Abstract
Background
Klebsiella pneumoniae (KP) accounts for high antimicrobial resistance and mortality rates of bloodstream infections (BSIs).
Objectives
To investigate incidence, antimicrobial resistance and risk factors for mortality of KP BSIs in East China.
Methods
A retrospective study of patients with KP BSIs was conducted in a tertiary care hospital from 2018 to 2022. Medical records of all hospitalised patients with KP BSIs were reviewed and analysed. The incidence, antimicrobial resistance and mortality of KP BSIs were evaluated. The Kaplan-Meier method was used to plot survival curves and logistic regression was used to analyse risk factors for crude 30-day mortality.
Results
A total of 379 inpatients with KP BSIs were enrolled. The incidence of patients with KP BSIs was fluctuating between 4.77 and 9.40 per 100,000 patient-days. The crude 30-day mortality rate of these patients was 26.39%. Of the 379 KPisolates, 197 (51.98%) were carbapenem-resistant (CR) and 252 (66.49%) were multidrug-resistant (MDR). All isolates showed the lowest resistance to tigecycline (13.77%) and polymyxin B (14.61%). Cases with MDR/CR isolates had significantly longer length of hospital stay, higher crude 30-day mortality and medical costs than non-MDR/non-CR isolates. Age, CR phenotype, paracentesis, indwelling central venous catheter (CVC), use of carbapenems, tetracyclines, polymyxins B, and irrational empiric treatment were independently associated with crude 30-day mortality.
Conclusion
MDR/CR KP BSIs are associated with increased mortality, healthcare costs and prolonged hospitalisation. Patients with advanced age, CR phenotype, paracentesis, CVC, exposure to some antibiotics, and irrational empirical antibiotic treatment are at higher mortality risk.
Introduction
Bloodstream infections (BSIs) are one of the leading causes of mortality. The growing number of immunocompromised individuals, use of invasive procedures and medications contributed to the high incidence of BSIs, which is receiving increasing attention worldwide [Citation1]. Klebsiella pneumoniae (KP) is an important Gram-negative bacillus causing pneumonia, urinary tract infections, BSIs and abdominal infections [Citation2]. It is ranked as the second Gram-negative organism causing BSIs [Citation3]. Mortality of BSIs caused by KP ranged between 20% and 40% [Citation4].
Carbapenems are broad-spectrum antimicrobials for treatment of infectious diseases and are also used as the last line drug in the treatment of Gram-negative bacteria [Citation5]. In recent years, with the wide usage of cephalosporins, the proportion of extended-spectrum beta-lactamase producing KP has increased rapidly [Citation6], resulting in significant increase of carbapenems usage and proportion of carbapenem-resistant KP (CRKP) [Citation7]. According to the China Bacterial Resistance Monitoring Network, the resistance rate of CRKP increased by tenfold from 3% to 30% between 2005 and 2023 in China [Citation8]. In addition, multidrug-resistant KP (MDRKP) has been on the rise in China in recent years [Citation9], and infections caused by MDRKP result in mortality rates as high as 40%–50% [Citation10]. Moreover, hypervirulent KP (hvKP) has been reported to be widespread in Asia-Pacific region, Europe and North America, the Middle East and Africa in recent years [Citation11–13]. In China, the proportion of hvKP has been reported as high as 57.14% [Citation14].
Mortality rate of BSIs is closely related to the drug-resistant phenotype and virulence of pathogens [Citation15]. However, there were few studies on the epidemiology of KP BSIs in the context of high virulence and high drug resistance. In this study, we conducted a comparative analysis of the clinical characteristics, incidence, drug-resistant phenotypes and risk factors for 30-day mortality of KP BSIs among patients in a large tertiary care hospital in the eastern region of China.
Materials and methods
Ethics
The study was approved by the ethics committee of Ruijin Hospital (KY2023-083). Because of the retrospective nature of the study, the committee waived informed consent. Patient data was obtained from medical record systems and analysed anonymously to protect patient privacy.
Study design and setting
This is a retrospective study performed at Ruijin Hospital from 1 January 2018 to December 31 2022. Ruijin Hospital located in the eastern part of China, is a large-scale, tertiary-level A-class hospital with approximately 3400 beds. It has approximately 5.36 million outpatient and emergency visits and nearly 150,000 hospitalized patients per year. Patients with at least one positive blood culture of KP were enrolled in our study. Patients will be excluded in the following cases: (i) positive culture results are considered as contaminants (confirmed by physicians caring for the patient); (ii) patients with incomplete/inaccurate medical records; (iii) patients insisted on discharge from hospital against medical advice. Only the first episode of each patient was included and each patient was included only once.
