Abstract
Objective
To study the effect of plitidepsin antiviral treatment in immunocompromised COVID-19 patients with underlying haematological malignancies or solid tumours, particularly those who have undergone anti-CD20 therapies.
Design
We conducted a retrospective observational study, involving 54 adults treated with plitidepsin on compassionate use as an antiviral drug. Our analysis compared outcomes between patients with solid tumours and those with haematological malignancies, and a cohort of cases treated or not with anti-CD20 monoclonal antibodies.
Results
Patients with a history of anti-CD20 therapies showed a prolonged time-to-negative RT-PCR for SARS-CoV-2 infection compared to non-treated patients (33 d (28;75) vs 15 (11;25); p = .002). Similar results were observed in patients with solid tumours in comparison to those with haematological malignancies (13 (10;16) vs 26 (17;50); p < .001). No serious adverse events were documented.
Conclusions
Patients with haematological malignancies appear to be at a heightened risk for delayed SARS-CoV-2 clearance and subsequent clinical complications. These findings support plitidepsin as a well-tolerated treatment in this high-risk group. A phase II clinical trial (NCT05705167) is ongoing to evaluate plitidepsin as an antiviral drug in this population.
Haematological patients face an increased risk for severe COVID-19.
Anti-CD20 therapies could increase fatal outcomes in COVID-19 patients.
Persistent viral replication is increased in immunocompromised patients.
Plitidepsin does not lead to new serious adverse events in immunocompromised patients.
KEY POINTS
Background
The dynamic landscape of the coronavirus disease 2019(COVID-19) pandemic has been significantly influenced by the emergence of novel, immune-evasive severe acute respiratory syndrome–related coronavirus 2 (SARS-CoV-2) variants, most notably the Omicron lineage. Concurrently, widespread vaccination campaigns [Citation1] and prior infections have altered the clinical trajectory of COVID-19. Despite these advancements, a subset of the population, particularly individuals with solid tumours and haematological malignancies, remains vulnerable to severe outcomes [Citation2,Citation3].
Patients undergoing cancer therapy often experience immunocompromise, elevating their risk of developing severe COVID-19 pneumonia, potentially leading to treatment delays. Recognising the need for effective antiviral interventions, researchers have sought strategies to improve primary outcomes, such as mortality, and prevent the persistence of SARS-CoV-2 viral replication [Citation4,Citation5].
This study presents the results and clinical characteristics derived from a compassionate use of plitidepsin as an antiviral therapy. This investigation serves as an extension of the previously reported Spanish cohort [Citation6], focusing on hospitalised adult patients at high risk of severe COVID-19 due to solid tumours or haematological malignancies. This cohort notably includes individuals treated with anti-CD20 monoclonal antibodies (mAb), adding a layer of complexity to their vulnerability.
Methods
This study encompassed individuals admitted to general wards with confirmed COVID-19, undergoing treatment with a minimum three-day course of plitidepsin (1.5 or 2.5 mg/d, intravenous (IV), with premedication following available protocols [Citation7]). Eligibility criteria included clinical evidence of a total lymphocyte count < 1 × 103 cells/mm3 or persistent SARS-CoV-2 replication for over 14 d.
Ethical approval was obtained from the Jimenez Diaz Foundation Ethics Committee (EO065-22) v2.3 on January 30, 2023. Data collection spanned from November 2020 to January 2023 across six Spanish centres: Hospital Universitario Quirónsalud Madrid (coordinator), Hospital Universitario HM Sanchinarro, Hospital MD Anderson Cancer Centre Madrid, Hospital Universitario de Burgos, Hospital HLA Universitario Moncloa, and Hospital Clínic Barcelona. Patient monitoring continued until two consecutive negative reverse transcription-polymerase chain reaction (RT-PCR) tests for SARS-CoV-2 or until deceased.
The methods were previously detailed [Citation6] and are also summarised in the supplementary material. Briefly, the primary outcomes included time to respiratory recovery (saturation/fraction of inspired O2 [SpFi] ≥ 315), 30-day cumulative mortality from the first plitidepsin infusion, and time to undetectable SARS-CoV-2 RNA (nasopharyngeal swab samples, RT-PCR). Additional objectives explored correlations between anti-CD20 monoclonal antibody-based therapy and patient mortality, as well as recovery time.
