Abstract
Introduction
Convalescent plasma (CP) emerged as potential treatment for COVID-19 early in the pandemic. While efficacy in hospitalised patients has been lacklustre, CP may be beneficial at the first stages of disease. Despite multiple new variants emerging, no trials have involved analyses on variant-specific antibody titres of CP.
Methods
We recruited hospitalised COVID-19 patients within 10 days of symptom onset and, employing a double-blinded approach, randomised them to receive 200 ml convalescent plasma with high (HCP) or low (LCP) neutralising antibody (NAb) titre against the ancestral strain (Wuhan-like variant) or placebo in 1:1:1 ratio. Primary endpoints comprised intubation, corticosteroids for symptom aggravation, and safety assessed as serious adverse events. For a preplanned ad hoc analysis, the patients were regrouped by infused CP’s NAb titers to variants infecting the recipients i.e. by titres of homologous HCP (hHCP) or LCP (hLCP).
Results
Of the 57 patients, 18 received HCP, 19 LCP and 20 placebo, all groups smaller than planned. No significant differences were found for primary endpoints. In ad hoc analysis, hHCPrecipients needed significantly less respiratory support, and appeared to be given corticosteroids less frequently (1/14; 7.1%) than those receiving hLCP (9/23; 39.1%) or placebo (8/20; 40%), (p = 0.077).
Discussion
Our double-blinded, placebo-controlled CP therapy trial remained underpowered and does not allow any firm conclusions for early-stage hospitalised COVID-19 patients. Interestingly, however, regrouping by homologous – recipients’ variant-specific – CP titres suggested benefits for hHCP. We encourage similar re-analysis of ongoing/previous larger CP studies.
Trial registration
ClinTrials.gov identifier: NCT0473040
Introduction
In the early days of the SARS-CoV-2 pandemic, treatment options were urgently needed. Various valuable tools for prevention and treatment of COVID-19 vaccines, antivirals, monoclonal antibodies, and immunomodulatory therapies were not yet available. The concept of applying convalescent plasma (CP) as therapy for COVID-19 felt intriguing because of a potentially ample supply of CP. Before COVID-19, CP had been used to treat other viral infections, such as the 1918 and 2009 H1N1 influenza pandemics, SARS-CoV-1, and Middle East Respiratory Syndrome (MERS). CP therapy appeared to be of benefit particularly when given early in the course of infection [Citation1,Citation2], but the evidence was derived from a small number of studies lacking control groups [Citation2].
In April 2020, the European Commission (EC) issued advice on the use of CP for COVID-19 in research settings, and a database was created to collect data from clinical trials [Citation3]. While some potential adverse effects of CP treatment, such as transfusion-related acute lung injury (TRALI) and disease aggravation by antibody-dependent enhancement (ADE), were initially of concern, it is important to note that such events have proved very rare. In contrast, at present, CP treatment is considered generally safe [Citation4,Citation5].
Early in the pandemic, three randomised controlled trials (RCT) suggested that CP therapy is ineffective late in disease progression [Citation6–8]. However, there were indications that high titre CP given soon after symptom onset could be beneficial [Citation9,Citation10]. Accordingly, we focused on treatment provided soon after hospital admission using a placebo-controlled, randomised, double-blinded study design including comparison between low- and high-titre CP.
Methods
Trial design and study objectives
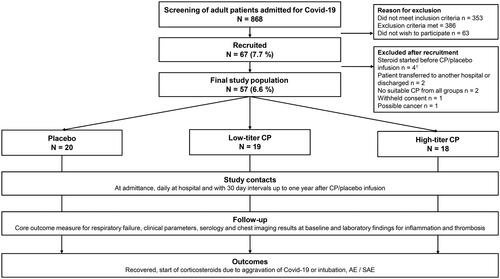
We conducted a prospective double-blinded RCT on the efficacy and safety of convalescent plasma (CP) treatment of hospitalised adult COVID-19 patients at the HUS Helsinki University Hospital between 2 February 2021 and 19 January 2022. Patients were randomised 1:1:1 to receive either low-titre CP, high-titre CP or placebo. The titres were categorised by level of neutralising antibodies (NAb) against the SARS-CoV-2 ancestral strain (B.1 Wuhan-like original variant) as measured by a microneutralization test (MNT) from donated CP. The donors were recruited among RT-PCR-confirmed patients from the Helsinki Metropolitan area. Primary endpoints were intubation and initiation of a systemic corticosteroid for aggravation of COVID-19 within 21 days, and safety, assessed as rates of serious adverse events (SAE). A more detailed list of study objectives is presented in Supplementary Table S1. and conduct of the trial in . Degree of respiratory failure and other clinical and laboratory parameters were monitored throughout hospitalisation. Participants were followed up for 360 days.
