Abstract
Background
Although the diverse communities of tick-borne viruses (TBVs) have recently been proposed, the threat of infection and exposure to TBVs among humans across Kenya has been poorly understood.
Objective
Severe fever with thrombocytopenia syndrome virus (SFTSV) is an emerging tick-borne viral agent associated with the epidemic of severe fever with thrombocytopenia syndrome (SFTS) disease in East Asian countries. This study investigated the seroprevalence of SFTSV among humans in Kenya.
Methods
Serum samples were collected from 459 healthy people in Kenya and tested for anti-SFTSV antibodies, which were further confirmed by immunofluorescence assays. Micro neutralization assays were performed to identify neutralising antibodies against SFTSV and SFTSV-related viruses.
Results
A high seroprevalence (162/459, 35.3%) of SFTSV was found in the samples from nine of the ten surveyed counties in Kenya, with higher rates in the eastern plateau forelands, semiarid and arid areas, and coastal areas than in the area aside Rift valley. The seropositive rate was slightly higher in women than in men and was significantly higher in the 55-64 age group. Neutralising activity against SFTSV was detected in four samples, resulting in a rate of 0.9%. No cross-neutralising activity against the SFTSV-related Guertu virus and Heartland virus was detected in the anti-SFTSV positive serum samples.
Conclusion
The results provide serologic evidence of human exposure to SFTSV in Kenya and extend our understanding of SFTSV prevalence from Asia to Africa. The findings suggest an increasing threat of exposure to emerging TBVs and the need to investigate tick viromes in Kenya.
Introduction
Kenya, situated in East Africa, has long been threatened by tick-borne viruses (TBVs), such as Crimean-Congo haemorrhagic fever virus (CCHFV, genus Orthonairovirus in family Nairoviridae of order Bunyavirales), which caused infections in livestock and humans [Citation1]. Although metagenomic analyses have suggested diverse tick-borne viral communities from ticks in Kenya in recent years [Citation2,Citation3], the threat of infection and exposure to tick-borne viruses in humans has been poorly understood.
Severe fever with thrombocytopenia syndrome virus (SFTSV, genus Bandavirus of the family Phenuiviridae in the order Bunyavirales) is an emerging tick-borne virus associated with severe febrile illness in humans, with a fatality rate of 5%-30% in different regions [Citation4]. Since its first identification in patients in China in 2009, SFTSV infection has been reported in Japan and South Korea, and later serologic exposure was found in humans in Pakistan and Vietnam [Citation5–8]. However, the prevalence and threat of SFTSV in other countries were unclear.
This study investigated the seroprevalence of SFTSV among healthy people in Kenya and confirmed the neutralising activity specific to SFTSV. We analysed the association of seroprevalence rates among tested human samples with their location, age, and sex. The results provided serologic evidence of human exposure to SFTSV in Pakistan, and revealed different levels of risk from SFTSV exposure in regions from west to east, which promotes understanding of potential risks from novel tick-borne viruses and would benefit human disease surveillance among humans in Kenya.
Materials and methods
Sample collection and ethics statement
Serum samples were collected from 459 healthy individuals in ten counties in Kenya between 2016 and 2018 and tested for anti-SFTSV antibody responses. Serum samples from two convalescent SFTS patients stored at the National Virus Resource Centre (Wuhan, China; YB17WIVS286 and YB17WIVS294) and serum samples from three healthy individuals were used as the positive and negative controls, respectively.
We recorded and summarised the test results by sex, age, and geographic location (). The collection of human serum samples and subsequent testing were reviewed and approved by the ethics committees of the Government College University, Faisalabad, Pakistan (approval number: GCUF/MICRO/18/1598). Written informed consent was obtained from adult participants and parents of participants <18 years of age.
Table 1. Seroprevalence of severe fever with thrombocytopenia syndrome virus in Kenya based on ELISA and MNT results.
Cells, virus, and antibodies
The Vero cells (CCL-81, ATCC) were maintained in Dulbecco’s modified Eagle medium (DMEM, New Zongke Biotech, Wuhan, China)supplemented with 10% or 2% foetal bovine serum (FBS, Gibco®, Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin-streptomycin-glutamine at 37 °C with 5% CO2.
