article. Other authors report no competing interests to declare.
Additional information
Funding
References
- Baker WS, Gray GC. A review of published reports regarding zoonotic pathogen infection in veterinarians. J Am Vet Med Assoc. 2009;234(10):1271–1278. doi: 10.2460/javma.234.10.1271.
- Bernard H, Brockmann SO, Kleinkauf N, et al. High seroprevalence of Coxiella burnetii antibodies in veterinarians associated with cattle obstetrics, Bavaria, 2009. Vector Borne Zoonotic Dis. 2012;12(7):552–557. doi: 10.1089/vbz.2011.0879.
- Fowler HN, Holzbauer SM, Smith KE, et al. Survey of occupational hazards in Minnesota veterinary practices in 2012. J Am Vet Med Assoc. 2016;248(2):207–218. doi: 10.2460/javma.248.2.207.
- Kinnunen PM, Matomäki A, Verkola M, et al. Veterinarians as a risk group for zoonoses: exposure, knowledge and protective practices in Finland. Saf Health Work. 2022;13(1):78–85. doi: 10.1016/j.shaw.2021.10.008.
- Sánchez A, Prats-van der Ham M, Tatay-Dualde J, et al. Zoonoses in veterinary students: a systematic review of the literature. PLoS One. 2017;12(1):e0169534. doi: 10.1371/journal.pone.0169534.
- Constable PJ, Harrington JM. Risks of zoonoses in a veterinary service. Br Med J (Clin Res Ed). 1982;284(6311):246–248. doi: 10.1136/bmj.284.6311.246.
- Dowd K, Taylor M, Toribio JA, et al. Zoonotic disease risk perceptions and infection control practices of Australian veterinarians: call for change in work culture. Prev Vet Med. 2013;111(1–2):17–24. doi: 10.1016/j.prevetmed.2013.04.002.
- Epp T, Waldner C. Occupational health hazards in veterinary medicine: zoonoses and other biological hazards. Can Vet J. 2012;53(2):144–150.
- Gummow BA. Survey of zoonotic diseases contracted by South African veterinarians. J S Afric Vet Assoc. 2003;73(3):a514. doi: 10.4102/jsava.v74i3.514.
- Thomas-Lopez D, Müller L, Vestergaard LS, et al. Veterinary students have a higher risk of contracting cryptosporidiosis when calves with high fecal Cryptosporidium loads are used for fetotomy exercises. Appl Environ Microbiol. 2020;86(19):e01250–20. doi: 10.1128/AEM.01250-20.
- Virta A, Jokelainen P, Kinnunen PM, et al. Zoonoses in the veterinary work. [Zoonoosit eläinlääkärin työssä; in Finnish]. Työterveyslääkäri. 2021;1:10–14.
- Finnish Food Authority. Animal diseases in Finland 2021. Report. Finnish Food Authority Publications. 2022;4. Available at https://www.ruokavirasto.fi/globalassets/tietoa-meista/julkaisut/julkaisusarjat/julkaisuja/elaimet/ruokaviraston_julkaisuja_4_2022_elaintaudit_suomessa_2021.pdf.
- EFSA/ECDC. EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2021 Zoonoses Report. EFSA J. 2022;20(12):e7666. doi: 10.2903/j.efsa.2022.7666.
- Kantala T, Kinnunen PM, Oristo S, et al. Hepatitis E virus antibodies in Finnish veterinarians. Zoonoses Public Health. 2017;64(3):232–238. doi: 10.1111/zph.12312.
- Siponen AM, Kinnunen PM, Koort J, et al. Toxoplasma gondii seroprevalence in veterinarians in Finland: older age, living in the countryside, tasting beef during cooking and not doing small animal practice associated with seropositivity. Zoonoses Public Health. 2019;66(2):207–215. doi: 10.1111/zph.12550.
- Verkola M, Järvelä T, Järvinen A, et al. Infection prevention and control practices of ambulatory veterinarians: a questionnaire study in Finland. Vet Med Sci. 2021;7(4):1059–1070. doi: 10.1002/vms3.464.
- Väisänen E, Mohanraj U, Kinnunen PM, et al. Global distribution of human protoparvoviruses. Emerg Infect Dis. 2018;24(7):1292–1299. doi: 10.3201/eid2407.172128.
- Mikola N, Oborina V, Jokelainen P. Knowledge about emerging zoonotic vector-borne parasites Dirofilaria immitis and Dirofilaria repens in Finland: questionnaire survey to medical doctors and veterinarians. Vector Borne Zoonotic Dis. 2020;20(1):27–32. doi: 10.1089/vbz.2019.2502.
- Glen S. Snowball sampling: definition, advantages and disadvantages. StatisticsHowTo.com: Elementary Statistics for the rest of us! 2022. Available at: https://www.statisticshowto.com/snowball-sampling/.
- Pelkola K, Dillard K. Pernarutto suomalaisella naudalla. [Anthrax in a Finnish cow; in Finnish]. Kansanterveys. 2004;9:7–9. Available at https://www.julkari.fi/bitstream/handle/10024/102262/2004_9.pdf?sequence=1&isAllowed=y.
- Zoonoses Centre. Zoonoses in Finland in 2000-2010. Report. 2012, Helsinki. Available at https://www.ruokavirasto.fi/globalassets/teemat/zoonoosikeskus/zoonoosit/zoonosesinfinland_final_nettiversio.pdf.
