Abstract
Objectives
To investigate receipt of antibiotics among patients with neuroborreliosis after initial antibiotic treatment, likely attributable to posttreatment symptoms.
Methods
We performed a nationwide, matched, population-based cohort study in Denmark (2009-2021). We included all Danish patients with neuroborreliosis, i.e. a positive Borrelia burgdorferi intrathecal antibody index test and a cerebrospinal fluid leukocyte count ≥10 × 106/l, and initially treated with doxycycline. To form a comparison cohort, we randomly extracted individuals from the general population matched 1:10 to patients with neuroborreliosis on date of birth and sex. The main outcome was receipt of doxycycline, and the secondary outcome was receipt of phenoxymethylpenicillin. We calculated short-term (<1 year) and long-term (≥1 year) hazard ratios (HR) with 95% confidence intervals (95%CI).
Results
We included 463 patients with neuroborreliosis and 2,315 comparison cohort members. Compared with the comparison cohort members, patients with neuroborreliosis initially treated with doxycycline had increased receipt of additional doxycycline within 1 year (HR: 38.6, 95%CI: 17.5–85.0) and ≥1 years (HR: 3.5, 95%CI: 1.9–6.3). Compared with comparison cohort members, patients with neuroborreliosis had no increased receipt of phenoxymethylpenicillin (<1 year HR 1.0, 95%CI: 0.7–1.3; ≥1 years HR 1.2, 95%CI: 0.9–1.5).
Conclusions
After initial antibiotic treatment, patients with neuroborreliosis have increased receipt of doxycycline particularly within one year after initial antibiotic therapy but also subsequently. The lack of increased receipt of phenoxymethylpenicillin suggests that the receipt of doxycycline was not merely due to differences in healthcare-seeking behaviour, increased risk of early Lyme borreliosis due to exposure, or differences in antibacterial usage in general.
Introduction
Neuroborreliosis is a nervous system infection caused by the tick-borne bacteria Borrelia burgdorferi sensu lato complex (Bb) [Citation1]. Symptoms include painful meningoradiculitis, lymphocytic meningitis, and/or cranial nerve palsy [Citation1].
While most patients with neuroborreliosis respond well to appropriate antibiotic treatment, some patients report persistent posttreatment symptoms such as fatigue, pain, and cognitive impairment, which may impact quality of life [Citation2–5]. Posttreatment symptoms may lead to concerns of persistent infection and prolonged or supplementary courses of antibiotic treatment [Citation3]. However, no previous study has investigated the use of doxycycline among patients with neuroborreliosis following the initial course of antibiotic therapy.
Therefore, in the present study, we investigated the receipt of additional doxycycline after initial doxycycline treatment among patients with neuroborreliosis.
Methods
We performed a nationwide, population-based, matched registry-based cohort study between May 1, 2009, and January 31, 2021. We compared patients with neuroborreliosis with initial doxycycline treatment with an age- and sex matched comparison cohort from the background population.
Setting
During the years of study inclusion, Denmark had a population of 5.2–5.8 million individuals, and all Danish residents received tax-supported healthcare free of charge [Citation6,Citation7]. Each Danish resident is assigned an individual and unique 10-digit personal identification number at birth or upon immigration, which was used to track individuals through the Danish health and administrative registers [Citation6].
Data sources
We obtained data on all positive Bb intrathecal antibody index test (Bb-AIs) from all Danish departments of clinical microbiology, that performed these tests between May 2009 and January 2021 (Supplementary Table 1). This data source is a complete record of the Bb-AI diagnostic activity in Denmark. Bb specific intrathecal antibody production was measured by capture ELISA using native purified flagella antigen. Correction for blood brain barrier with supplementary measurements of albumin and total IgG is not required for this method. An increased proportion of specific antibodies in cerebrospinal fluid (CSF) directly reflect intrathecal antibody synthesis. The test result was expressed as a specific capture AI index OD-CSF/OD-serum x (OD-CSF- OD-serum). An elevated (positive) Bb-AI was assumed if the specific capture IgG and/or IgM AI was ≥ 0.3 in combination with ODCSF ≥ 0.15. The departments of clinical microbiology used the IDEIA™ Lyme Neuroborreliosis assay, Oxoid limited, UK [Citation8]. At Statens Serum Institut an original in-house version of the same assay was used [Citation9]. This assay was thoroughly validated on consecutive clinical cases [Citation10,Citation11]. Additionally, we obtained data from all the Danish departments of clinical microbiology on all positive CSF cultures, PCRs, and herpes simplex virus AI, and varicella zoster virus AIs between February 1985 and February 2021.