Figure 1. Study design.
Abbreviations: COVID-19 = Coronavirus Disease, CP = convalescent plasma, AE = adverse event, SAE = Serious adverse event.
1One of these participants was administered corticosteroids before the study drug infusion but was given the infusion by mistake. A decision to exclude was made the same day.
Ethics clearance and monitoring
The four study protocols involved were each approved by the HUS Ethical Committee. Three of them – referred jointly as C_COVID master study – covered recruitment for antibody analyses: Clin_COVID (HUS/1238/2020), Commun_COVID (HUS/1239/2020), and SARS-CoV-2 / COVID-19 (HUS/853/2020). The fourth, Plasma_COVID (HUS/1637/2020), included plasma donation and the clinical trial presented here. Written informed consent was obtained from all participants. The study was monitored by an external monitor (HUCH Institute Ltd) and registered at Clinical Trials (NCT04730401).
Convalescent plasma products
Patients with an RT-PCR-confirmed COVID-19 infection in the HUS area were recruited into the C_COVID master study [Citation11–14]. Healthy seropositive individuals aged 18–65 were invited to participate in the present investigation, Plasma_COVID, as donors. Convalescent plasma was collected at the Finnish Red Cross Blood Service (FRCBS) in Helsinki, with a concurrent plasma sample collected for serological tests. Plasma with a NAb titre of 1:20–1:80 against the ancestral strain by MNT (microneutralization assay) was considered low-titre plasma (LCP), and that with ≥1:160 high-titre plasma (HCP).
Preparation of plasma products at FRCBS
Plasmapheresis was conducted with the Trima Accel Automated Blood Collection System (Terumo BCT Inc, Lakewood, USA), software version 6.0, applying the protocol described elsewhere [Citation15]. The recommendations of the European Commission on CP collection [Citation3] and the guidance of the EDQM [Citation16] were followed. In addition to routine testing for blood-borne infectious diseases, all donors were screened for interferon alpha 2b (IFN-α2b), and women for HLA antibodies. Those with a positive finding were excluded from the donor pool.
Recruitment of COVID-19 patients
We recruited ≥18-year-old patients hospitalised for RT-PCR-confirmed COVID-19 at HUS. For inclusion, symptom duration was not to exceed 10 days. Our exclusion criteria (Supplementary Table S2) included current systemic corticosteroids, immunosuppression or immunosuppressive/-modulating therapy over the past six months and do-not-resuscitate order or decision to withhold ICU treatment. Eligibility was re-evaluated immediately before administering CP/placebo. Unblinded study personnel randomised the patients only after verifying availability of both low- and high-titre blood type-compatible CP.
At baseline, the participants filled out a questionnaire on their general health, COVID-19 risk factors and symptoms. They were randomised into one of three groups to receive 1) high-titre NAb CP (HCP), 2) low-titre NAb CP (LCP), or 3) as placebo, intravenous isotonic saline solution. Once new variants began to emerge, we gave the most recently collected plasma to increase the probability of matching donors’ and recipients’ virus variants.
Infusion of CP/placebo and treatment in the hospital
The unblinded study nurses administered plasma or placebo as a single 200 mL intravenous 60-minute infusion (blinded to patients and ward personnel). Apart from the intervention, all participants received standard care: for those with increasing need for supplemental oxygen, and clinical or radiological suspicion of COVID-19 pneumonitis, local guidelines instructed dexamethasone after > 7 days from symptom onset. The decision on dexamethasone, like all other decisions regarding patient care, was made by treating physicians, who instructed its administration. The researchers were not involved in the clinical decisions. Antiviral medications for immunocompetent patients were not in use at HUS over the study period.
ABO and RhD blood groups were determined from all participants; pregnancy was ruled out for women of child-bearing age. Sera for anti-SARS-CoV-2 antibodies and nasopharyngeal SARS-CoV-2 swabs for RT-PCR and sequencing were collected before the study infusion. Clinical and laboratory parameters were retrieved from electronic patient records daily. As baseline laboratory values and clinical parameters, we used those from the morning of infusion day or (for participants with no laboratory tests taken that day) the preceding day. The degree of respiratory failure was recorded as Core Outcome Measure for Respiratory Failure (COMRF) score [Citation17], adopting score 10 (death) from the WHO clinical progression scale [Citation18] (Supplementary Table S3).