Definitions
KP BSIs were defined as a positive blood culture of KP with fever, chills, shiver, stun, cutaneous mucous haemorrhage, hypotension, multiple organ dysfunction, increased heart rate (>90 times/min), increased respiratory rate (>20 times/min), low partial arterial carbon dioxide pressure (<32 mmHg), abnormal number of peripheral blood white blood cell or elevated inflammatory indexes such as C-reactive protein and procalcitonin [Citation16]. The presence of KP BSIs was defined as the moment when the first blood culture was positive for KP. A case was defined as a patient diagnosed with KP BSIs. KP BSIs incidence was defined as the number of cases per 100,000 patient-days. Polymicrobial BSIs was defined as a set of blood cultures simultaneously isolating KP and other microorganisms [Citation17]. According to the Centres for Disease Control and Prevention’s criteria, hospital-associated BSI (HABSI) was defined as a BSI that occurred more than 48 h after admission to hospital or within 48 h of the last hospital discharge [Citation18]. Primary BSIs were defined as bacteria for which there was no documented focal source. Source of infection was defined as the most possible origin of infection responsible for KP BSIs according to medical records. MDR was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories [Citation19]. CR was defined as resistance to any carbapenem [Citation20]. Inappropriate empirical antimicrobial therapy was defined as empirical dosing that did not include any drug with in-vitro activity before blood culture report, whereas inappropriate definitive antimicrobial therapy refers to the moment of receiving antimicrobial susceptibility test results [Citation21,Citation22].
Data collection
The following clinical information of the patient was extracted from the hospital electronic medical record system: gender, age, admission department, intensive care unit (ICU) stay, length of hospital stay, site of complicated infection, underlying diseases, history of invasive clinical procedures, special treatments (glucocorticoids, immunosuppressive agents, radiotherapy and chemotherapy, and dialysis), data of antibiotic therapy. Definition of each variable corresponding to these data was listed in Supplementary Table 1.
Microbiology
Isolates were identified using MALDITOF MS (bioMérieux, Marcy l‘Etoile, France) and drug sensitivity tests were performed using VITEK®2 Compact (bioMérieux, Marcy l‘Etoile, France). Drug sensitivity tests were interpreted in accordance with Clinical and Laboratory Standard Institute standard [Citation23]. For tigecycline, the results were analysed based on the breakpoint of European Committee On Antimicrobial Susceptibility [Citation24].
Statistical analysis
Resistance rates of MDR/non-MDR, CR/non-CR groups of commonly used drugs were compared using Pearson’s chi-square test or Fisher’s exact test. Continuous variables that conform to normal distribution were expressed as mean and standard deviation, and continuous variables that did not conform to normal distribution were expressed as median and interquartile range (IQR). Categorical variables were compared using Pearson’s chi-square test and Fisher’s exact test. Logistic regression was used to analyse risk factors for crude 30-day mortality. Correlation and relevant interactions between variables with p < 0.05 in univariate analysis were checked. After removing variables with high-level correlation (correlation coefficient ≥0.70), the remaining variables were considered for inclusion in the multivariate model to assess independent risk factors associated with 30-day mortality. Odds ratio (OR) and 95% confidence interval (95% CI) were calculated to determine the strength of these associations. The Kaplan–Meier method was used to plot 30-day survival curves after the day of the first positive blood culture for KP and differences between survival curves by antimicrobial resistant phenotypes were evaluated by the log-rank test. P values were two-tailed and p < 0.05 was considered statistically significant. SPSS version 26.0 (IBM, Armonk, NY, USA) and GraphPad Prism version 9.0.0 (GraphPad Software, San Diego, CA, USA) were used for statistical analysis and data generation.
Results
Demographic and clinical details
A total of 379 cases were included in this study (Supplementary Figure 1). The clinical characteristics of enrolled patients are shown in . Their age ranged from 2 to 95 years, with a median age of 63 years, and 42.22% were 65 years or older. Males accounted for 71.24%. Of the 379 cases, 313 patients were HABSIs (82.59%) and 193 were primary BSIs (50.92%). There were 60 patients with polymicrobial BSIs (60/379, 15.83%). Of the 186 (49.08%) cases with known origin, gastrointestinal, abdominal, and pelvic infections were the most common source (86/186, 46.2%), followed by respiratory tract infections (51/186, 27.4%) and skin and soft tissue infections (35/186, 18.8%). About half of the cases were from surgical (174/379, 45.91%), 34.75% from haematology (41/118, 34.75%) and 22.96% from ICU (87/379, 22.96%).
