Abstract
Background
Hospital-acquired pneumonia (HAP) is the most common hospital-acquired infection (HAI). HAP is associated with a high burden of morbidity and mortality, but the diagnosis is difficult to establish and the incidence uncertain.
Methods
Patients aged ≥ 18 years hospitalised with radiologically verified non-ventilator hospital acquired pneumonia (NV-HAP) during 2018 were retrospectively identified at Drammen Hospital, a Norwegian general hospital. Infectious Diseases Society of America and the American Thoracic Society’s definition of HAP was used.
Results
In total 119 cases of NV-HAP were identified among 27,701 admissions. The incidence was 4.3 per 1000 admissions and 1.2 per 1000 patient-days. The median age was 74 years, 63% were male and median Charlson comorbidity index was 5. Coronary heart disease (42%) was the most common comorbidity. Median length of stay was 17.2 days. A blood culture was obtained in 53.8% of patients, while samples from lower airways were seldom obtained (10.9%). In-hospital mortality was 21%, accumulated 30-day mortality was 27.7% and accumulated 1-year mortality was 39.5%. Thirty-day readmission rate among survivors was 39.4%.
Conclusion
NV-HAP was present in approximately 1 in 250 hospitalisations, most had multiple comorbidities, and 1 in 5 died in hospital. Although thorough microbiological sampling is recommended when NV-HAP is suspected, our data indicate that airway sampling is infrequent in clinical practice. Our findings underscore the need to develop microbiological diagnostic strategies to achieve targeted antimicrobial treatment that may improve patient outcomes and reduce broad-spectrum antibiotic usage.
Introduction
Hospital-acquired pneumonia (HAP) is the most common hospital-acquired infection (HAI) in Europe [Citation1]. HAP is often divided into non-ventilator hospital-acquired pneumonia (NV-HAP) and ventilator-associated pneumonia (VAP). Pneumonia accounts for about 22% of the burden of all HAIs, of which 61% can be attributed to NV-HAP, and 39% attributed to VAP [Citation2]. The reported incidence of NV-HAP ranges from 4.9 to 21.2 per 1000 hospital admissions and 1.25 to 5.9 per 1000 patient days [Citation3–7]. The variation in incidence is due to inherent difficulties in establishing a true diagnosis, different diagnostic criteria used, including International Classification of Diseases (ICD) coding, Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) criteria [Citation8] and Center for Disease Control (CDC) surveillance criteria [Citation9]. In clinical practice, a tentative diagnosis of HAP and the decision to initiate antibiotic treatment is often based on clinical suspicion alone, as illustrated in the IDSA/ATS guidelines. Consequently, it is difficult to procure objective HAP surveillance data. A recently published study explored 10 candidate definitions for NV-HAP based upon various combinations of parameters including worsened oxygenation, fever, high or low leukocyte levels, chest X-ray (CXR) being obtained, microbiological sampling from lower airways and three or more days of antibiotic treatment [Citation6]. Depending on how these parameters were weighted and combined, NV-HAP incidence ranged from 2 to 34 per 1000 hospital admissions and 4 to 64 per 1000 patient-days.
The burden of HAP is significant, both for affected patients and hospitals. Reported mortality for NV-HAP ranges from 13 to 30% [Citation2, Citation5–7,Citation10–13]. Together, NV-HAP and VAP are the leading causes of death among HAIs, underscoring their clinical importance [Citation14]. Furthermore, such infections are associated with an increase in average length of stay (LOS) of 4.0–15.9 days [Citation2, Citation11]. The average cost of an NV-HAP episode is estimated to be $38,242 [Citation15] and $49,444 [Citation3] (Adjusted for inflation from year of publication to current (2023) using the Bureau of Labor Statistics CPI Inflation Calculator).
Despite its relatively high frequency, most hospitals do not track, report or target NV-HAP for preventive measures [Citation16]. In addition, the mortality increases when multidrug resistant (MDR) pathogens are present [Citation17]. Due to this risk, patients are empirically treated with broad-spectrum antibiotics, which may contribute to antimicrobial resistance and result in undesired side effects. Previous studies of NV-HAP have mainly been performed in intensive care units (ICUs) and are based on combined cohorts of NV-HAP and VAP [Citation18–21]. Thus, there is minimal data on incidence, microbiological sampling and microbiological aetiology specifically for NV-HAP which primarily occur in general wards.