We obtained data on biochemical CSF analyses from the Danish Laboratory Database, established in 2008 [Citation12].
We obtained data on date of birth, sex, date of immigration, date of emigration, country of origin and date of death from the Danish Civil Registration System, which was established in 1967 [Citation13].
We obtained data on annual taxable income from the Income Statistics Register [Citation14].
We obtained data on diagnoses codes and date of diagnoses from the Danish National Patient Registry (DNPR) [Citation15,Citation16].
We obtained data on date of prescription medication, including type and date of redemption, from the Danish National Prescription Registry which was established in 1995 [Citation17,Citation18]. Medications are classified according to the Anatomical Therapeutic Chemical (ATC) Classification System [Citation19].
Study population
Neuroborreliosis cohort
We included all Danish residents who had a first-time positive Bb-AI combined with a CSF leukocyte count ≥ 10x106/L and reception of ≥20 Defined Daily Doses of doxycycline (ATC code: J01AA02) within −2 to 28 days from the positive Bb-AI. We excluded all patients with neuroborreliosis who had a positive CSF culture or PCR for other pathogens, herpes simplex virus AI, or varicella zoster virus AI at any point between February 1987 and up to 14 days after the positive Bb-AI. We defined the date of study inclusion as 14 days after receipt of doxycycline or positive Bb-AI whichever came last.
Comparison cohort
To create a comparison cohort, we randomly extracted individuals without a positive Bb-AI from the Danish Civil Registration System matched 1:5 to patients with neuroborreliosis on date of birth and sex. The comparison cohort members were assigned the same date of study inclusion as the corresponding patient with neuroborreliosis.
Outcomes
The primary outcome was time-to receipt of doxycycline. To control for potential confounding, we included time-to receipt of phenoxymethylpenicillin (ATC code: J01CE02) as a measure of healthcare-seeking behaviour, treatment for early Lyme borreliosis as per Danish guidelines [Citation20], or antibiotic use unrelated to neuroborreliosis.
Statistical analyses
We calculated observation time from study inclusion until event of interest, date of death, date of emigration, loss to follow-up, 6 years after study inclusion or September 1, 2021, whichever occurred first. We used cumulative incidence function with death as a competing risk to calculate cumulative incidences and used a Cox-regression model to calculate hazard ratios (HR) and corresponding 95% confidence intervals (95%CIs) within <1 year and ≥1 years after study inclusion.
Stratified analyses
We stratified the analyses according to age at study inclusion (<16 years, and ≥16 years), and according to the CSF leukocyte count of patients in the neuroborreliosis cohort (10–69 × 106/l, 70–199 × 106/l, and ≥200 × 106/l). The choice of intervals for CSF leukocytes counts was based on stratifying the neuroborreliosis patients in tertiles.
Sensitivity analysis
We performed a sensitivity analysis excluding patients in the neuroborreliosis cohort (and their corresponding comparison cohort members) without a hospital assigned diagnosis of borreliosis in the Danish National Patient Registry (International Classification of Diseases, tenth revision: A69.2) within 30 days of the positive Bb-AI. This diagnosis code does not distinguish between the different clinical manifestations of borreliosis.
Results
We included 463 patients in the neuroborreliosis cohort initially treated with doxycycline, and 2,315 comparison cohort members with a total observation time of 10,741 years (). In both cohorts, the median age was 57 years, 10% of individuals were younger than 16 years, and the proportion of females was 40%. The patients in the neuroborreliosis cohort had higher annual taxable income and a greater proportion was born in Denmark than among comparison cohort members. A fifth of both patients in the neuroborreliosis cohort and comparison cohort had a Charlson Comorbidity Index score ≥2. Among patients in the neuroborreliosis cohort, 386 (84%) were assigned a hospital diagnosis code of borreliosis.
Table 1. Characteristics and observation time of patients in the neuroborreliosis cohort and comparison cohort members. Values are shown in numbers (percentage) unless otherwise stated.