Table 1. Clinical characteristics of 379 Klebsiella pneumoniae bloodstream infections cases.
Incidence
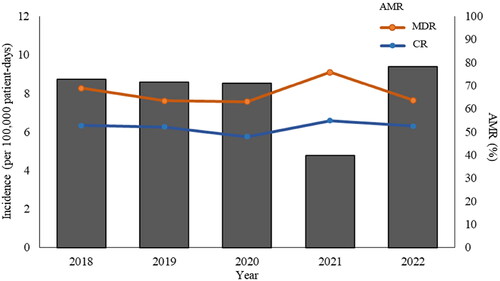
The incidence of KP BSIs remained stable between 8.52 and 8.72 per 100,000 patient-days in 2018–2020 followed by a significant decline in 2021 (4.77 per 100,000 patient-days, p = 0.0005) (). The mean and standard deviation of incidence of KP BSIs is 7.99 per 100,000 patient-days and 1.65, respectively.
Figure 1. Incidence of Klebsiella pneumoniae bloodstream infections and percentage of antimicrobial resistant phenotypes between 2018 and 2022. Bar chart represents the incidence of K. pneumoniae BSIs. Scatter plot and line chart illustrate percentage of multidrug resistant (MDR) and carbapenem resistant (CR) K. pneumoniae isolates.
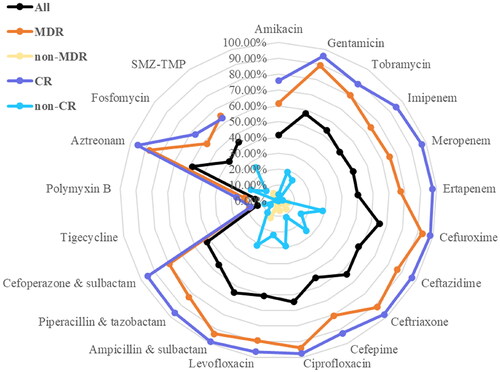
Antimicrobial resistance
Isolates with MDR and CR phenotypes accounted for 66.49% (252/379) and 51.98% (197/379), respectively. All isolates showed low resistance to tigecycline (13.77%) and polymyxin B (14.61%) (). By contrast, resistance rate was the highest to cefuroxime (65.22%) (). It is noteworthy that for all antibiotics tested, resistant rates of MDR isolates were higher than that of non-MDR isolates, and resistant rates of CR isolates were higher than that of non-CR isolates ().
Risk factors for crude 30-day mortality
The crude 30-day mortality rate of KP BSIs was 26.39%. Cases infected with MDR/CR isolates had significantly lower crude 30-day survival rates than these cases infected with non-MDR/non-CR isolates () (p < 0.001). Total length of hospital stay and length of hospital stay after KP isolation were significantly longer in cases with MDR/CR isolates than those with non-MDR/non-CR isolates () (p < 0.001). Healthcare costs of cases with MDR/CR isolates were also significantly higher than cases with non-MDR/non-CR isolates () (p < 0.001).
Figure 3. Survival (in days) of 379 Klebsiella pneumoniae bloodstream infections cases and comparison by antimicrobial resistant phenotypes. MDR, multidrug resistant; non-MDR, non-multidrug resistant; CR, carbapenem resistant; non-CR, non-carbapenem resistant.
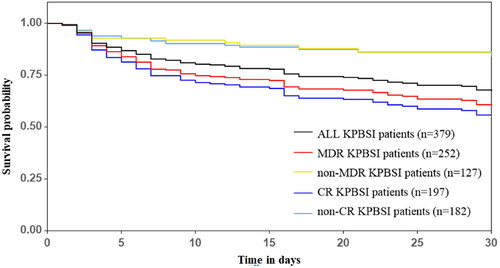
Table 2. Comparison of total length of hospital stay and healthcare costs of 379 Klebsiella pneumoniae bloodstream infections cases by antimicrobial resistant phenotypes.