More data is needed, especially on NV-HAP acquired outside of the ICU to improve diagnosis and management. Available data on the incidence of pneumonia in Norway (not limited to NV-HAP and including all ages) estimate incidence rates of 5.28–5.35/1000 person-years [Citation22]. To our knowledge, there are no published studies on the incidence of HAP in Norway.
The purpose of this study was retrospectively to determine the incidence, LOS and mortality associated with NV-HAP in a Norwegian general hospital. We also wanted to review antimicrobial treatment, microbiological sampling techniques and evaluate microbiological findings in the NV-HAP retrospective cohort.
Materials and methods
Study design and participants
We performed a single-centre retrospective study of patients hospitalized at Drammen Hospital, Norway, throughout 2018. During the study period, the 344-bed general hospital had 27,701 admissions and 96,239 patient-days among 20,182 unique patients. The ICUs had 2278 admissions and 3096 patient-days.
Our definition of NV-HAP was based on the Infectious Diseases Society of America (IDSA) and the American Thoracic Society’s (ATS) guidelines for treatment of HAP [Citation8]. NV-HAP was defined as a new or progressive radiographic infiltrate in a patient who had been admitted to the hospital for more than 48 h or readmitted less than 48 h after discharge plus at least two of the following clinical features: Temperature ≥38.0 °C or <36.0 °C, white blood cell (WBC) count >11.0 or <3.5 × 109 cells/L, purulent airway secretions or reduced oxygenation defined as peripheral oxygen saturation (SpO2) decline of ≥5% with unchanged fraction of inspired oxygen (FiO2) or any reduction in ratio of arterial oxygen partial pressure (PaO2) to FiO2 (P/F-ratio). The presence of a radiographic infiltrate was based on a radiology specialist’s interpretation.
In 2018, the national Norwegian guidelines for NV-HAP recommended empirical treatments adapted to the risk of an infection with a multiresistant microbe (MRM) [Citation23]. When an infection without the risk of MRMs was suspected, the initial recommendation was benzylpenicillin or ampicillin combined with gentamicin. Accepted alternative regimens were amoxicillin-clavulanic acid, cefuroxime and cefotaxime. When an infection with the risk of MRMs was suspected, the empirical recommendations included piperacillin-tazobactam, ceftazidime or meropenem combined with gentamicin, tobramycin or ciprofloxacin [Citation23]. The risk factors for MRMs were defined as (1) hospital stay > 4–5 days, (2) previous treatment with antibiotics, (3) immunosuppression and (4) local high prevalence of multi-resistant microbes. The national guidelines did not specify the number of risk factors needed to justify broadening the empirical therapy. For this study, we decided to define the risk of MRMs as when two or more risk factors were present.
Data source, method for selection and eligibility criteria
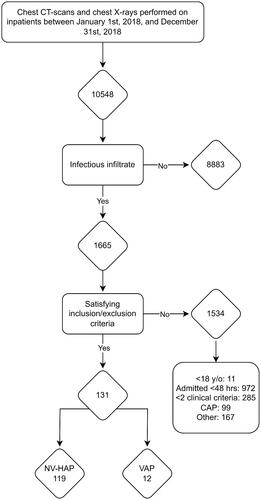
A search in the hospital radiology software was set up to include all chest X-rays and computed tomography (CT) thorax-scans performed on adult inpatients ≥18 years of age, between January 1st, 2018, and December 31st, 2018, and a list of radiology reports was compiled. All radiology exams are routinely reviewed by either a specialist in radiology, or a junior radiologist with subsequent review by a specialist in radiology. The radiology reports extracted from the search were then reviewed by one of the study investigators to ascertain if the description contained findings of a possible infectious origin. Double-checking was not performed in the screening process due to a lack of resources to review the large amount of radiology reports.
For all patients considered eligible, electronic patient journals were systematically reviewed and evaluated for inclusion according to the IDSA/ATS definition. We excluded cases where (1) the patient received treatment for community-acquired pneumonia (CAP) with no suspicion of secondary infection, (2) the treating clinician did not consider pneumonia to be a likely diagnosis, (3) the patient was already included in the study, (4) the patient was transferred from a different hospital with ongoing treatment for NV-HAP and (5) the patient was treated with invasive mechanical ventilation for more than 48 h in the immediate days up to the potential inclusion point. Patients who had been treated with invasive mechanical ventilation for less than 48 h were considered eligible. Previous mechanical ventilation during the hospital stay was not considered a reason for exclusion.