Results
A total of 54 cases were analysed. Of them, 23 (42.6%) were women, 46 (85.2%) were vaccinated against COVID-19, 98.1% had cancer, and 32 (59.3%) were treated with anti-CD20 mAb ( and Supplementary Table 1). Deceased patients had a mean age at hospital admission of 75.6 [standard deviation (SD) 5.9] years, while survivors had a median age of 66.1 (SD 11.8) years (). Of the total of cases with hematological malignancies (n = 42), 73.8% had received therapy with anti-CD20 monoclonal antibodies.
Table 1. Descriptive analysis associated to COVID-19 related mortality.
The median time [95% confidence interval (CI)] from COVID-19 symptoms onset to hospital admission was 14 d (12; 22). Standard care involved dexamethasone and low-molecular-weight heparin, with additional treatments including sotrovimab (46.3%), remdesivir (14.8%), and nirmatrelvir/ritonavir (3.7%) (). The first decile for survival, representing 10% of mortality, was 8 d (6;20). Cumulative mortality at day 30 stood at 21.7% (10.2;35.4) (Supplementary Figure 1).
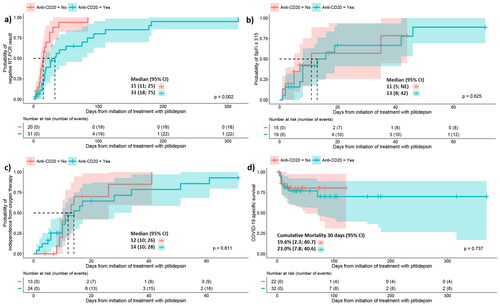
Comparative analysis revealed delayed SARS-CoV-2 clearance in patients under anti-CD20 mAb therapy compared to those without this treatment (log-rank test p-value = .002), showing median times of: 33 d (28;75) vs 15 (11; 25), respectively (). However, no differences were observed in time to reach SpFi ≥ 315 (), independence from oxygen support (), or COVID-19 cumulative mortality at day 30 (no, 19.6% (2.3;40.7) vs yes, 23% (7.8;40.6) ().
Figure 1. Comparative analysis of patients treated with anti-CD20 therapies or not since first administration of plitidepsin. (a) The cumulative incidence curve displays the time to achieve a negative result for nasopharyngeal SARS-CoV-2 RT-PCR. The median time (95% CI) to reach a SARS-CoV-2 RT-PCR negative result was 33 d (18;75) for patients who received anti-CD20 therapy and 15 d (11;25) for those who did not. The log-rank test indicated significant differences (p-value = .002). The analysis included 31 patients previously treated with anti-CD20 therapies and 20 not treated. Three patients were excluded because no RT-PCR was obtained after plitidepsin treatment. (b) The cumulative incidence curve shows the time to reach SpFi ≥315 value after the infusion of plitidepsin. The median time (95% CI) to reach SpFi ≥315 value was 11 d (5;not estimable) for patients who received anti-CD20 therapy and 13 days (8;42) for those who did not. The log-rank test did not reveal significant differences (p-value = 0.625). The analysis involved 19 patients previously treated with anti-CD20 therapies and 15 not treated. Excluded patients never reachedSpFi ≤315 values. (c) The cumulative incidence curve depicts the time to reach independence from oxygen therapy. The median time (95% CI) to reach independence from oxygen therapy was 14 d (10;28) for patients who received anti-CD20 therapy and 12 d (10;26) for those who did not. The log-rank test did not show significant differences (p-value = .611). The analysis included 24 patients previously treated with anti-CD20 therapies and 13 not treated. Excluded patients did not required oxygen therapy after plitidepsin treatment. (d) The estimation of COVID-19-related mortality during follow-up since the first plitidepsin administration is presented. The first decile survival was 8 d (6;71) for patients who received anti-CD20 therapy and 7 d (4;not estimable) days for those who did not. Cumulative mortality at 30 d (95% CI) was 23.0% (7.8;40.6) for patients who received anti-CD20 therapy and 19.6% (2.3;40.7) for those who did not. The log-rank test did not reveal significant differences (p-value = .737). The analysis involved 32 patients previously treated with anti-CD20 therapies and 22 not treated. Crosses on the graphs indicate censored patients. CI: confidence interval; RT-PCR: real time polymerase chain reaction; SpFi: saturation/fraction of inspired oxygen; NE: not estimable.