The univariate analysis showed that age, MDR/CR phenotype, some medical exposures in the 90 days prior to the onset of BSI, cardiovascular disease, respiratory disease, several invasive procedures, use of certain medications in the 90 days before diagnosis of BSI, irrational empirical drug use and irrational targeted treatments were associated with crude 30-day mortality. The multivariate analysis showed that age, CR phenotype, paracentesis, indwelling CVC, carbapenems, tetracyclines, polymyxins and irrational empirical therapies were independent risk factors for crude 30-day mortality.
Discussion
This study provided a comprehensive analysis of the epidemiology of patients with KP BSIs and evaluated the incidence of and risk factors for mortality. To our knowledge, this is the first report to assess the epidemiology of KP BSIs in eastern China which is an area with high prevalence of resistant and virulent isolates in recent years.
The incidence of KP BSIs fluctuated between 9.40 and 4.77 per 100,000 patient-days during 2018–2022. The coronavirus disease 2019 (COVID-19) pandemic began in 2020, and some literature suggests that incidence of BSIs increased during COVID-19 pandemic [Citation25]. However, maybe due to strict controls by the Chinese government and the closed-loop transfer of COVID-19 patients to sentinel hospitals, the incidence of KP BSIs reduced by nearly half to 4.77 per 100,000 patient-days in 2021. In contrast, the incidence of KP BSIs returned to pre-pandemic levels with the downgraded management of COVID-19 in 2022.
The crude 30-day mortality rate of KP BSIs patients was 26.39%, which is much higher than Japan [Citation26]. Therefore, it is necessary to evaluate risk factors for mortality of KP BSIs. Consistent with other study, advanced age was found to be an independent variable that can significantly increase crude 30-day mortality in this study as it represented a vulnerable population with several underlying diseases for drug resistant pathogen infections [Citation27]. Among comorbidities, ischaemic heart disease and pulmonary infection were associated with mortality. Bacteraemia which was an important driver of a fatal outcome generally begins with respiratory tract infections [Citation28]. Moreover, this study showed that 27.42% of all the cases stemmed from respiratory tract infection (). Similar to other studies, time at risk, length of hospital stay and ICU stay were associated with crude 30-day mortality which may be explained by the increased risk of contracting resistant bacterial infection over time and antimicrobial selection pressure due to antibiotics used for infections at other sites given that hospital is a reservoir of resistant bacteria [Citation29, Citation30]. As reported in the literature [Citation31], invasive procedures are independent risk factors for mortality, our study also found that patients who experienced invasive procedures had a higher mortality rate. Previous studies have shown that intravenous catheter insertion was strongly associated with disease severity and increased the risk of poor prognosis [Citation28, Citation31]. Paracentesis such as thoracentesis and abdominal paracentesis were independent risk factors for CRKP infections and associated with higher mortality rate [Citation28]. Also, paracentesis was an independent risk factor for 30-day mortality in this study. It is well-known that KP typically colonises the respiratory and gastrointestinal tract of the human body, and invasive procedures or devices could increase the probability of bacterial colonisation or infection by rupturing the mucous membranes of the respiratory and gastrointestinal tracts, thereby increasing the chances of KP entering the bloodstream [Citation32]. It is noteworthy that invasive procedures should be interpreted with caution as they may present surrogate markers of critical illness rather than reflecting a direct association with mortality. Consistent with this interpretation, our study also found that ICU stay was associated with crude 30-day mortality ().
Table 3. Risk factors associated with crude 30-day mortality of 379 Klebsiella pneumoniae bloodstream infections cases.
Our results showed a high rate of resistance in KP, except for two drugs, tigecycline and polymyxin B, which are consistent with other studies [Citation20]. Polymyxin B and tigecycline are often used as last resort drugs for CR infections while carbapenems are often used as last resort drugs for MDR infections [Citation33]. Use of polymyxin B, tetracyclines and amount of carbapenems 90 days before diagnosis of KP BSIs were determined as independent risk factors for crude 30-day mortalityin this study (). Majority of tetracyclines used in this study was tigecycline (39/46, 84.78%). This may be explained by the fact that, non-survivors were more extensively treated with these antibiotics than survivors as the proportion of non-survivors with CR/MDR infections were significantly higher than that of survivors (). Moreover, combination therapy including tigecycline, polymyxins and carbapenems were reported to be more effective than active monotherapy for KP BSIs [Citation34, Citation35]. However, antimicrobial toxicity of combination therapy may increase the probability of adverse effects and the risk of mortality afterwards [Citation36]. Additionally, use of carbapenems may induce the production of carbapenemases, which is one of the main resistant mechanisms of CRKP [Citation37]. Again, this could be explained by critical illness as these antibiotics are last-line therapeutic options for MDR/CR infections.