Data collection and management
Demographic variables including sex, age, comorbidities, admission types, LOS and mortality were registered. Inflammatory and clinical parameters such as C-reactive protein (CRP), WBC count, national early warning score 2 (NEWS2), Sequential Organ Failure Assessment (SOFA) and a Charlson Comorbidity Index (CCI) score were compiled. We also collected data on microbiological sampling, microbiological results and whether the patient received antibiotic treatment in accordance with recommendations in national guidelines. We collected data on risk factors for MRMs and considered whether the prescribed regimen was appropriate, too narrow spectrum or too broad spectrum. De-identified data were collected and managed using the approved electronic registry tool Ledidi (Ledidi.com). A list of codes was stored in a separate approved software, MedInsight (Medinsight.no).
Statistical analysis
Statistical analyses were performed using integrated statistical analysis tools in Ledidi. The results are expressed as the number of patients (%) for categorical variables. The Shapiro-Wilk test was used to assess normality for continuous variables. Due to some variables not being normally distributed, continuous variables are expressed as median (IQR lower-IQR-upper). Incidence data are expressed as per 1000 admissions and per 1000 patient-days.
Results
We identified 10,548 radiological thorax examinations () among inpatients between January 1st, 2018, and December 31st, 2018, of which 1665 examinations contained a description of a new or progressing infiltrate. Of these, 119 cases of NV-HAP were identified. Leukocytosis or leukopoenia was present in 87 patients (73.1%), fever or hypothermia in 72 (60.5%), purulent secretions in 43 (36.1%) and reduced oxygenation in 99 (83.2%).
Within all hospital wards the incidence of NV-HAP was 4.3 per 1000 admissions and 1.2 per 1000 patient-days. In the ICUs the incidence was 7.5 per 1000 ICU admissions and 5.5 per 1000 patient-days.
The median time from admission to diagnosis of NV-HAP was 6.6 (IQR 4.2–13.0) days, 73.9% were emergency admissions, 85.7% were diagnosed in regular wards and 14.3% in ICUs. Patient characteristics at time of diagnosis are listed in . Among patients diagnosed outside of the ICUs, 42.9% were admitted to an ICU, with 10.9% requiring intubation and mechanical ventilation during their ICU stay. Clinical outcomes are listed in . In-hospital mortality was 21%, and 39.4% of surviving patients were readmitted within 30 days.
Table 1. Baseline demographics, clinical and biochemical features at time of inclusion.
Table 2. Clinical outcomes.
Seventy (58.8%) patients had some form of microbiological sampling performed. Blood cultures were the most common sampling method (53.8%), followed by nasopharynx sampling for polymerase chain reaction (24.4%). Only 14 lower airway samples were obtained among 13 individual patients (10.9%). The microbiological culture results were considered clinically significant in 69.2% of the 14 samples. Only two of the isolated microbes were MDR, one Escherichia coli found in blood culture and one Proteus mirabilis found in mini-BAL. Microbiological sampling and findings are detailed in . Antimicrobial resistance testing is detailed in Supplementary Table 1.
Table 3. Microbiological sampling and findings.
The median antibiotic treatment duration was 9.0 (7.0–12.0) days. Sixty-two (52.1%) patients had at least two risk factors for MRMs present. Hospital stay >4–5 days was the most common risk factor (73.9%) and previous antibiotic treatment the second most common (57.1%). Immunosuppression was present in 16.8% of patients. At the time of the study, there were no outbreaks of MRM-related infections in the hospital. An empirical antibiotic regimen listed in national guidelines [Citation23] was prescribed to 85.7% of patients. Considering risk factors, patients could be prescribed a regimen which was appropriate, too narrow, or too broad spectrum. We classified patients into two groups, either with the risk of MRMs or without the risk of MRMs, based upon the presence of at least two risk factors. With our interpretation of national guidelines, 52.9% of the patients were prescribed a regimen considered appropriate, 36.1% of the patients were prescribed a regimen considered too narrow and 10.9% were prescribed a regimen considered too broad spectrum. Adjuvant treatment with antifungal or antiviral drugs was administered to 7.2% and 3.5% of the patients, respectively.