Adverse events
The reporting period for adverse events (AE) was six hours, and for thromboembolic and cardiovascular AE and serious adverse events (SAEs) 360 days after the study infusion. To differentiate between respiratory failure caused by CP infusion (TRALI), and aggravation of COVID-19 per se, the reporting period was 12 h for respiratory failures classified as SAE.
Follow-up after hospital discharge
The post-hospitalisation follow-up comprised a serum sample for anti-SARS-CoV-2 antibodies, a phone call, and an electronic questionnaire filled out by each participant to survey symptoms, possible SAEs and new medications at 15, 30, 60, 90, 120, 150, 180, 270, and 360 days after infusion. Patient records were rechecked at the end of the 360-day follow-up for changes in study endpoints.
Ad hoc groups
To compensate for the possibility of donors and recipients being infected with different virus variants – resulting in patients receiving heterologous CP – we conducted an ad hoc analysis, regrouping the recipients by CP’s pseudovirus-neutralising antibody (pNAb) titre against their (homologous) virus variants. Plasma with a homologous pNAb titre ≥160 was defined as homologous high-titre plasma (hHCP) and others as homologous low-titre plasma (hLCP).
Statistics
Power calculations are described in the supplementary file. Statistical analyses were designed before database lock and unblinding. Randomisation was done by Granitics CFR software with a fixed block size of two times the number of subject groups.
The Shapiro-Wilk test was used to assess normality of continuous variables. One-way analysis of variance was employed to compare age and BMI between the groups. The differences in non-normally distributed continuous variables were tested using the Kruskall-Wallis test which was also chosen for analysis of differences in COMRF score (ordinal variable). Pearson’s chi-square or Fisher’s exact test was used for categorical variables. P-values below 0.05 were considered statistically significant, and two-sided tests were employed. Statistical analyses were carried out with IBM SPSS Statistics 28 (IBM Corp., Armonk, NY).
Laboratory methods
SARS-CoV-2 PCR
RNA was extracted from nasopharyngeal swab samples with Trizol (Thermo Scientific) according to manufacturer’s instructions. It was then tested with RdRp targeting RT-PCR as previously described [Citation19]. RT-PCR was conducted with Stratagene Mx3005P (Agilent Technologies).
Sequencing
The nasopharyngeal swab samples were sequenced as described previously [Citation20] and analysed using HAVoC pipeline [Citation21]. For further analyses, the sequences were categorized into Wuhan-like ancestral strain, Alpha, Beta, Delta, and Omicron variants.
Microneutralization and pseudovirusneutralization assays
Microneutralization test (MNT) was used for initial determination of NAbs when selecting the CP for those randomised to receive LCP or HCP. As described earlier in detail [Citation22,Citation23], MNT was carried out in a biosafety-level 3 laboratory in duplicate in Vero E6 cells from 1:20 serum dilution onwards with FIN-1: Vero E6-passaged strain from the 1st Finnish patient in 2020 [Citation22]. The pseudovirus neutralisation test was used for analyses of CP’s variant-specific neutralising antibody titres and evaluation of the recipients’ seropositivity upon enrolment. It was based on a recombinant lentivirus with a luciferase reporter pseudotyped with S proteins of different SARS-CoV-2 variants of concern that circulated in Finland over the study period [Citation20, Citation24] as described previously [Citation25,Citation26].
Detection of IFN-α2b antibodies by ELISA
Patient sera were screened for antibodies against recombinant IFN-α2b protein (ImmunoTools, Friesoythe, Germany) by a validated in-house enzyme-linked immunosorbent assay (ELISA) as previously described [Citation27]. Absorbance was read at 450 nm with a photometer and the mean value of the triplicate samples was used, deduced by the background OD value of buffer-only wells. The cut-off level was set on the basis of reference healthy donor sample values at OD-signal 0.04.
Data availability
We report a group with potentially recognisable participants; to protect their anonymity, individual-level data are not provided. Any other relevant data are available from the corresponding author upon reasonable request.
Results
Participant groups
Over the study period, 868 patients admitted to HUS for RT-PCR-confirmed COVID-19 were screened for eligibility. Initially, 67 patients were recruited to participate, but upon pre-infusion re-evaluation of eligibility, nine more were excluded: three for dexamethasone given before the infusion; two for being discharged or transferred to a non-study hospital; two for either low- or high-titre blood type compatible plasma lacking; one for withheld consent; and one for findings compatible with a malignancy. Finally, one was excluded upon infusion by the blinded PI because of dexamethasone ordered shortly before but not spotted in time.