The crude 30-day mortality rate increased to 34.12% in MDRKP BSI patients and higher in CRKP BSI patients (40.10%). CR phenotype was an independent risk factor for crude 30-day mortality. In this study, the majority of CR isolates (n = 197) are also MDR isolates (n = 194). MDR and CR phenotypes were widely reported to be predictors of poorer prognosis due to quite few effective therapeutic options for treatment [Citation22, Citation38, Citation39]. We found that irrational empirical antibiotic therapy was an independent predictor for 30-day mortality. Empirical antibiotics are started before the availability of microbiological results. This is vital because inappropriate, inadequate or delayed administration of active antibiotics has been reported to be associated with increased mortality [Citation4, Citation22, Citation34]. Therefore, better antibiotic stewardship and appropriate drug use is critical.
Interestingly, we found that total days of hospitalisation, days of hospitalisation after KP separation, and total hospitalisation costs of cases with MDR/CR phenotype were significantly higher than those of cases with non-MDR/non-CR phenotype. It is well-known that prolonged hospitalisation is a risk factor for drug-resistant bacterial infections [Citation40]. Approximately 10% of hospitalisations are complicated by healthcare-associated infections, and up to 75% of infections are due to microorganisms resistant to first-line therapeutic antibiotics [Citation41]. Increased medical costs are due to higher drug costs for MDR/CR BSIs, increased frequency of procedures such as intravenous antibiotics, and increased risk of complications [Citation42]. Drug-resistant bacterial infections make the disease more severe and complex, making treatment more difficult and ultimately leading to longer hospital stays and higher healthcare costs.
This study has some limitations. First, it is a retrospective, single-center study that may subject to selection and recall bias. Second, we have not molecularly characterised these clinical isolates to dissect their potential resistance mechanisms and virulence which may contribute to high mortality. Bioinformatics and phylogenetic studies are needed in future studies to better understand the phylogeny and pathogenicity of these isolates.
Conclusion
The incidence of KP BSIs was fluctuating during the study period. The crude 30-day mortality rate of KP BSIs patients was 26.39%. Awareness is required to prevent widespread of highly resistant KP. KP BSIs cases with non-MDR/non-CR phenotypes had lower mortality, shorter hospital stay, and lower healthcare costs compared to those of cases with MDR/CR phenotypes. Age, CR phenotype, paracentesis, CVC, exposure to some antibiotics, and irrational empirical treatment are independent risk factors for mortality.
Ethics approval and consent to participate
The study was approved by the ethics committee of Ruijin Hospital (KY2023-083). Because of the retrospective nature of the study, the committee waived informed consent. Patient data was obtained from medical record systems and analysed anonymously to protect patient privacy.
Supplementary_R3.docx
Download MS Word (40.6 KB)Acknowledgement
We would like to thank all the technicians of Department of Clinical Microbiology of Ruijin Hospital for their assistance in data collection.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets used during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Delle Rose D, Pezzotti P, Fontana C, et al. An in-depth analysis of nosocomial bloodstream infections due to gram-negative bacilli: clinical features, microbiological characteristics and predictors of mortality in a 1 year, prospective study in a large tertiary care Italian hospital. Infect Dis (Lond). 2019;51(1):12–22. doi: 10.1080/23744235.2018.1492149.
- Pau CK, Ma FF, Ip M, et al. Characteristics and outcomes of Klebsiella pneumoniae bacteraemia in Hong Kong. Infect Dis (Lond). 2015;47(5):283–288. doi: 10.3109/00365548.2014.985710.
- Holmes CL, Anderson MT, Mobley HLT, et al. Pathogenesis of gram-negative bacteremia. Clin Microbiol Rev. 2021;34(2):e00234-20. doi: 10.1128/CMR.00234-20.
- Chen J, Ma H, Huang X, et al. Risk factors and mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection in a tertiary-care hospital in China: an eight-year retrospective study. Antimicrob Resist Infect Control. 2022;11(1):161. doi: 10.1186/s13756-022-01204-w.
- Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16(1):18. doi: 10.1186/s12941-017-0191-3.