Discussion
We observed an NV-HAP incidence of 4.3 per 1000 admissions and 1.2 per 1000 patient days, which is comparable to other published studies. Sopena and Sabrià [Citation5] found an incidence of 3 ± 1.4/1000 hospital admissions in a prospective multicentre study. Jones et al. [Citation7] using an electronic surveillance definition applied to clinical data estimated 5.5 NV-HAP events per 1000 hospitalisations, which is slightly higher than our findings. Giuliano et al. [Citation3] found an incidence of 1.9% representing a rate of 3.63 per 1000 patient-days in the 2012 US National Inpatient Sample dataset.
Ji et al. [Citation6] evaluated possible surveillance definitions for NV-HAP. The incidence ranged from 2 to 34 per 1000 admissions and 0.4 to 6.4 per 1000 patient-days depending on the chosen clinical criteria. This highlights the difficulty of defining NV-HAP in hospital populations. There is general agreement that NV-HAP is a pneumonia developing at least 48 h after admission, in a patient not on invasive mechanical ventilation. However, different studies choose different clinical, radiological, and microbiological NV-HAP criteria. Furthermore, the methods of screening and patient inclusion vary greatly. We chose radiology reports as the first step in delineating the study population. Other studies have used ICD coding for pneumonia as a secondary diagnosis [Citation3,Citation4], and others have used microbiological sampling from airways as the first step [Citation11]. Consequently, the choice of study design and case definition will influence incidence rates.
We decided to use the IDSA/ATS criteria as basis for our inclusion criteria as the IDSA/ATS case definition has been used in much of the existing HAP literature. We used screening of radiology reports as our initial step in delineating the study population because the radiological criterion is obligate in the IDSA/ATS definition of pneumonia and as such, we would not miss any potential inclusions. Chest CT is the reference standard for the radiological diagnosis of pneumonia [Citation24–27]. However, routinely performing CT-scans of all patients with suspected pneumonia is not feasible due to a high cost. Although less sensitive and specific, the conventional CXR is cheap, quick and easy to conduct. For identifying pneumonia, the reported sensitivity of CXR ranges from 32% to 77.7% and specificity from 58.8% to 94% [Citation24,Citation25,Citation28,Citation29]. Temperature, leucocyte numbers and oxygenation values are easily obtained for most patients, but the presence or absence of purulent sputum might not be registered in the medical records. Retrospective studies of HAP have used an ICD code for pneumonia as a secondary diagnosis as an inclusion criterion, however, the sensitivity of coding for NV-HAP is only 40% to 60% [Citation6]. Unfortunately, an ICD code specifically for HAP does not exist. The use of microbiological sampling as an obligatory criterion would have resulted in a vast underestimation of incidence in our study due to the low frequency of airway sampling. Although time consuming, we believe our screening and inclusion procedure is the most comprehensive for such a retrospective study.
Hospital- or healthcare-acquired infections (HAI) are responsible for high morbidity, mortality and increases in length of hospital stay. Identifying patients particularly at risk of developing NV-HAP and applying evidence based preventative measures would therefore be beneficial to reduce the burden for the patient at risk, a cost saving measure for the hospital, as well as contributing to reduced use of broad-spectrum antibiotics. Our study’s in-hospital mortality rate of 21.0% is consistent with the current literature [Citation2,Citation5–7,Citation10–13]. The patients had a median CCI-score of 5, similar to what Micek et al. [Citation11] found. While higher CCI-scores reflect the burden of chronic disease, there are also examples of patients with few to no comorbidities in our study. With such a high mortality rate, it is surprising that NV-HAP is not a surveilled disease with focus on preventative measures. There are some studies on prevention [Citation30,Citation31], but further research and clear recommendations are needed. ICU staff are very aware of the risk of VAP, and surveillance programs are in development. Considering the mortality and morbidity of NV-HAP combined with low awareness in regular wards and non-existing surveillance, an awareness campaign may be warranted.
We found a 30-day readmission rate of almost 40%, high long-term mortality rates, a high CCI-score, a median NEWS2-score of 8, and SOFA-score of 4 at the time of diagnosis. This reflects a population with several comorbidities and a short life-expectancy who become severely ill when they develop NV-HAP.