The SFTSV strain HB29 (GenBank accession number: KP202165, NVRC accession number: IVCAS 6.6311), Guertu virus (GTV) strain DXM (GenBank accession number: KT328591, NVRC accession number: IVCAS 6.6106), and Heartland virus (HRTV) strain patient 1 (GenBank accession number: NC024496, NVRC accession number: IVCAS 6.6330) are preserved in the National Virus Resource Centre and were cultured and titrated on Vero cells with the DMEM containing 2% FBS. The SFTSV infection assays were performed in a biosafety level 2 laboratory according to the catalogue of pathogenic microorganisms transmitted between humans, published by the National Health Commission in 2023 (https://zwfw.nhc.gov.cn/kzx/tzgg/gzbxbywswsyhdsp_230/202311/t20231101_2629.html).
Polyclonal antibodies against SFTSV nucleoprotein (NP), GTV NP, HRTV NP, and the N-fragments of HRTV glycoproteins (Gn) were prepared in-house as previously described [Citation5,Citation9,Citation10]. Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488, ab150077; Abcam, Cambridge, UK), and goat anti-human IgG H&L (FITC) (ab6854; Abcam) were used as the secondary antibodies for immunofluorescence assays.
Indirect enzyme linked immunosorbent assay (ELISA)
The commercial SFTSV human IgG ELISA kit (New Zongke Biotech, Wuhan, China), which uses SFTSV nucleocapsid protein (NP) as the viral antigen, was used to detect anti-SFTSV antibodies in the human serum samples as previously described [Citation6]. Serum samples at a dilution of 1:20 were incubated as the primary antibodies and then incubated with horseradish peroxidase (HRP)-conjugated anti-human IgG monoclonal antibody for colour development according to the manufacture’s instruction. OD values were measured using a multifunctional enzyme marking instrument (Synergy H1, BioTek, USA). Samples were considered IgG-positive if the absorbance was >2.1 times the mean absorbance of the negative control.
Microneutralization test (MNT)
The neutralising activity of the anti-SFTSV positive human samples was further tested by MNT at a dilution of 1:20 as previously described [Citation6]. Briefly, Vero cells (1 × 104/well) were seeded overnight in 96-well culture plates. Serum samples were diluted and mixed with equal volumes of SFTSV (100 TCID50/well). After 1 h of incubation, the virus-serum mixture (100 μL/well) was added to 96-well plates in triplicate. After 1 h, the supernatants were removed and replaced with fresh DMEM containing 2%FBS. Cells were further maintained at 37 °C for 4-7 days. In case that the production of SFTSV cytopathic effect (CPE) is not evident, the inhibition of SFTSV infection in cells was determined by a specific immunofluorescence assay. If no cells with SFTSV infection were identified in any of the triplicate wells, the serum sample was considered to have SFTSV-specific neutralising activity. Neutralisation to GTV or HRTV was also tested by incubating serum samples with GTV or HRTV using the same procedure.
Indirect immunofluorescence assays (IFAs)
IFA was performed to verify the validity of the commercial ELISA and to exclude viral infection of cells in MNT. Briefly, SFTSV-infected cells were fixed with 4% paraformaldehyde for 15 min, followed by permeabilization with 0.2% Triton-X 100 in PBS for 15 min, and blocked with 5% BSA in phosphate buffer saline (PBS) for 1h at 37 °C. After incubation with serum samples (1:20dilution) overnight at 4 °C, the cells were washed and incubated with Goat anti-human IgG H&L (FITC) antibody according to the manufacturer’s instructions. Images were taken with a fluorescence microscope (Olympus, Japan).
Statistical analysis
The prevalence of SFTSV with 95% confidence intervals (CIs) was calculated based on the ELISA and MNT results. The association of SFTSV seroprevalence rates with age, sex, and location of sample collection were statistically analysed using chi-squared or Fisher’s exact test. For all analyses, p < 0.05 was considered significant.