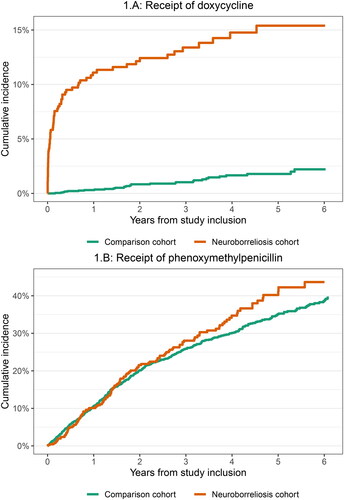
Compared with comparison cohort members, patients in the neuroborreliosis cohort had increased receipt of doxycycline (<1 year HR: 38.6, 95%CI: 17.5–85.0; ≥1 year HR: 3.5, 95%CI: 1.9–6.3) ().
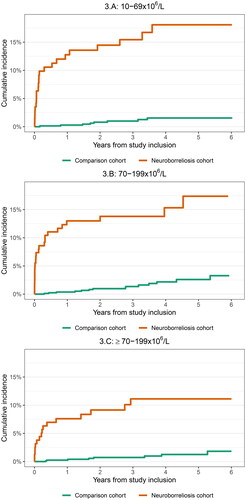
When we stratified the analyses for age and CSF leukocyte count, the risk estimates did not change substantially. Both patients in the neuroborreliosis cohort aged <16 years and ≥16 years had increased receipt of doxycycline compared with the comparison cohort: <1 year HR not available due to no outcomes among the comparison cohort members and 37.2: 16.9–82.1 respectively and > 1 year 3.6: 0.9–15.1 and 3.5: 1.8––6.8. Patients in the neuroborreliosis cohort with low CSF leukocyte count (10–69 × 106/l) had slightly higher risk of receipt of doxycycline than patients with higher leukocyte count (70–199 × 106/l and >200 × 106/l): <1 year HR 48.0, 95%CI: 11.1–207.1, 37.3: 11.1–125.0 and 31.2: 7.0–139.2 respectively and > 1 year 7.2: 2.4–21.5, 2.4: 0.8–7.0, 3.0: 1.0–9.0. The cumulative incidence of receipt of doxycycline was lower among patients <16 years and patients with high CSF leukocyte count ( and ).
Figure 2. Cumulative incidence of redemption of doxycycline stratified on age groups (<16 years, and ≥16 years old).
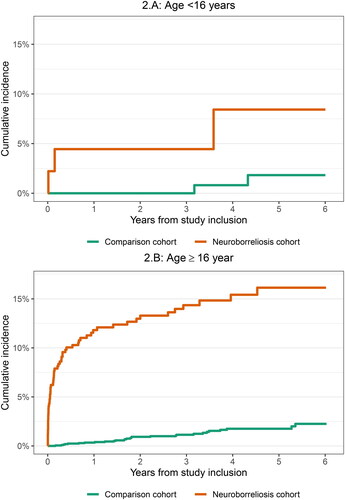
Figure 3. Cumulative incidence of redemption of doxycycline stratified on cerebrospinal fluid leukocyte count (10-69 × 106/l, 70-199 × 106/l, and ≥200 × 106/l).
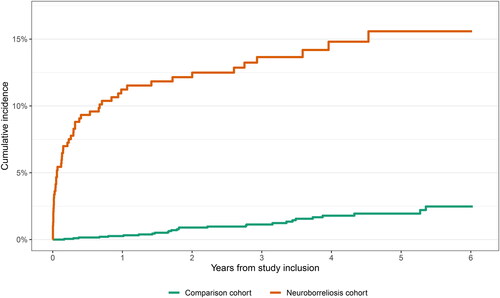
Exclusion of patients in the neuroborreliosis cohort without an assigned diagnosis of borreliosis did not change the results substantially (). After exclusion of patients in the neuroborreliosis cohort without an assigned diagnosis of borreliosis, patients in the neuroborreliosis cohort had increased receipt of doxycycline (<1 year HR 45.4, 95%CI: 18.0–114.7; > 1 year HR 2.6, 95%CI: 1.4–5.2) compared with comparison cohort members.
Figure 4. Cumulative incidence of receipt of doxycycline after exclusion of patients with neuroborreliosis without an assigned diagnosis of borreliosis (international classification of diseases, 10th revision: A69.2).
Compared with comparison cohort members, patients in the neuroborreliosis cohort had no increased receipt of phenoxymethylpenicillin (<1 year HR 1.0, 95%CI: 0.7–1.3; ≥1 years HR 1.2, 95%CI: 0.9–1.5) ().
Discussion
In this nationwide, population-based, matched cohort study, we found that patients with neuroborreliosis initially treated with doxycycline had increased short- and long-term receipt of additional doxycycline compared with the background population.