Only 10.9% of patients had an airway sample for pathogen detection obtained. Given that national guidelines recommend lower airway samples should be obtained, this number is surprisingly low. This may be due to lack of awareness, busy clinical situations and poor understanding of the therapeutic value of airway sampling. The fact that empirical treatments have generally been considered clinically effective in Norway due to a low burden of antimicrobial resistance might also affect willingness for microbiological sampling. In the few airway samples obtained, a high percentage had findings (), and many were considered relevant. We acknowledge that Candida spp. are usually not considered a respiratory pathogen, but since some of the cases were considered relevant by the treating clinician and resulted in antifungal treatment we choose to report them as such. The numbers in our study are too small to draw any conclusions, but improved patient outcomes and reduced broad spectrum antibiotic usage is potentially achievable with better airway sampling. A comparison with other studies is somewhat difficult as some studies have chosen to use microbiological sampling as an obligate criterion [Citation11] and some studies have yet to report the rate of microbiological sampling at all [Citation3,Citation10]. Sopena et al. [Citation5] had sputum cultures performed in 53.9% of their patients, however, their study was prospective by active, bimonthly 1-week surveillance and as such awareness might increase microbiological sampling.
About half of the patients in this study, according to the Norwegian antibiotic treatment guidelines, had at least two risk factors for MRMs present. For this study, we decided to define that at least two of four risk factors should be present to warrant use of one of the broader spectrum regimens recommended by guidelines. Using this definition 52.9% received an appropriate regimen, while 36.1% received a regimen considered too narrow spectrum. Due to the low rate of microbiological airway sampling, it is not possible to ascertain whether treatment failure was associated with the chosen antimicrobial regimen. We do not know if the deviation from national guidelines impacted endpoints since the study was not designed to answer these questions. Furthermore, we do not have data to explain the deviation from use of appropriate antibiotic treatment, and it is therefore surprising that the majority of non-appropriate regimens were too small spectrum. One possible reason is that the high mortality and morbidity of NV-HAP is under-communicated.
One of the isolated Streptococcus pneumoniae was resistant to doxycycline and tetracycline. Resistance to tetracycline is uncommon among S. pneumoniae isolates in Norway [Citation32]. The Proteus mirabilis isolate was resistant to amoxicillin, ampicillin, amoxicillin-clavulanic acid, gentamycin, tobramycin and trimethoprim-sulfamethoxazole, making this isolate more resistant than expected [Citation33]. The Moraxella catarrhalis isolate was resistant to amoxicillin and ampicillin as expected [Citation34]. The extended spectrum beta-lactamase (ESBL) producing E. coli was susceptible to cefoxitin, ertapenem, meropenem, nitrofurantoin and mecillinam. While E. coli ESBL isolates with resistance to carbapenems are rare in Norway, many isolates are classified as susceptible, increased exposure or susceptible to piperacillin-tazobactam and amoxicillin-clavulanic acid unlike our isolate, making it more resistant than expected [Citation32]. In line with surveillance data the Pseudomonas aeruginosa isolate was susceptible to all tested antibiotics [Citation35]. None of the isolated Candida albicans were resistant to antifungals.
A major strength of the study is the meticulous screening process and completeness of the material. With the inclusion criteria derived from the IDSA/ATS definition, and our screening method, we are confident that we have obtained as close to a complete dataset as possible from a retrospective study.
The study has several limitations; (1) missed NV-HAP cases due to lack of a radiological examination carried out or the use of a radiological examination with poor sensitivity, such as ordinary X-ray, (2) it is a single centre study conducted in 2018, before the covid-19 pandemic, (3) the number of included patients is low. Collecting data for more years than one would strengthen the study, (4) we may have missed some inclusions due to only one clinical criterion being positive as the presence or absence of purulent sputum was undocumented in several of the patient charts, (5) we could not include data on attributable mortality and risk factors due to not having the resources to create a matched cohort, (6) we were not able to assess aspiration events or risk of aspiration due to lack of information in the medical records. Nevertheless, the study has important findings that need to be corroborated in well-designed multicentre retrospective studies that include the period following COVID-19, as well as multicenter prospective studies.
Conclusion
The incidence of NV-HAP in Norway is comparable to studies from other countries. Patients who develop NV-HAP have many comorbidities, poor short-term and long-term prognosis, and high readmission rates. Identifying patients at risk for NV-HAP to provide preventative measures has the potential to save hospital resources and funds, lessen the burden of disease for the patient and reduce usage of broad-spectrum antibiotics.