The final participant group comprised 57 patients, of whom 18 were given HCP, 19 LCP, and 20 placebo (). The ad hoc regrouping by homologous titre yielded 14 hHCP and 23 hLCP recipients (8 from HCP group transferred to hLCP, 4 from LCP to hHCP group). Reasons for exclusion are presented in Supplementary Table S4.
Termination of the study
Recruitment was extended beyond its initially planned termination date (September 2021) in response to slow recruitment, mainly ascribed to late hospitalisation (>10 days after symptom onset) and early start of dexamethasone. The study concluded on 30 January 2022 due to recruitment difficulties (see discussion). The emergence of the Omicron variant (B.1.1.529) against which our CP preparations were expected to have poor neutralising activity, as they had been collected during predominance of other variants [Citation23,Citation28–30], expedited the decision (see discussion). Continuing the trial without variant-compatible CP was deemed unethical, and collecting a new CP bank seemed futile when so few eligible patients were being admitted.
Baseline data about randomised groups
Our study population comprised 57 participants with a mean age of 51.7 years; 27 (47%) were women.
We identified the following variants: Alpha for 24 patients (42.1%), Delta for 21 (36.8%), Beta for nine (15.8%), Wuhan-like ancestral strain for two, and Omicron variant for one. The median time span from symptom onset to intervention was eight (IQR 6.5–9) days. The infusion was given on recruitment day to 35 patients, and 22 received it the following day.
Between the randomised groups, no significant differences were observed in age, sex, BMI, chronic illnesses, vaccination status, seropositivity at baseline, or proportions of virus variants. Likewise, the groups did not differ in their symptom duration prior to hospitalisation or need for supplemental oxygen.
Baseline data about ad hoc groups
For ad hoc analyses, the recipients were regrouped by donated CPs’ titres to their infecting variants. The results showed significantly fewer (p = 0.043) needing supplemental oxygen at baseline in the hHCP group (3/14; 21.4%) than the hLCP (11/23; 47.8%) or placebo (13/20; 65%) groups. The hHCP group also appeared less likely to have dyspnoea or tightness in chest as one of the main presenting symptoms, but this difference did not reach statistical significance. provides the baseline characteristics in detail.
Table 1. Baseline characteristics.
Outcomes for randomised and ad hoc groups
The HCP, LCP and placebo groups did not differ significantly in their rates of SAE/AE (), length of hospitalisation, or number of patients intubated or given corticosteroid (). For the first three variables, no differences were seen between the ad hoc groups either. However, in the hHCPgroup corticosteroids were begun for disease aggravation only for 1/14 of patients (7.1%), while the respective rate in the hLCPgroup was 9/23 (39.1%) and placebo group 8/20 (40.0%); the difference did not reach statistical significance (p = 0.077). The lowest respiratory failure score (COMRF) seen for the hHCP group was significantly (p = 0.029) below that of the other groups: only four (4/14 = 28.6%) required supplemental oxygen during hospitalisation compared with 10/23 (43.5%) in the hLCP and 12/20 (60%) in the placebo groups. None in the hHCP group needed more intensive respiratory support than supplemental oxygen compared with 5/23 (21.8%) in the hLCPand 2/20 (10%) in the placebo group. depicts the main outcomes.
Table 2. Comparison of primary and secondary outcomes between randomised and ad hoc groups.
Table 3. Adverse (AE) and severe adverse events (SAE) for hospitalised COVID-19 patients receiving convalescent plasma containing a high (HCP) or low (LCP) titre of anti-SARS-CoV-2 neutralising antibodies, or placebo.
Discussion
The prevailing view in scientific literature and guidelines appears to be that CP therapy is not an effective treatment for hospitalised COVID-19 patients, particularly for advanced disease [Citation31–33].
In other studies, however CP has appeared beneficial for early-stage outpatients [Citation34–36] and, by some reports, early-stage inpatients [Citation28,Citation37]. In addition, a recent open label randomised study found CP effective for mechanically ventilated ICU patients when given in a sufficiently high dose [Citation38]. To further explore the potential of CP therapy for early-stage patients, our double-blinded randomised prospective study focused on hospitalised patients for whom CP therapy would be realistic in a real-life scenario, striving to recruit patients within ten days of symptom onset.