Results
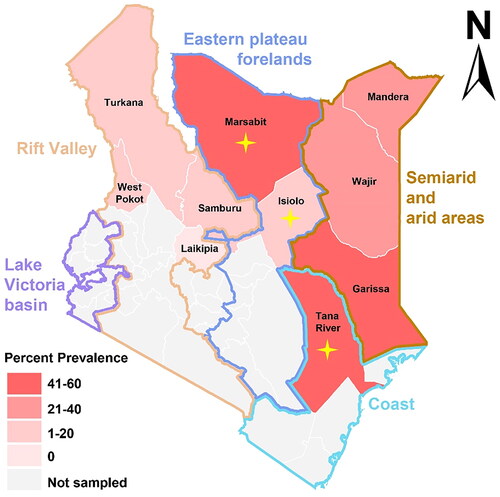
ELISA results showed that 162 out of 459 serum samples were antibody positive, resulting in a high seroprevalence (35.3%, 95% CI: 31.1%-39.8%) of SFTSV. Of the 162 ELISA-positive serum samples, 127 samples were confirmed by IFA (Supplementary Figure 1), resulting in the confirmation rate of 78.4% of the total. Spatial analysis showed that these serum samples originated from nine of the ten surveyed counties in Kenya (, ). In general, the semi-arid and arid areas had high seroprevalence rates of SFTSV, including Garissa (55.9%, 95% CI: 44.1%-67.1%), which is the highest of all regions surveyed, followed by Mandera (38.9%, 95% CI: 20.3%-61.4%) and Wajir (31.1%, 95% CI: 19.5%-45.7%). Seroprevalence rates were also high in the coastal area (Tana River: 52.2%, 95% CI: 43.9%-60.3%)and in the eastern Plateau foreland (Marsabit: 47.4%, 95% CI: 27.3% −68.3%), which were slightly lower than that in Garissa. Unlikely, seroprevalence rates in the plateaus areas aside the valley was lower than in the other regions (), including Samburu (18.6%, 95% CI: 9.7%-32.6%), Isiolo (16.7%, 95% CI: 8.7%-29.6%), Turkana (10.5%, 95% CI: 4.2%-24.1%), and West Pokot (5.4%, 95% CI: 1.0%-17.7%).
Figure 1. Seroprevalence of severe fever with thrombocytopenia syndrome virus in Kenya determined by ELISA results. The regions where individuals with neutralisation activity to SFTSV were identified are indicated by a yellow cross-shaped star.
There were slightly more positive women (38.4%, 95% CI: 32.4%-44.9%) than men (33.8%, 95% CI: 27.9%-40.3%), but this difference was not significant. Significantly higher (p < 0.05) prevalence was observed in the age group 55-64 years followed by ≥65 years (45.0%, 95% CI: 33.1%-57.5%), 3-14 years (42.9%, 95% CI: 21.4%-67.4%), 35-44 years (32.6%, 95% CI: 23.9%-42.7%), 15-24 years (31.4%, 95% CI: 20.2%-45.2%), 25-34 years (28.6%, 95% CI: 20.3%-38.6%), and 45-54 years (25.0%, 95% CI: 16.4%-36.1%) ().
We further confirmed SFTSV infection by MNT, which detected neutralising activity in four individuals (sample 144, 223, 390, and 460) out of 459 samples tested, resulting in a rate of neutralising antibodies (0.9%, 95% CI: 0.3%-2.2%) (). The four samples exhibited neutralisation titres of 40, 20, 20, and 20, respectively (Supplementary Figure 2). All the four individuals kept livestock at home, including two individuals (36-year-old female and 55-year-old male) from Tana River (2/72, 2.8%), one (60-year-old male) from Marsabit (1/9, 11.1%), and the other (49-year-old female) from Isiolo (1/8, 12.5%) (). The high seroprevalence rate of 35.3% in contrast to the low rate of neutralising activity rate may indicate the presence of other viruses serologically related to SFTSV. Therefore, we examined neutralising activities specific for the SFTSV-related GTV and HRTV, however, did not identify neutralisation positive in any of the 162 anti-SFTSV positive samples (Supplementary Figure 3).