Very few NV-HAP patients seem to undergo lower airway microbiological sampling, which may cause under- and overtreatment due to lack of targeted antimicrobial treatment. We argue for an increased health care focus on adequate airway sampling in NV-HAP. Increased airway sampling will provide a better understanding of the microbiological aetiology of NV-HAP and a more targeted antibiotic treatment for the patients.
Authors’ contributions
This study was conceived and designed by JAF, KEM and LH. JAF and KEM acquired the data. JAF, KEM, HMSG, EU and LH analysed and interpreted the data. JAF, KEM and LH drafted the article, which was critically revised by HMSG and EU.
Ethical considerations
The study protocol was approved by the local data protection officer (PVO 21/08639-1/005). An inquiry was made to the regional ethics committee who clarified that a formal application was not required due to the study design. In accordance with EU GDPR article 6 nr. 1e, and article 9 nr. 2h, and The Personal Data Act §9 and by evaluation of the local data protection officer, the study qualifies as non-intervention research which waives the need for signed informed consent of the participants. In accordance with local regulations, surviving patients were retrospectively informed of the study, including its aims and usage of anonymized data extracted from medical records. They were given the opportunity to withdraw if they did not consent to their medical data being used.
Supplemental Material
Download MS Word (14.9 KB)Acknowledgements
The authors wish to thank Hanne Opsand and Bjørn Martin Woll for their support in collecting and registering data. We also thank Hanan Smedberg for her support in setting up the screening system in the radiology software.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The datasets generated and analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Suetens C, Latour K, Kärki T, et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018;23(46):1800516 doi: 10.2807/1560-7917.ES.2018.23.46.1800516.
- Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801.
- Giuliano KK, Baker D, Quinn B. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am J Infect Control. 2018;46(3):322–327. doi: 10.1016/j.ajic.2017.09.005.
- Quinn B, Baker DL, Cohen S, et al. Basic nursing care to prevent nonventilator hospital-acquired pneumonia. J Nurs Scholarsh. 2014;46(1):11–19. doi: 10.1111/jnu.12050.
- Sopena N, Sabria M, Neunos 2000 Study Group. Multicenter study of hospital-acquired pneumonia in non-ICU patients. Chest. 2005;127(1):213–219. doi: 10.1378/chest.127.1.213.
- Ji W, McKenna C, Ochoa A, et al. Development and assessment of objective surveillance definitions for nonventilator hospital-acquired pneumonia. JAMA Netw Open. 2019;2(10):e1913674. doi: 10.1001/jamanetworkopen.2019.13674.
- Jones BE, Sarvet AL, Ying J, et al. Incidence and outcomes of non-ventilator-associated hospital-acquired pneumonia in 284 US hospitals using electronic surveillance criteria. JAMA Netw Open. 2023;6(5):e2314185. doi: 10.1001/jamanetworkopen.2023.14185.
- Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353.
- Centers for Disease Control and Prevention. Pneumonia events – Ventilator-associated and non-ventilator-associated Penumonia (PNEU); 2021. Available from: https://www.cdc.gov/nhsn/psc/pneu/index.html.
- Davis J. A second breadth: hospital-acquired pneumonia in pennsylvania nonventilated versus ventilated patients. PA Patient Saf Advis. 2018;15(3):1–12.
- Micek ST, Chew B, Hampton N, et al. A case-control study assessing the impact of nonventilated hospital-acquired pneumonia on patient outcomes. Chest. 2016;150(5):1008–1014. doi: 10.1016/j.chest.2016.04.009.
- See I, Chang J, Gualandi N, et al. Clinical correlates of surveillance events detected by National Healthcare Safety Network Pneumonia and Lower Respiratory Infection Definitions-Pennsylvania, 2011–2012. Infect Control Hosp Epidemiol. 2016;37(7):818–824. doi: 10.1017/ice.2016.74.
- Sopena N, Heras E, Casas I, et al. Risk factors for hospital-acquired pneumonia outside the intensive care unit: a case-control study. Am J Infect Control. 2014;42(1):38–42. doi: 10.1016/j.ajic.2013.06.021.
- Cassini A, Plachouras D, Eckmanns T, et al. Burden of six healthcare-associated infections on European population health: estimating incidence-based disability-adjusted life years through a population prevalence-based modelling study. PLoS Med. 2016;13(10):e1002150. doi: 10.1371/journal.pmed.1002150.