Size of study population
Instead of the initially planned 400, our study population came to comprise 57 patients. This was the sum of several factors: (1) fewer patients were hospitalised than anticipated on the basis of other countries’ reports, thus limiting the number of eligible patients within the planned timeframe; (2) after epidemiological changes and large-scale vaccinations, most of the admitted patients turned out to be multimorbid and thus ineligible; (3) selecting starting of systemic corticosteroids for COVID-19 as our primary endpoint (in addition to intubation and SAE) proved unsuccessful because of subsequent changes in clinical practice: initially, steroids were recommended in the event of increasing need for oxygen supplementation [Citation39], but, later on, dexamethasone was also begun in relatively mild hypoxaemia, further challenging our search for eligible patients. Thus, in the end, with the limited study population, our trial lacks the power to demonstrate other than very substantial differences in outcomes.
Primary outcome
Our study was underpowered to reliably compare the primary outcomes for hospitalised COVID-19 patients receiving high-titre CP, low-titre CP or placebo. The majority of other RCTs conducted on hospitalised patients have not shown a benefit for CP. The first RCT by Li et al. in 2020 reported no decrease in mortality; however, they focused on individuals with severe disease, with a median time from symptom onset to randomisation totalling 30 days [Citation7]. Likewise, lack of efficacy was also reported by the Indian multicentre PLACID trial in 2020; however, most of their study patients received low-titre CP and/or already had an advanced disease at recruitment [Citation6]. Also, the trial by Simonovich et al. failed to show a significant clinical benefit for CP administered to patients with severe COVID-19 pneumonia [Citation8]. In May 2021, the large RECOVERY trial reported no benefit for CP over placebo in 28-day survival of hospitalised COVID-19 patients [Citation10], though those given high-titre plasma or therapy <7 days after symptom onset appeared to thrive better (28-day mortality lower, RR 0.92, p = 0.06). The most recent Cochrane systematic review update, which included 23 RCTs comparing CP to placebo or standard care, provided data about 22020 participants with moderate/severe COVID-19: CP treatment was not effective in reducing death or risk of invasive mechanical ventilation [Citation31]. However, in a more recent meta-analysis, CP therapy was associated with a small mortality benefit [Citation40].
Following several RCTs with no efficacy for CP in hospitalised patients, a recent randomised open-label study by Misset et al. [Citation38] deserves attention. CP was found successful among patients on ventilator who had progressed to acute respiratory distress syndrome. The median time from symptom onset was 12 days. This finding challenges the current understanding of only early-stage CP being potentially effective. Compared to previous studies, Misset et al. administered a higher quantity of antibodies: their CP had a verified high-titre of NAb (≥1:160, 82.3% had ≥1:320) and a large volume (400–500 ml) [Citation28,Citation41,Citation42].
NAb titres and timing may at least partly account for the hospitalised patients’ poor results, since the key requirements of CP therapy success have appeared to be specific neutralising antibodies infused in a high dose early in the course of disease [Citation28,Citation41,Citation42]. Indeed, shortly before we began recruiting patients, Libster et al. published a double-blinded RCT showing efficacy of early high-titre CP treatment administered to non-hospitalised COVID-19 patients at high risk of severe disease [Citation35]. This result was corroborated in a recent meta-analysis concluding that CP prevents hospitalisation particularly when given in high titres and within five days of symptom onset [Citation34]. Although potentially efficacious as an antiviral therapy preventing progression into severe inflammation, CP is not primarily an anti-inflammatory agent and should therefore not be expected to perform well at later stages of disease [Citation28,Citation42]. Thus, although a late transfusion of CP seemed futile, the success of high-titre CP treatment soon after hospital admission a narrow window of opportunity deserved further study.
Variant-specific analyses
The constantly changing landscape of the pandemic proved challenging for research on CP [Citation29], since the emergence of the new SARS-CoV-2 variants led to administration of products with non-matching variants.
Of note, later in the pandemic, the availability of CP from convalescents with hybrid immunity (resulting from both vaccination and natural infection) may help to address the challenge posed by non-matching variants [Citation43].
The CP preparations in our randomised trial were initially categorised into LCP and HCP by their MNT titre against the Wuhan-like ancestral strain. However, these products may have been administered to participants having an infection with a more recently emerged – heterologous – variant, thus the possible efficacy could only have been based on cross-reactive antibodies with lower neutralising activity [Citation23,Citation28–30]. The variant-specific titres were studied by pseudoneutralization test (using lentiviruses), which allows rapid and robust analysis of sera with larger panels of emerging variant spikes, and a good correlation to MNT titres [Citation44]
Ideally, if sequencing data became quickly available, randomisation could be carried out by antibody titre against the variant infecting each recipient. To compensate for such initial lack of virus variant data, we conducted an ad hoc analysis, to which end we analysed plasma of each donor for variant-specific pNAbs and regrouped the participants by titre of administered plasma against their infecting variant. This ad hoc classification was decided before unblinding. Franchini et al. [Citation45] used a similar approach to treat immunocompromised patients hospitalised for COVID-19: they transfused CP with a high NAb titre against the recipients’ specific VOCs with some apparent success, but their case series lacks a control group.