- Quan J, Zhao D, Liu L, et al. High prevalence of ESBL-producing Escherichia coli and Klebsiella pneumoniae in community-onset bloodstream infections in China. J Antimicrob Chemother. 2017;72(1):273–280. doi: 10.1093/jac/dkw372.
- Wang Z, Du M, Cao H, et al. Epidemiology and risk factors of nosocomial infections in a Chinese tertiary-care hospital: a 10-year retrospective case-control study. Infect Dis (Lond). 2024;56(4):320–329. doi: 10.1080/23744235.2024.2310647.
- Hu F, Guo Y, Yang Y, et al. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis. 2019;38(12):2275–2281. doi: 10.1007/s10096-019-03673-1.
- Dong N, Yang X, Chan E, et al. Klebsiella species: taxonomy, hypervirulence and multidrug resistance. EBioMedicine. 2022;79:103998. doi: 10.1016/j.ebiom.2022.103998.
- Bassetti M, Righi E, Carnelutti A, et al. Multidrug-resistant Klebsiella pneumoniae: challenges for treatment, prevention and infection control. Expert Rev Anti Infect Ther. 2018;16(10):749–761. doi: 10.1080/14787210.2018.1522249.
- Lin Y-T, Siu LK, Lin J-C, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol. 2012;12(1):13. doi: 10.1186/1471-2180-12-13.
- Arena F, Menchinelli G, Di Pilato V, et al. Resistance and virulence features of hypermucoviscous Klebsiella pneumoniae from bloodstream infections: results of a nationwide Italian surveillance study. Front Microbiol. 2022;13:983294. doi: 10.3389/fmicb.2022.983294.
- Pomakova DK, Hsiao C-B, Beanan JM, et al. Clinical and phenotypic differences between classic and hypervirulent Klebsiella pneumonia: an emerging and under-recognized pathogenic variant. Eur J Clin Microbiol Infect Dis. 2012;31(6):981–989. doi: 10.1007/s10096-011-1396-6.
- Zhang Y, Zhao C, Wang Q, et al. High prevalence of hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrob Agents Chemother. 2016;60(10):6115–6120. doi: 10.1128/AAC.01127-16.
- Chang H, Wei J, Zhou W, et al. Risk factors and mortality for patients with Bloodstream infections of Klebsiella pneumoniae during 2014-2018: clinical impact of carbapenem resistance in a large tertiary hospital of China. J Infect Public Health. 2020;13(5):784–790. doi: 10.1016/j.jiph.2019.11.014.
- Chen Y, Chen Y, Liu P, et al. Risk factors and mortality for elderly patients with bloodstream infection of carbapenem resistance Klebsiella pneumoniae: a 10-year longitudinal study. BMC Geriatr. 2022;22(1):573. doi: 10.1186/s12877-022-03275-1.
- Song F, Zhang K, Huang J, et al. Clinical characteristics, risk factors, and outcomes of patients with polymicrobial Klebsiella pneumoniae bloodstream infections. Biomed Res Int. 2021;2021:6619911–6619910. doi: 10.1155/2021/6619911.
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002.
- Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x.
- Liu KS, Tong YS, Lee MT, et al. Risk factors of 30-day all-cause mortality in patients with carbapenem-resistant Klebsiella pneumoniae bloodstream infection. J Pers Med. 2021;11(7):616. doi: 10.3390/jpm11070616.
- Chumbita M, Puerta-Alcalde P, Gudiol C, et al. Impact of empirical antibiotic regimens on mortality in neutropenic patients with bloodstream infection presenting with septic shock. Antimicrob Agents Chemother. 2022;66(2):e0174421. doi: 10.1128/AAC.01744-21.
- Xiao T, Zhu Y, Zhang S, et al. A retrospective analysis of risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae bacteremia in nontransplant patients. J Infect Dis. 2020;221(Suppl 2):S174–S183. doi: 10.1093/infdis/jiz559.
- Humphries R, Bobenchik AM, Hindler JA, et al. Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100, 31st edition. J Clin Microbiol. 2021;59(12):e0021321. doi: 10.1128/JCM.00213-21.
- Giske CG, Turnidge J, Cantón R, et al. Update from the European Committee on Antimicrobial Susceptibility Testing (EUCAST). J Clin Microbiol. 2022;60(3):e0027621. doi: 10.1128/JCM.00276-21.
- Valik JK, Hedberg P, Holmberg F, et al. Impact of the COVID-19 pandemic on the incidence and mortality of hospital-onset bloodstream infection: a cohort study. BMJ Qual Saf. 2022;31(5):379–382. doi: 10.1136/bmjqs-2021-014243.