- Metlin AE, Holopainen R, Tuura S, et al. Imported case of equine rabies in Finland: clinical course of the disease and the antigenic and genetic characterization of the virus. J Equine Vet Sci. 2006;26(12):584–587. doi: 10.1016/j.jevs.2006.11.001.
- THL. Finnish Institute for Health and Welfare. Veriviljelyt, kaikki ikäryhmät 2011–2020. [Blood cultures, all age groups, 2011–2020; In Finnish]. National Infectious Disease Register. Last updated 7 June 2021. Available at https://thl.fi/fi/web/infektiotaudit-ja-rokotukset/seurantajarjestelmat-ja-rekisterit/tartuntatautirekisteri/tartuntatautien-esiintyvyystilastot/veri-ja-selkaydinnesteloydokset.
- Suokorpi A, Autio T, Ruotsalainen E, et al. Why do cryptosporidiosis cases increase in Finland? Duodecim. 2019;135(17):1635–1643.
- THL. Finnish Institute for Health and Welfare. Statistical Database of the National Infectious Disease Register. Last updated 22 April 2023. Available at https://sampo.thl.fi/pivot/prod/fi/ttr/shp/fact_shp.
- Enbom T, Suominen K, Laitinen S, et al. Cryptosporidium parvum: an emerging occupational zoonosis in Finland. Acta Vet Scand. 2023;65(1):25. doi: 10.1186/s13028-023-00684-z.
- Finnish Food Authority. Animal diseases in Finland 2020. Report. Finnish Food Authority Publications. 2021;4. Available at https://www.ruokavirasto.fi/globalassets/tietoa-meista/julkaisut/julkaisusarjat/julkaisuja/elaimet/ruokaviraston_julkaisuja_4_2021_elaintaudit_suomessa_2020.pdf.
- Airola K. Vartalon ja päänahan silsa (sieni-infektio). [Ringworm of body and scalp (fungal infection.); in Finnish]. In Lääkärikirja Duodecim. Helsinki: Kustannus Oy Duodecim; 2020. Available at https://www.terveyskirjasto.fi/dlk00837.
- Animal Health ETT. Pälvisilsa. [Ringworm; in Finnish]. 2021. Available at https://www.ett.fi/nauta/taudit/palvisilsa/.
- Friman M, Kakko L, Constantin C, et al. An atypical Bacillus anthracis infection in a bull – A potential occupational health hazard. Reprod Domest Anim. 2019;54(9):1279–1283. doi: 10.1111/rda.13532.
- Nyberg M, Kulonen K, Neuvonen E, et al. An epidemic of sylvatic rabies in Finland – descriptive epidemiology and results of oral vaccination. Acta Vet Scand. 1992;33(1):43–57. doi: 10.1186/BF03546935.
- THL. Finnish Institute for Health and Welfare. Tartuntataudit Suomessa. 2020. Infectious diseases in Finland 2020; in Finnish]. Report. 2021b. Available at https://thl.fi/documents/533963/7590511/Tartuntataudit+Suomessa + 2020_28.9.2021.pdf.
- Rimhanen-Finne R, Ollgren J, Gadd T, et al. Notifications of suspected rabies exposure increased in Finland: 26 years of one health surveillance, 1995-2020. Infect Dis (Lond). 2023;55(7):458–466. doi: 10.1080/23744235.2023.2203761.
- Lindh E, Lounela H, Ikonen N, et al. Highly pathogenic avian influenza A(H5N1) virus infection on multiple fur farms in the South and Central Ostrobothnia regions of Finland, July 2023. Euro Surveill. 2023;28(31):2300400. doi: 10.2807/1560-7917.ES.2023.28.31.2300400.
- Hästbacka J, Hynninen M, Kolho E. Capnocytophaga canimorsus bacteremia: clinical features and outcomes from a Helsinki ICU cohort. Acta Anaesthesiol Scand. 2016;60(10):1437–1443. doi: 10.1111/aas.12752.
- World Health Organization. Rabies vaccines: WHO position paper, April 2018 – recommendations. Vaccine 2018;36(37):5500–5503. doi: 10.1016/j.vaccine.2018.06.061.
- Shayam C, Duggal AK, Kamble U, et al. Post-exposure prophylaxis for rabies. JIACM. 2006;7(1):39–46.
- Meyerhoff P, Manekeller S, Saleh N, et al. Rabies post-exposure prophylaxis in Germany – what are the challenges? Epidemiol Infect. 2021;149:e119. doi: 10.1017/S0950268821000601.
- Robin C, Bettridge J, McMaster F. Zoonotic disease risk perceptions in the British veterinary profession. Prev Vet Med. 2017;136:39–48. doi: 10.1016/j.prevetmed.2016.11.015.
- Zelenik K, Avberšek J, Pate M, et al. Cutaneous listeriosis in a veterinarian with the evidence of zoonotic transmission – A case report. Zoonoses Public Health. 2014;61(4):238–241. doi: 10.1111/zph.12075.
- Kivelä SL. Pernaruton esiintyminen eläimillä Suomessa vuosina 1940–1990. [Occurrence of anthrax in animals in Finland, years 1940–1990] Licentiate Thesis. 1993. Faculty of Veterinary Medicine, University of Helsinki. Available at http://hdl.handle.net/1975/1271.