In our ad hoc analysis, participants given homologous high-titre CP (hHCP) managed better, needing significantly less respiratory support than those given homologous low-titre CP (hLCP) or placebo. In addition, a smaller proportion of the hHCP patients needed corticosteroids, the finding not reaching statistical significance, though. Owing to our small sample size, these groups showed differences at baseline: other risk factors for severe disease remained evenly distributed, but supplemental oxygen had been required by fewer participants in the hHCP than the other groups. Thus, it remains somewhat uncertain whether our findings reflect a true benefit of hHCP.
Strengths and limitations
Our major limitation consisted in failing to reach the volume planned: our study population size remained underpowered for reliable evaluation of anything else but a major benefit from CP.
Reassessment of eligibility prior to intervention can be considered either a strength or a weakness. It was necessary because of the rapidly changing clinical conditions and the possibility of patients being administered dexamethasone (an endpoint) or even discharged in the short period between recruitment and intervention. To minimise the theoretical bias, a blinded physician was given the decision to exclude whenever needed. Thanks to this approach ten patients were not included who either could not have been administered plasma/placebo or had already reached an endpoint prior to our potential intervention.
While we strived to recruit patients as soon as possible after symptom onset and used a limit of 10 days as an inclusion criterion, it might be that CP needs to be administered even sooner. We considered setting the time limit to seven days but that would have reduced our study population to less than half, since most patients were not admitted that early ().
The greatest strength of our trial was its double-blind, randomised, and controlled study design in a real-life environment. In the constantly changing pandemic environment, our ad hoc analyses of homologous variants proved a relevant approach not previously employed in any RCT. Grading was based on neutralising antibody titres, not only on ELISA, unlike in most published RCTs [Citation31]. Furthermore, our locally collected plasma bank could be trusted to contain antibodies against regionally circulating variants [Citation28,Citation42].
Yet another strength was that antivirals and immunomodulatory treatments did not interfere with our results, as they were not used for immunocompetent patients in our hospitals at the time. For safety, we screened potential donors for interferon alpha autoantibodies suggested to be associated with severe COVID-19 [Citation46].
Conclusion
As our double-blinded randomised study remained underpowered, its failure to show statistically significant differences in endpoints between the randomised groups should not be interpreted to indicate lack of efficacy. In fact, our ad hoc analysis of participants regrouped by the CP’s homologous variant antibody titres suggested efficacy for recipients of high-homologous titre CP. Our study highlights the concept of variant-specific titres as a key factor impacting CP treatment efficacy. Thus, we suggest similar (post-study) ad hoc analyses for larger RCTs to see whether plasma with high NAb titers against recipients’ virus variants would indeed prove beneficial for moderately ill COVID-19 patients, if administered soon after hospital admission.
Supplemental Material
Download MS Word (267.5 KB)Acknowledgements
We thank our study nurses: Ville and Emilia Saksa, Sadeta Hulkko, Mirja Tervo, Riitta Hannuksela and Rauni Pakarinen who agreed to work flexible hours. Leena Palmunen and Mira Utriainen deserve credit for performing the serology assays. We are grateful to the staff of the wards for allowing us to conduct the study amid all the rush of COVID-19 pandemic as well as to the staff of the Meilahti Hospital Blood Bank, especially to Dr Katja Salmela. We want to express our greatest gratitude to all the study participants and convalescents who donated plasma.
Disclosure statement
The authors report there are no competing interests to declare.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/23744235.2024.2336320)
Additional information
Funding
References
- Luke TC, Kilbane EM, Jackson JL, et al. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139.
- Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90. doi: 10.1093/infdis/jiu396.
- Directorate-General for Health and Food Safety An EU programme of COVID-19 convalescent plasma collection and transfusion: guidance on collection, testing, processing, storage, distribution and monitored use. Brussels: european Comission; 2021.
- Joyner MJ, Bruno KA, Klassen SA, et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95(9):1888–1897. doi: 10.1016/j.mayocp.2020.06.028.
- Franchini M, Cruciani M, Casadevall A, et al. Safety of COVID-19 convalescent plasma: a definitive systematic review and meta-analysis of randomized controlled trials. Transfusion. 2023;64(2):388–399. doi: 10.1111/trf.17701.