Discussion
Although previous studies have highlighted the threat posed by TBVs, such as CCHFV [Citation1], Jingmen tick virus [Citation11], Dhori virus, and Dugbe virus[Citation12], there have been no reports in the literature of SFTSV detection in ticks or animals in Kenya. Nevertheless, our study demonstrated serologic evidence of human exposure to SFTSV. Among the counties surveyed, Garissa and Tana River had the highest seroprevalence rates. A recent study reported diverse virus populations in Hyalomma ticks collected from camels in Garissa and Tana River, Kenya, and showed that the camels were serologically exposed to the viruses identified in the parasitic ticks [Citation2], suggesting the circulation of novel TBVs between ticks and animal hosts. However, no evidence of SFTSV prevalent in ticks or camels from either Garissa or Tana River was found. This may be because Hyalomma ticks are not the competent vectors of SFTSV. We identified SFTSV-neutralising antibodies in human samples from Tana River, demonstrating the significant prevalence of SFTSV.
The high total seroprevalence of SFTSV (35.3%) in contrast to its low neutralisation rate (2.5%) may be affected by the time of sampling and the condition and period of sample preservation. It also suggests that there may be other viruses serologically related to SFTSV, which have caused serologic exposure to humans in Kenya; however, the anti-serum failed to cross-neutralize SFTSV. The recent study has identified novel viruses from camel ticks in Kenya, including Iftin tick virus (IFTV) and Mbalambala tick virus (MATV), which are closely related to viruses of the genus Uukuvirus of the family Phenuiviridae[Citation2]. The RNA-dependent RNA polymorases (RdRps) of IFTV and MATV shared amino acid (aa) sequence identities of 29.24% and 29.22% with SFTSV RdRp, respectively, while the nucleoprotein (NP) of IFTV and MATV shared 22.01% and 19.84% aa identities with SFTSV NP, respectively. These two viruses could not cross-react serologically with SFTSV due to the limited sequence similarity. Bhanja virus (BHAV), belonging to the genus Bandavirus of family Phenuiviridae, and Kupe virus (KUPV) and CCHFV, belonging to the genus Orthonariovirus of the family Nairoviridae, have been found in arid and semiarid regions of the eastern and northeastern of Kenya [Citation1, Citation13]. BHAV shared 34.87%, 41.70% and 25.51% aa sequence similarity with SFTSV RdRp, NP, and GP, respectively. A previous study reported that the BHAV antigen can react with antibodies against SFTSV [Citation14]. Therefore, we speculate that anti-BHAV sera may be present in the SFTSV antibody-positive samples. However, we were unable to characterise the cross-reactive activity of the SFTSV-neutralising serum samples to prevent BHAV infection due to the lack of this virus. Serologic cross-reaction of SFTSV with KUPV and CCHFV may be much less likely to happen due to their low sequence similarity comparing to SFTSV (KUPV: 10.32% for RdRp, 8.55% for NP and 9.91% for GP; CCHFV: 14.42% for RdRp, 10.08% for NP and 9.26% for GP) and phylogeny distinct from SFTSV. Guertu virus (GTV) and Heartland virus (HRTV), belonging to the genus Bandavirus of the family Phenuiviridae, are the viruses most closely related to SFTSV according to their phylogeny [Citation5]. GTV was prevalent in Xinjiang, China, bordering with Pakistan, and has been identified with serologic cross-reaction to SFTSV [Citation5]. HRTV was prevalent only in the United States [Citation15], while the serologic cross-reaction between HRTV and SFTSV has been suggested by using monoclonal antibodies specific for conserved motifs of both viruses [Citation10]. We did not detect neutralising activity from the SFTSV antibody-positive serum samples cross-reacting with the two viruses, suggesting that the high seroprevalence of SFTSV in Kenya is not due to GTV and HRTV. However, due to the limited understanding of tick viromes in Kenya, it is still important to conduct virus investigations related to SFTSV.