The increased receipt of doxycycline could be caused by (1) persistent symptoms after initial antibiotic therapy for neuroborreliosis, (2) change in healthcare-seeking behaviour, (3) reinfection with Bb due to tick-exposure and subsequent treatment, or (4) differences in antibacterial usage unrelated to neuroborreliosis.
Frequent posttreatment symptoms after antibiotic therapy have been reported among patients with neuroborreliosis or disseminated borreliosis [Citation2–5]. Such posttreatment symptoms may lead to concerns by either the patient or the treating clinician regarding persistent infection or co-infection with other tick-borne pathogens despite completed antibiotic treatment. The frequency of posttreatment symptoms is more pronounced immediately after antibiotic therapy [Citation4]. Our results show a similar pattern of receipt of doxycycline after the initial treatment of neuroborreliosis, which supports the notion that posttreatment symptoms likely contribute to repeated antibiotic therapy.
Nordberg et al. demonstrated that patients with neuroborreliosis and low CSF leukocyte counts experienced longer symptom duration prior to diagnosis, and that longer symptom duration was associated with poorer outcome [Citation2]. Conversely, we could not demonstrate differences in the risk of receiving doxycycline based on the CSF leukocyte count. Although we observed a trend where patients with neuroborreliosis and low CSF leukocyte counts more frequently received doxycycline compared to those with higher CSF leukocyte counts, the wide confidence intervals for the estimates suggest that this could be a chance finding.
Potential causes for persistent symptoms include sustained inflammation or persistent infection after initial antibiotic treatment. Although, patients with previous borreliosis with persistent symptoms did not show inflammation in the CSF [Citation21,Citation22], those with persistent symptoms after treatment for neuroborreliosis have higher blood levels of interferon-α which supports the hypothesis of sustained inflammation [Citation23,Citation24]. Although limited by low sensitivity, CSF cultures and PCRs for Bb in patients with persistent symptoms can not demonstrate persistent infection [Citation21,Citation25].
Regardless of whether persistent symptoms could be caused by sustained inflammation or persistent infection, randomised clinical trials have not demonstrated an overall beneficial effect of repeated antibiotic therapy for posttreatment symptoms in patients with borreliosis [Citation21,Citation22,Citation26–28] or in patients with neuroborreliosis [Citation29]. Furthermore, repeated antibiotic treatment may cause adverse effects [Citation22,Citation30,Citation31] and contribute to development of antimicrobial resistance [Citation32]. Therefore, our results could indicate unnecessary and unwarranted use of antibiotics in patients with neuroborreliosis.
The increased receipt of doxycycline among patients with neuroborreliosis after initial antibiotic therapy could also be attributed to a change in healthcare-seeking behaviour, or differences in antibacterial usage unrelated to neuroborreliosis. However, such confounding would likely also lead to an increased receipt of phenoxymethylpenicillin among the patients in the neuroborreliosis cohort, but we found no such increase.
The increased receipt of doxycycline could in part be due to treatment of new Bb infections. Patients with previous neuroborrelioses may be at increased risk of new tick-bite due to geographical location and risk behaviour. However, reinfections alone cannot convincingly explain the notably increased receipt of doxycycline within the first year after the initial antibiotic treatment for neuroborreliosis. Patients with previous neuroborreliosis likely actively reduce the risk of new tick-bites or are keener on removing ticks, which would minimise the risk of reinfection. As phenoxymethylpenicillin is the recommended antibiotic treatment for erythema migrans in Denmark [Citation20], and we could not demonstrate an increased risk of receipt of phenoxymethylpenicillin, it is unlikely that reinfections could explain the increased risk of receipt of doxycycline.
Although smaller in magnitude than the short-term increased risk, the long-term increased risk of receipt of doxycycline could be attributed to persistent symptoms or to reinfections.
Strengths and limitations
The strengths of this study include the nationwide design and the high-quality data accessible at an individual level in the Danish national health and administrative registries.
We only had access to data on medication redeemed at a pharmacy in Denmark, and the registration of the intravenous antibiotic treatments in the Danish Patient Registry is incomplete, why it was not included in our analyses. Thus, we lacked data on antibiotics handed out or given intravenously at a clinic or redeemed abroad. While we assume that this potential misclassification is non-differential and, therefore, unlikely to impact the relative risk estimates, it is possible that the cumulative incidence of receipt of antibiotics may have been underestimated due to this misclassification. Finally, we lacked data on indication for the prescribed antibiotic treatments, which could have provided valuable insights into the reason for the repeated receipt of doxycycline.