- Agarwal A, Mukherjee A, Kumar G, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID trial). BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939.
- Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044.
- Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in covid-19 severe pneumonia. N Engl J Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304.
- Joyner MJ, Senefeld JW, Klassen SA, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-Month experience. medRxiv. 2020;2020.08.12.20169359. doi: 10.1101/2020.08.12.20169359.
- Abani O, Abbas A, Abbas F, et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. The Lancet. 2021;397(10289):2049–2059. doi: 10.1016/S0140-6736(21)00897-7.
- Cabrera LE, Pekkarinen PT, Alander M, et al. Characterization of low-density granulocytes in COVID-19. PLoS Pathog. 2021;17(7):e1009721. doi: 10.1371/journal.ppat.1009721.
- Jalkanen P, Kolehmainen P, Häkkinen HK, et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun. 2021;12(1):3991. doi: 10.1038/s41467-021-24285-4.
- Kantele A, Lääveri T, Kareinen L, et al. SARS-CoV-2 infections among healthcare workers at helsinki university hospital, Finland, spring 2020: serosurvey, symptoms and risk factors. Travel Med Infect Dis. 2021;39:101949. doi: 10.1016/j.tmaid.2020.101949.
- Kurki SN, Kantonen J, Kaivola K, et al. APOE epsilon4 associates with increased risk of severe COVID-19, cerebral microhaemorrhages and post-COVID mental fatigue: a finnish biobank, autopsy and clinical study. Acta Neuropathol Commun. 2021;9(1):199. doi: 10.1186/s40478-021-01302-7.
- Al‐Riyami AZ, Burnouf T, Yazer M, et al. International forum on the collection and use of COVID‐19 convalescent plasma: protocols, challenges and lessons learned: summary. Vox Sang. 2021;116(10):1117–1135. doi: 10.1111/vox.13113.
- European Directorate for the Quality of Medicines & HealthCare (EDQM). Guide to the preparation, use and quality assurance of blood components. 20th ed. Strasbourg: Council of Europe; 2020.
- Tong A, Baumgart A, Evangelidis N, et al. Core outcome measures for trials in people with coronavirus disease 2019: respiratory failure, multiorgan failure, shortness of breath, and recovery. Crit Care Med. 2021;49(3):503–516. doi: 10.1097/CCM.0000000000004817.
- Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. The Lancet Infect Dis. 2020;20(8):e192–e7. doi: 10.1016/S1473-3099(20)30483-7.
- Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveillance: bulletin Europeen Sur Les Maladies Transmissibles = Eur Commun Dis Bull. 2020;25(3):23–30.
- Vauhkonen H, Nguyen PT, Kant R, et al. Introduction and rapid spread of SARS-CoV-2 omicron variant and dynamics of BA.1 and BA.1.1 sublineages, Finland, december 2021. Emerg Infect Dis. 2022;28(6):1229–1232. doi: 10.3201/eid2806.220515.
- Truong Nguyen PT, Plyusnin I, Sironen T, et al. HAVoC, a bioinformatic pipeline for reference-based consensus assembly and lineage assignment for SARS-CoV-2 sequences. BMC Bioinformatics. 2021;22(1):373. doi: 10.1186/s12859-021-04294-2.
- Haveri A, Smura T, Kuivanen S, et al. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, january to february 2020. Euro Surveillance: bulletin Europeen Sur Les Maladies Transmissibles = Eur Commun Dis Bull. 2020;25(11):16–21.
- Virtanen J, Uusitalo R, Korhonen EM, et al. Kinetics of neutralizing antibodies of COVID-19 patients tested using clinical D614G, B.1.1.7, and B 1.351 isolates in microneutralization assays. Viruses. 2021;13(6):996. doi: 10.3390/v13060996.
- Kant R, Nguyen PT, Blomqvist S, et al. Incidence trends for SARS-CoV-2 alpha and beta variants, Finland, spring 2021. Emerg Infect Dis. 2021;27(12):3137–3141. doi: 10.3201/eid2712.211631.
- Fred SM, Kuivanen S, Ugurlu H, et al. Antidepressant and antipsychotic drugs reduce viral infection by SARS-CoV-2 and fluoxetine shows antiviral activity against the novel variants in vitro. Front Pharmacol. 2021;12:755600. doi: 10.3389/fphar.2021.755600.
- Knip M, Parviainen A, Turtinen M, et al. SARS-CoV-2 and type 1 diabetes in children in Finland: an observational study. Lancet Diabetes Endocrinol. 2023;11(4):251–260. doi: 10.1016/S2213-8587(23)00041-4.