Our collection sites were in arid environments in Kenya and samples were collected from camel owners. The Tana River and Garissa sample sites shared camels, especially at the auction markets. Their serologic similarity may be explained by the weekly movement of camels between these sites. The Pokot camel population is a subset of the Turkana. This is partly due to camel smuggling between the two counties. During trade, nomadism, and smuggling, camels move around with their ticks and thus can be infected with and/or exposed to SFTSV. Neutralisation specific for SFTSV was found in Tana River, Marsabit, and Isiolo (, ). These places are the typical agricultural and pastoral areas that are quite dry, and the temperatures are quite suitable for ticks, where animal parasitic ticks are commonly found. The four individuals with neutralising antibodies to SFTSV all keep domestic animals, suggesting the need to investigate the circulation of SFTSV among ticks, animal hosts and human populations.
A few studies have described tick species in Kenya [Citation1, Citation11, Citation16,Citation17]. Several tick species have been found to carry SFTSV, including Rhipicephalus microplus, Rhipicephalus sanguinensis [Citation18,Citation19], Haemaphysalisconcinna, Haemaphysalislongicornis [Citation20–22], and Haemaphysa-lisflava [Citation7] in China, South Korea, and Japan, in Kenya. Of those species, only Rhipicephalus sanguinensishas been found prevalent among dogs and wildlife in Kenya and may serve as a vector for transmitting certain diseases [Citation23]. It is important to perform surveys to further investigate the potentials of different tick species to vector SFTSV, which would provide a better understanding of SFTSV prevalence in the world.
Conclusion
This study provides serologic evidence for the presence of SFTSV and the occurrence of SFTSV exposure in humans in Kenya. This extends our understanding of SFTSV prevalence from Asia to Africa, and suggests an increasing threat of infection and exposure to emerging TBVs. Due to the lack of understanding of the viromes of different tick species and the capacity for virus spill-over to hosts, it is still important to ensure continued surveillance of the prevalence of TBVs and prepare for the threats posed by emerging TBVs, especially in countries and areas where agriculture and livestock are the main industries.
Ethical statement
The Study received ethical clearance from University of Nairobi - Kenyatta National Hospital (KNH-UoN) under permit number: P210/04/2017. Written informed consent was given by participants over the age of 18 and parents of participants < 18 years of age.
Supplemental Material
Download TIFF Image (510.8 KB)Supplemental Material
Download TIFF Image (2.1 MB)Supplemental Material
Download TIFF Image (2.6 MB)Acknowledgments
We thank the County Governments of Kenya, The National Government and the Ministry of Health support. We thank the Mrs. Min Zhou in National Virus Resource Center (Wuhan, China) for providing the virus-infected Vero cells (accession No. IVCAS14.0001).
Disclosure statement
All authors declare no conflicts of interest.
Additional information
Funding
References
- Chiuya T, Masiga DK, Falzon LC, et al. Tick-borne pathogens, including Crimean-Congo haemorrhagic fever virus, at livestock markets and slaughterhouses in western Kenya. Transbound Emerg Dis. 2021;68(4):2429–2445. doi:10.1111/tbed.13911.
- Zhang Y, Hu B, Agwanda B, et al. Viromes and surveys of RNA viruses in camel-derived ticks revealing transmission patterns of novel tick-borne viral pathogens in Kenya. Emerg Microbes Infect. 2021;10(1):1975–1987. doi:10.1080/22221751.2021.1986428.
- Ogola EO, Kopp A, Bastos ADS, et al. Phlebovirus diversity in ticks from livestock in arid ecologies in Kenya. Ticks Tick-Borne Dis. 2023;14(1):102087. doi:10.1016/j.ttbdis.2022.102087.
- Zhan J, Wang Q, Cheng J, et al. Current status of severe fever with thrombocytopenia syndrome in China. Virol Sin. 2017;32(1):51–62. doi:10.1007/s12250-016-3931-1.
- Shen S, Duan X, Wang B, et al. A novel tick-borne phlebovirus, closely related to severe fever with thrombocytopenia syndrome virus and Heartland virus, is a potential pathogen. Emerg Microbes Infect. 2018;7(1):1–14. doi:10.1038/s41426-018-0093-2.
- Zohaib A, Zhang J, Saqib M, et al. Serologic evidence of severe fever with thrombocytopenia syndrome virus and related viruses in Pakistan. Emerg Infect Dis. 2020;26(7):1513–1516. doi:10.3201/eid2607.190611.