- Namikawa H, Niki M, Niki M, et al. Clinical and virulence factors related to the 30-day mortality of Klebsiella pneumoniae bacteremia at a tertiary hospital: a case-control study. Eur J Clin Microbiol Infect Dis. 2019;38(12):2291–2297. doi: 10.1007/s10096-019-03676-y.
- Hussein K, Raz-Pasteur A, Finkelstein R, et al. Impact of carbapenem resistance on the outcome of patients’ hospital-acquired bacteraemia caused by Klebsiella pneumoniae. J Hosp Infect. 2013;83(4):307–313. doi: 10.1016/j.jhin.2012.10.012.
- Cao Z, Yue C, Kong Q, et al. Risk factors for a hospital-acquired carbapenem-resistant Klebsiella pneumoniae bloodstream infection: a five-year retrospective study. Infect Drug Resist. 2022;15:641–654. doi: 10.2147/IDR.S342103.
- Barchiesi F, Montalti R, Castelli P, et al. Carbapenem-resistant Klebsiella pneumoniae influences the outcome of early infections in liver transplant recipients. BMC Infect Dis. 2016;16(1):538. doi: 10.1186/s12879-016-1876-5.
- Tian L, Tan R, Chen Y, et al. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control. 2016;5(1):48. doi: 10.1186/s13756-016-0145-0.
- Babich T, Naucler P, Valik JK, et al. Risk factors for mortality among patients with Pseudomonas aeruginosa bacteraemia: a retrospective multicentre study. Int J Antimicrob Agents. 2020;55(2):105847. doi: 10.1016/j.ijantimicag.2019.11.004.
- Kim YA, Lee SJ, Park YS, et al. Risk factors for carbapenemase-producing enterobacterales infection or colonization in a korean intensive care unit: a case-control study. Antibiotics (Basel). 2020;9(10):680. doi: 10.3390/antibiotics9100680.
- Tran TB, Velkov T, Nation RL, et al. Pharmacokinetics/pharmacodynamics of colistin and polymyxin B: are we there yet? Int J Antimicrob Agents. 2016;48(6):592–597. doi: 10.1016/j.ijantimicag.2016.09.010.
- Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase–producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55(7):943–950. doi: 10.1093/cid/cis588.
- Wang T, Liu H, Huang H, et al. Colistin monotherapy or combination for the treatment of bloodstream infection caused by Klebsiella pneumoniae: a systematic review and meta-analysis. BMC Infect Dis. 2024;24(1):161. doi: 10.1186/s12879-024-09024-6.
- Paul M, Carmeli Y, Durante-Mangoni E, et al. Combination therapy for carbapenem-resistant gram-negative bacteria. J Antimicrob Chemother. 2014;69(9):2305–2309. doi: 10.1093/jac/dku168.
- Orsi GB, Bencardino A, Vena A, et al. Patient risk factors for outer membrane permeability and KPC-producing carbapenem-resistant Klebsiella pneumoniae isolation: results of a double case-control study. Infection. 2013;41(1):61–67. doi: 10.1007/s15010-012-0354-2.
- Li Y, Li J, Hu T, et al. Five-year change of prevalence and risk factors for infection and mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection in a tertiary hospital in North China. Antimicrob Resist Infect Control. 2020;9(1):79. doi: 10.1186/s13756-020-00728-3.
- Ben-David D, Kordevani R, Keller N, et al. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect. 2012;18(1):54–60. doi: 10.1111/j.1469-0691.2011.03478.x.
- Cohen MJ, Anshelevich O, Raveh D, et al. Acquisition of multidrug-resistant organisms among hospital patients hospitalized in beds adjacent to critically ill patients. Infect Control Hosp Epidemiol. 2006;27(7):675–681. doi: 10.1086/505919.
- Lautenbach E, Perencevich EN. Addressing the emergence and impact of multidrug-resistant gram-negative organisms: a critical focus for the next decade. Infect Control Hosp Epidemiol. 2014;35(4):333–335. doi: 10.1086/675592.
- Thaden JT, Li Y, Ruffin F, et al. Increased costs associated with bloodstream infections caused by multidrug-resistant gram-negative bacteria are due primarily to patients with hospital-acquired infections. Antimicrob Agents Chemother. 2017;61(3):e01709-16. doi: 10.1128/AAC.01709-16.