Conclusion
Within the first year after initial doxycycline treatment for neuroborreliosis, approximately every tenth patient received additional doxycycline. The receipt of doxycycline among patients with neuroborreliosis diminished after the first year but continued to be increased. Thus, patients with neuroborreliosis frequently receive repeated antibiotic treatment. Future research should focus on causes for the repeated antibiotic use and how to reduce it.
Authors’ contributions
MMT, LO and NO have full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. MMT was responsible for concept and statistical analyses and drafted the first version of manuscript. RBD, SE, NSA, CSJ, MP, JB, KKS, JB, ACN, and JKM were responsible for the data collection and edited the manuscript. LHO and NO were responsible for statistical analyses, concept and edited the manuscript. AML were responsible for concept and edited the manuscript. All authors contributed to the data interpretation. All authors amended and approved the final version of the manuscript. MMT assumes full responsibility for the integrity of the submission, from inception to published article.
Role of the funder
The Independent Research Fund Denmark, the Research Fund of Copenhagen University Hospital - Rigshospitalet, and the Lundbeck Foundation had no role in the design and conduct of the study; collection, management, analyses, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supplemental Material
Download MS Word (15.5 KB)Acknowledgments
Emma E. Graham, MD., provided a revision of the language and grammar in this manuscript.
Disclosure statement
MMT has received travel grants outside this work from GlaxoSmithKline Pharma A/S. RBD has advisory board activity with Pfizer. AML reports speakers’ honorarium/travel grants/advisory board activity and unrestricted grant from Gilead, speakers honorarium/travel grants from GSK, speaker’s honorarium/advisory board activity from Pfizer outside this work. AML was supported by a grant from the Lundbeck foundation. None of the authors report any conflict of interests in connection with this article.
Data availability statement
The ethical approval of this study from the Danish Data Protection Agency states the data that has been used in this article cannot be shared publicly.
Additional information
Funding
References
- Stanek G, Wormser GP, Gray J, et al. Lyme borreliosis. Lancet. 2012; 379(9814):461–473. doi:10.1016/S0140-6736(11)60103-7.
- Nordberg CL, Bodilsen J, Knudtzen FC, et al. Lyme neuroborreliosis in adults: a nationwide prospective cohort study. Ticks Tick Borne Dis. 2020; 11(4):101411.
- Knudtzen FC, Andersen NS, Jensen TG, et al. Characteristics and clinical outcome of lyme neuroborreliosis in a high endemic area, 1995-2014: a retrospective cohort study in Denmark. Clin Infect Dis. 2017; 65(9):1489–1495. doi:10.1093/cid/cix568.
- Ursinus J, Vrijmoeth HD, Harms MG, et al. Prevalence of persistent symptoms after treatment for lyme borreliosis: a prospective observational cohort study. Lancet Reg Health Eur. 2021; 6:100142.
- Dersch R, Sommer H, Rauer S, et al. Prevalence and spectrum of residual symptoms in Lyme neuroborreliosis after pharmacological treatment: a systematic review. J Neurol. 2016; 263(1):17–24. doi:10.1007/s00415-015-7923-0.
- Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi:10.2147/CLEP.S179083.
- Statistics Denmark. Population figures [Internet]. [cited Feb 24, 2022]. Available from: https://www.dst.dk/en/Statistik/emner/borgere/befolkning/befolkningstal.
- Oxoid - Product Detail. [Internet]. [cited Feb 1, 2022]. Available from: http://www.oxoid.com/UK/blue/prod_detail/prod_detail.asp?pr=K602811&c=UK&lang=EN.
- Hansen K, Lebech AM. Lyme neuroborreliosis: a new sensitive diagnostic assay for intrathecal synthesis of Borrelia burgdorferi–specific immunoglobulin G, A, and M. Ann Neurol. 1991; 30(2):197–205.
- Hansen K. Lyme neuroborreliosis: improvements of the laboratory diagnosis and a survey of epidemiological and clinical features in Denmark 1985-1990. Acta Neurol Scand Suppl. 1994;151:1–44.
- Hansen K, Lebech AM. The clinical and epidemiological profile of Lyme neuroborreliosis in Denmark 1985-1990. A prospective study of 187 patients with Borrelia burgdorferi specific intrathecal antibody production. Brain. 1992; 115 (Pt 2)(2):399–423. doi:10.1093/brain/115.2.399.