- Takahashi T, Maeda K, Suzuki T, et al. The first identification and retrospective study of Severe Fever with Thrombocytopenia Syndrome in Japan. J Infect Dis. 2014;209(6):816–827. doi:10.1093/infdis/jit603.
- Tran XC, Yun Y, Van An L, et al. Endemic severe fever with thrombocytopenia syndrome, Vietnam. Emerg Infect Dis. 2019;25(5):1029–1031. doi:10.3201/eid2505.181463.
- Zhang YF, Shen S, Shi JM, et al. Isolation, characterization, and phylogenic analysis of three new severe fever with thrombocytopenia syndrome bunyavirus strains derived from Hubei Province, China. Virol Sin. 2017;32(1):89–96. doi:10.1007/s12250-017-3953-3.
- Qian J, Fu LY, Wu XL, et al. Developing and characterizing monoclonal antibodies of Guertu bandavirus nucleoprotein for developing methods of Guertu bandavirus and severe fever with thrombocytopenia syndrome virus detection. Braz J Microbiol. 2023;54(3):1433–1445. doi:10.1007/s42770-023-00982-8.
- Ogola EO, Kopp A, Bastos ADS, et al. Jingmen tick virus in ticks from Kenya. Viruses-Basel. 2022;14(5):1041. doi:10.3390/v14051041.
- Sang R, Onyango C, Gachoya J, et al. Tickborne arbovirus surveillance in market livestock, Nairobi, Kenya. Emerg Infect Dis. 2006;12(7):1074–1080. doi:10.3201/eid07.060253.
- Johnson BK, Chanas AC, Squires EJ, et al. Arbovirus isolations from ixodid ticks infesting livestock, Kano Plain, Kenya. Trans R Soc Trop Med Hyg. 1980;74(6):732–737. doi:10.1016/0035-9203(80)90188-1.
- Matsuno K, Weisend C, Travassos da Rosa APA, et al. Characterization of the Bhanja Serogroup viruses (Bunyaviridae): a novel species of the genus phlebovirus and its relationship with other emerging tick-borne phleboviruses. J Virol. 2013;87(7):3719–3728. doi:10.1128/JVI.02845-12.
- McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367(9):834–841. doi:10.1056/NEJMoa1203378.
- Clifford CM, Kohls GM, Hoogstraal H. Ixodes walkerae, n. sp., from a bird in Kenya (Acarina: Ixodidae). J Med Entomol. 1968;5(4):513–514. doi:10.1093/jmedent/5.4.513.
- Madder M, Day M, Schunack B, et al. A community approach for pathogens and their arthropod vectors (ticks and fleas) in cats of sub-Saharan Africa. Parasit Vectors. 2022;15:321.
- Seo JW, Han SY, Sung SH, et al. Survey on tick distribution and tick-borne pathogens in Daejeon and adjacent areas in South Korea. Ticks Tick Borne Dis. 2021;12(4):101711. doi:10.1016/j.ttbdis.2021.101711.
- Seo MG, Noh BE, Lee HS, et al. Nationwide temporal and geographical distribution of tick populations and phylogenetic analysis of severe fever with thrombocytopenia syndrome virus in ticks in Korea, 2020. Microorganisms. 2021;9(8):1630. doi:10.3390/microorganisms9081630.
- Liu Q, He B, Huang SY, et al. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis. 2014;14(8):763–772. doi:10.1016/S1473-3099(14)70718-2.
- Kim EH, Park SJ. Emerging Tick-Borne Dabie bandavirus: virology, epidemiology, and prevention. Microorganisms. 2023;11(9):2309. doi:10.3390/microorganisms11092309.
- Hu YY, Zhuang L, Liu K, et al. Role of three tick species in the maintenance and transmission of Severe Fever with Thrombocytopenia Syndrome Virus. PLoS Negl Trop Dis. 2020;14(6):e0008368. doi:10.1371/journal.pntd.0008368.
- D’Amico G, Dumitrache MO, Široký P, et al. Altitudinal and seasonal differences of tick communities in dogs from pastoralist tribes of Northern Kenya. Vet Parasitol. 2015;212(3-4):318–323. doi:10.1016/j.vetpar.2015.08.025.