In a comparison between patients with haematological malignancies and those with solid tumours, individuals with solid tumours demonstrated a faster clearance of SARS-CoV-2, achieving a negative result in RT-PCR in half the time (log-rank test p-value <.001), with a median time of 13 d (10;16) compared to patients with haematological malignancies with 26 d (17;50) (Supplementary Figure 2a). The median time from the administration of plitidepsin to reach SpFi ≥ 315 was 13 d (8;42) in haematological malignancies vs 11 d (7; not estimable) in patients with solid tumours (Supplementary Figure 2b). Similar findings were observed in terms of independence from oxygen support (WHO scale < 4), with patients with haematological malignancies experiencing a median duration of 14 d (10;26) compared to 12.5 d (10;not estimable) for those with solid tumours) (Supplementary Figure 2c). COVID-19 cumulative mortality at day 30 was notably higher in patients with haematological malignancies (24.9%, 11.4;40.9) contrasting with 10.0% (0.0;31.1) in those with solid tumours (Supplementary Figure 2d). No significant differences were found in time to achieve a negative result in RT-PCR for SARS-CoV-2 when we compared haematological patients treated with anti-CD20 therapies with those treated with other therapies (Supplementary Figure 3).
We additionally conducted an analysis of adverse events related to plitidepsin administration (Supplementary Table 2). The data indicate no new serious adverse event in this population. This finding aligns with the observations from prior studies, whether involving plitidepsin in a phase I-II trial [Citation7] or under compassionate use [Citation6].
Discussion
Our investigation into the compassionate use of plitidepsin, a marine-derived cyclic depsipeptide, underscores its flexibility when is used as an antiviral agent for a subset of high-risk immunocompromised COVID-19 patients, including those with a history of anti-CD20 monoclonal antibody (mAb) therapy and hematological malignancies. The findings from our study confirm the safety profile of plitidepsin in the context of SARS-CoV-2 infection [Citation7]. Our observations suggest a potentially favourable impact on clinical outcomes, but merit further scrutiny through randomised clinical trials, looking for a reduction of the mortality rate and the prevention of persistent viral replication as primary outcomes.
It is imperative, however, to acknowledge the inherent limitations of our retrospective study. The absence of a control group and the non-standardized nature of our data collection protocol impose restrictions on the generalisability and robustness of our conclusions. The reliance on clinical judgement for timeline analysis and the relatively modest cohort size, particularly in relation to outcomes associated with anti-CD20 mAb therapy, underscore the numerical limitations that may impact the broader applicability of our findings. Despite these constraints, our study serves as a valuable initial exploration, laying the groundwork for future randomised, placebo-controlled clinical trials aimed at substantiating our presented results.
Notably, our study draws attention to the heightened vulnerability of patients with hematological malignancies, identified as a high-risk group for delayed SARS-CoV-2 clearance [Citation8], resulting in increased susceptibility to severe COVID-19 complications. The confirmed well-tolerated nature of plitidepsin as an antiviral treatment, as evidenced by our data, underscores its potential significance in managing COVID-19 in this particular patient population. Consequently, building on the outcomes of this study, an ongoing randomised phase II clinical trial (NCT05705167) seeks to rigorously evaluate the efficacy of plitidepsin as an antiviral agent in this vulnerable cohort. This ongoing trial holds promise for providing more robust evidence and informing future clinical approaches tailored to manage immunocompromised individuals facing heightened risks associated with COVID-19.
Authors contribution
PGV; JAG; JMJ; JAL conceived the original idea for the study. JAG; PGV; RL designed and drafted the study protocol. JAG and CF developed the case report form and conducted data analysis. JAG collected and assembled the data. CF provided statistical expertise. MGC analysed human samples. PVF; TGD; AFB; CNSF; LMB; FTA; EMMB; VPF; GSF; DCR treated and supplied patient data. JAL; XELE; JMJ; AGC provided scientific and logistical support. JAG; PGV; RL; AGC; CF; JMJ; contributed to the critical review of the article for intellectual content. The current manuscript has undergone review and approval by all authors.
Ethical approval
The study was approved by the Jimenez Diaz Foundation Ethics Committee (EO065-22) v2.3, dated January 30, 2023, in accordance with ethical principles outlined in the Declaration of Helsinki and the guidelines of the International Conference on Harmonization-Good Clinical Practice.