- Jokinen M, Edelman S, Krohn K, et al. Neutralizing natural anti-IL-17F autoantibodies protect autoimmune polyendocrine syndrome type 1 (APS-1) patients from asthma. Clin Immunol. 2020;219:108512. doi: 10.1016/j.clim.2020.108512.
- Focosi D, Franchini M, Pirofski LA, et al. COVID-19 convalescent plasma and clinical trials: understanding conflicting outcomes. Clin Microbiol Rev. 2022;35(3):e0020021. doi: 10.1128/cmr.00200-21.
- Jacobs JL, Haidar G, Mellors JW. COVID-19: Challenges of viral variants. Annu Rev Med. 2023;74(1):31–53. doi: 10.1146/annurev-med-042921-020956.
- Rössler A, Riepler L, Bante D, et al. SARS-CoV-2 omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med. 2022;386(7):698–700. doi: 10.1056/NEJMc2119236.
- Iannizzi C, Chai KL, Piechotta V, et al. Convalescent plasma for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2023;2(2):CD013600. doi: 10.1002/14651858.CD013600.pub5.
- Bhimraj AMR, Shumaker AH, Baden L, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19.: Infectious Diseases Society of America; 2023 [cited 2024 25th Jan]. Available from: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/.
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. [cited 2024 25th Jan]. Available from: https://www.covid19treatmentguidelines.nih.gov/.
- Levine AC, Fukuta Y, Huaman MA, et al. Coronavirus disease 2019 convalescent plasma outpatient therapy to prevent outpatient hospitalization: a meta-analysis of individual participant data from 5 randomized trials. Clin Infect Dis. 2023;76(12):2077–2086. doi: 10.1093/cid/ciad088.
- Libster R, Pérez Marc G, Wappner D, et al. Early high-titer plasma therapy to prevent severe covid-19 in older adults. N Engl J Med. 2021;384(7):610–618. doi: 10.1056/NEJMoa2033700.
- Sullivan DJ, Gebo KA, Shoham S, et al. Early outpatient treatment for covid-19 with convalescent plasma. N Engl J Med. 2022;386(18):1700–1711. doi: 10.1056/NEJMoa2119657.
- Bar KJ, Shaw PA, Choi GH, et al. A randomized controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia. J Clin Investig. 2021;131(24) doi: 10.1172/JCI155114.
- Misset B, Piagnerelli M, Hoste E, et al. Convalescent plasma for covid-19–induced ARDS in mechanically ventilated patients. N Engl J Med. 2023;389(17):1590–1600. doi: 10.1056/NEJMoa2209502.
- Horby P, Lim WS, Emberson JR, Recovery Collaborative Group., et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693–704.
- Senefeld JW, Gorman EK, Johnson PW, et al. Mortality rates among hospitalized patients with COVID-19 treated with convalescent plasma: a systematic review and meta-analysis. Mayo Clin Proc Innov Qual Outcomes. 2023;7(5):499–513. doi: 10.1016/j.mayocpiqo.2023.09.001.
- Bartelt LA, Markmann AJ, Nelson B, et al. Outcomes of convalescent plasma with defined high versus lower neutralizing antibody titers against SARS-CoV-2 among hospitalized patients: CoronaVirus inactivating plasma (CoVIP) study. mBio. 2022;13(5):e0175122. doi: 10.1128/mbio.01751-22.
- Casadevall A, Joyner MJ, Pirofski LA, et al. Convalescent plasma therapy in COVID-19: unravelling the data using the principles of antibody therapy. Expert Rev Respir Med. 2023;17(5):381–395.
- Sullivan DJ, Franchini M, Joyner MJ, et al. Analysis of anti-SARS-CoV-2 omicron-neutralizing antibody titers in different vaccinated and unvaccinated convalescent plasma sources. Nat Commun. 2022;13(1):6478. doi: 10.1038/s41467-022-33864-y.
- Cantoni D, Wilkie C, Bentley EM, et al. Correlation between pseudotyped virus and authentic virus neutralisation assays, a systematic review and meta-analysis of the literature. Front Immunol. 2023;14:1184362. doi: 10.3389/fimmu.2023.1184362.
- Franchini M, Focosi D, Percivalle E, et al. Variant of concern-Matched COVID-19 convalescent plasma usage in seronegative hospitalized patients. Viruses. 2022;14(7):1443. doi: 10.3390/v14071443.
- Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585.