- Kalsekar I, Amsden J, Kothari S, et al. Economic and utilization burden of hospital-acquired pneumonia (HAP): A systematic review and meta-analysis. Chest. 2010;138(4):739A. doi: 10.1378/chest.10337.
- Munro SC, Baker D, Giuliano KK, et al. Nonventilator hospital-acquired pneumonia: A call to action. Infect Control Hosp Epidemiol. 2021;42(8):991–996. doi: 10.1017/ice.2021.239.
- Rodrigo-Troyano A, Sibila O. The respiratory threat posed by multidrug resistant gram-negative bacteria. Respirology. 2017;22(7):1288–1299. doi: 10.1111/resp.13115.
- Esperatti M, Ferrer M, Giunta V, et al. Validation of predictors of adverse outcomes in hospital-acquired pneumonia in the ICU. Crit Care Med. 2013;41(9):2151–2161. doi: 10.1097/CCM.0b013e31828a674a.
- Gross AE, Schooneveld TCV, Olsen KM, et al. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob Agents Chemother. 2014;58(9):5262–5268. doi: 10.1128/AAC.02582-14.
- Ibrahim EH, Ward S, Sherman G, et al. A comparative analysis of patients with early-onset vs late-onset nosocomial pneumonia in the ICU setting. Chest. 2000;117(5):1434–1442. doi: 10.1378/chest.117.5.1434.
- Vallés J, Martin-Loeches I, Torres A, et al. Epidemiology, antibiotic therapy and clinical outcomes of healthcare-associated pneumonia in critically ill patients: a Spanish cohort study. Intensive Care Med. 2014;40(4):572–581. doi: 10.1007/s00134-014-3239-2.
- Gray S, Lambrelli D, Eriksson D, et al. Incidence of hospitalized all-cause pneumonia, and serotype distribution of hospitalized Streptococcus pneumoniae pneumonia in Norway 2008–2009. Eur Respir J. 2013;42(Suppl 57):P4204.
- National Centre for Disease Control, Directorate General of Health Services. National guideline for the use of antibiotics in hospitals (2013–2020); 2018.
- Claessens YE, Debray M-P, Tubach F, et al. Early chest computed tomography scan to assist diagnosis and guide treatment decision for suspected community-acquired pneumonia. Am J Respir Crit Care Med. 2015;192(8):974–982. doi: 10.1164/rccm.201501-0017OC.
- Esayag Y, Nikitin I, Bar-Ziv J, et al. Diagnostic value of chest radiographs in bedridden patients suspected of having pneumonia. Am J Med. 2010;123(1):88 e1-5. doi: 10.1016/j.amjmed.2009.09.012.
- Hayden GE, Wrenn KW. Chest radiograph vs. computed tomography scan in the evaluation for pneumonia. J Emerg Med. 2009;36(3):266–270. doi: 10.1016/j.jemermed.2007.11.042.
- Syrjala H, Broas M, Suramo I, et al. High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis. 1998;27(2):358–363. doi: 10.1086/514675.
- Corradi F, Brusasco C, Garlaschi A, et al. Quantitative analysis of lung ultrasonography for the detection of community-acquired pneumonia: a pilot study. Biomed Res Int. 2015;2015:868707. doi: 10.1155/2015/868707.
- Liu XL, Lian R, Tao Y, et al. Lung ultrasonography: an effective way to diagnose community-acquired pneumonia. Emerg Med J. 2015;32(6):433–438. doi: 10.1136/emermed-2013-203039.
- Quinn B, Giuliano KK, Baker D. Non-ventilator health care-associated pneumonia (NV-HAP): best practices for prevention of NV-HAP. Am J Infect Control. 2020;48(5S):A23–A27. doi: 10.1016/j.ajic.2020.03.006.
- Vignari M. Non-ventilator health care-associated pneumonia (NV-HAP): NV-HAP risk factors. Am J Infect Control. 2020;48(5S):A10–A13. doi: 10.1016/j.ajic.2020.03.010.
- NORM/NORM-VET. NORM/NORM-VET 2018. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway; 2019.
- NORM/NORM-VET. NORM/NORM-VET 2017. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway; 2018.
- NORM/NORM-VET. NORM/NORM-VET 2008. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway; 2009.
- NORM/NORM-VET. NORM/NORM-VET 2015. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway; 2016.