Supplemental Material
Download MS Word (25.9 KB)Supplemental Material
Download MS Word (17.9 KB)Supplemental Material
Download MS Word (16.1 KB)Supplemental Material
Download MS Word (34.1 KB)Supplemental Material
Download MS Word (829.6 KB)Supplemental Material
Download MS Word (110 KB)Acknowledgements
We express our gratitude to the patients and their families for their unwavering support and participation in the study. Without their collaboration, the progress and completion of this research would have been unattainable.
Disclosure statement
JMJ holds stocks of Pangaea Oncology, serves on the Scientific Advisory Board in a non-remunerated role, holds stocks in Promontory Therapeutics, and is a full-time employee and shareholder of PharmaMar, S.A. (Madrid, Spain), and a co-inventor of two patents for Plitidepsin (WO99-42125). JAL is a full-time employee and shareholder of PharmaMar, S.A (Madrid, Spain), and a co-inventor of a patent for Plitidepsin (WO2008135793A1). XELE is a full-time employee of PharmaMar, S.A. (Madrid, Spain). RL is a full-time employee of PharmaMar, S.A (Madrid, Spain). PGV received speaker fees from FLS Science, PharmaMar SA (Madrid, Spain) and GlaxoSmithKline (Spain); consulting fees from Angelini Pharma and PharmaMar SA; served as an advisory board member for Berlin Cures GmbH and PharmaMar SA; and meeting grants from GlaxoSmithKline and PharmaMar SA. DCR received speaking fees from GlaxoSmithKline and Gilead Sciences. JAG received meeting grant from PharmaMar SA and served as a scientific consultant. PVF received speaking fee from GlaxoSmithKline (Spain) and served as an advisory board member for PharmaMar SA. VPF received speaking fees from Janssen, Jazz Pharmaceuticals, and Roche; and consultant fees from Italfarmaco S.A. CF (Heorfy Consulting) has received fees from Hospital Universitario Quirónsalud Madrid for statistical analysis. CN received speaker fees from GILEAD and Pfizer and attending meetings from SEIMC.
All remaining authors have declared no conflicts of interest.
Additional information
Funding
References
- Tan WC, Tan JYJ, Lim JSJ, et al. COVID-19 severity and waning immunity after up to 4 mRNA vaccine doses in 73 608 patients with cancer and 621 475 matched controls in Singapore: a nationwide cohort study. JAMA Oncol. 2023;9(9):1221–1229. doi: 10.1001/jamaoncol.2023.2271.
- Potter AL, Vaddaraju V, Venkateswaran S, et al. Deaths due to COVID-19 in patients with cancer during different waves of the pandemic in the US. JAMA Oncol. 2023;9(10):1417–1422. doi: 10.1001/jamaoncol.2023.3066.
- Ofer J, Drozdinsky G, Basharim B, et al. Mortality and hospitalization risks in patients with cancer and the SARS-CoV-2 omicron variant. JAMA Oncol. 2023;10(1):e235042–e235138. doi: 10.1001/jamaoncol.2023.5042.
- Lee CY, Shah MK, Hoyos D, et al. Prolonged SARS-CoV-2 infection in patients with lymphoid malignancies. Cancer Discov. 2022;12(1):62–73. doi: 10.1158/2159-8290.CD-21-1033.
- Meyerowitz EA, Li Y. Review: the landscape of antiviral therapy for COVID-19 in the era of widespread population immunity and Omicron-Lineage viruses. Clin Infect Dis. 2023;78:908–917.
- Aguareles J, Fernández PV, Carralón-González MM, et al. Outcomes and clinical characteristics of the compassionate use of plitidepsin for immunocompromised adult patients with COVID-19. Int J Infect Dis. 2023;135:12–17.
- Varona JF, Landete P, Lopez-Martin JA, et al. Preclinical and randomized phase I studies of plitidepsin in adults hospitalized with COVID-19. Life Sci Alliance. 2022;5(4):e202101200. doi: 10.26508/lsa.202101200.
- Kang S-W, Kim J-W, Kim JY, et al. Virological characteristics and the rapid antigen test as deisolation criteria in immunocompromised patients with COVID-19: a prospective cohort study. J Med Virol. 2023;95(11):e29228.