- Danish Health Data Authority. The laboratory database [Internet]. [cited Jun 26, 2023]. Available from: https://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-nationale-sundhedsregistre/doedsaarsager-og-biologisk-materiale/laboratoriedatabasen.
- Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011; 39(7 Suppl):22–25. doi:10.1177/1403494810387965.
- Documentation of statistics: Income Statistics. [Internet]. [cited Jul 12, 2022]. Available from: https://www.dst.dk/en/Statistik/dokumentation/documentationofstatistics/income-statistics.
- Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011; 39(7 Suppl):30–33. doi:10.1177/1403494811401482.
- Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi:10.2147/CLEP.S91125.
- Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, et al. Data resource profile: the Danish national prescription registry. Int J Epidemiol. 2017; 46(3):798–798f. doi:10.1093/ije/dyw213.
- Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011; 39(7 Suppl):38–41. doi:10.1177/1403494810394717.
- WHO Collaborating Centre for Drug Statistics Methodology, Folkehelseinstituttet (Noruega). Guidelines for ATC classification and DDD assignment 2011. Oslo: WHO Collaborating Centre for Drug Statistics Methodology: Norwegian Institute of Public Health; 2010 [Internet]; [cited Jul 12, 2022]. Available from: http://www.whocc.no/filearchive/publications/2011guidelines.pdf.
- Dessau RB, Bangsborg J, Hansen K, et al. Lyme Borreliosis: clinical presentation, diagnostics and treatment in Denmark [Internet]. 2014. Danish Society of Clinical Microbiology, Danish Society of Infectious Diseases, and Danish Society of Neurology. Available from: https://www.infmed.dk/guidelines.
- Klempner MS, Hu LT, Evans J, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001; 345(2):85–92. doi:10.1056/NEJM200107123450202.
- Krupp LB, Hyman LG, Grimson R, et al. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology. 2003; 60(12):1923–1930. doi:10.1212/01.wnl.0000071227.23769.9e.
- Hernández SA, Ogrinc K, Korva M, et al. Association of persistent symptoms after lyme neuroborreliosis and increased levels of interferon-α in blood. Emerg Infect Dis. 2023; 29(6):1091–1101. doi:10.3201/eid2906.221685.
- Jacek E, Fallon BA, Chandra A, et al. Increased IFNα activity and differential antibody response in patients with a history of Lyme disease and persistent cognitive deficits. J Neuroimmunol. 2013; 255(1–2):85–91. doi:10.1016/j.jneuroim.2012.10.011.
- Klempner MS. Controlled trials of antibiotic treatment in patients with post-treatment chronic Lyme disease. Vector Borne Zoonotic Dis. 2002;2(4):255–263. doi:10.1089/153036602321653842.
- Fallon BA, Keilp JG, Corbera KM, et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. 2008; 70(13):992–1003. doi:10.1212/01.WNL.0000284604.61160.2d.
- Berende A, ter Hofstede HJM, Vos FJ, et al. Randomized trial of longer-term therapy for symptoms attributed to lyme disease. N Engl J Med. 2016; 374(13):1209–1220. doi:10.1056/NEJMoa1505425.
- Berende A, Ter Hofstede HJM, Vos FJ, et al. Effect of prolonged antibiotic treatment on cognition in patients with Lyme borreliosis. Neurology. 2019; 92(13):e1447–e1455. doi:10.1212/WNL.0000000000007186.
- Sjöwall J, Ledel A, Ernerudh J, et al. Doxycycline-mediated effects on persistent symptoms and systemic cytokine responses post-neuroborreliosis: a randomized, prospective, cross-over study. BMC Infect Dis. 2012; 12(1):186. doi:10.1186/1471-2334-12-186.
- Marzec NS, Nelson C, Waldron PR, et al. Serious bacterial infections acquired during treatment of patients given a diagnosis of chronic lyme disease—United States. MMWR Morb Mortal Wkly Rep. 2017;66(23):607–609. doi:10.15585/mmwr.mm6623a3.
- Holzbauer SM, Kemperman MM, Lynfield R. Death due to community-associated clostridium difficile in a woman receiving prolonged antibiotic therapy for suspected lyme disease. Clin Infect Dis. 2010; 51(3):369–370. doi:10.1086/654808.
- Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014; 5(6):229–241. doi:10.1177/2042098614554919.