ABSTRACT
Neurourbanism looks to understand the relationship between urban environments and mental well-being and is well placed to assess the role of these environments on the urbanised and ageing global population. This study builds on research using mobile electroencephalography (EEG) to understand the impact of urban environments (busy, quiet and green urban spaces) on brain activity. Ninety-five older participants aged over 65 years undertook one of six walks in an urban neighbourhood, transitioning between two distinct environmental settings. This study explores changes in alpha (associated with relaxation) and beta (associated with attention) brain activity recorded during walking in differing urban environments. Neural activity significantly varies as participants walk between urban busy and green settings, with reduced levels of low beta activity in the green setting, suggesting attention changes consistent with Attention Restoration Theory. Levels of alpha activity significantly varied between the urban busy and the urban quiet settings, with increases in the urban busy setting. There were no significant differences in EEG activity between the urban green and urban quiet settings, suggesting that the magnitude of environmental contrast between the urban busy context and other urban settings is an important factor in understanding the effects of these spaces on brain activity.
KEYWORDS:
Introduction
In the context of a need to better understand the role that urban environments play in human health, there is a well-established literature demonstrating that preferences exist for viewing natural scenes over urban scenes (Velarde et al. Citation2007), and this effect is evident across different cultures (Takayama et al. Citation2014, Conedera et al. Citation2015). Additionally, walking in some natural environments has been shown to be beneficial for both psychosocial well-being (Bratman et al. Citation2015, Gidlow et al. Citation2016) and cognition (Berman et al. Citation2008, Citation2012), owing perhaps to the restorative effect of natural spaces (Kaplan and Kaplan Citation1989, Kaplan Citation1995, Citation2001) that reduce the demand placed on attentional capacities by busy environments (Mulckhuyse and Theeuwes Citation2010). Alternative theories suggest that sensory elements of green spaces provide positive psychophysiological responses to green spaces leading, in turn, to mental restoration and/or stress reduction (Peschardt and Stigsdotter Citation2013, Roe et al. Citation2013b). What is less well understood is how spending time in urban environments may affect cognitive load and attentional capacity.
There have recently been attempts to understand the role of the environment on brain activity using various neuroimaging techniques as a means to validate subjective measures of affect, attention and activation. Functional magnetic resonance imaging (fMRI) studies have revealed distinct networks of neural activation while viewing static natural and urban images. Natural scenes are associated with increased activation in the frontal gyrus, precuenus and anterior cingulate while the urban scenes induce increased activation in a network including the hippocampus, amygdala and inferior frontal gyrus (Kim et al. Citation2010a). The authors suggest that distinct environments induce distinct networks of neural activation and that these activations are related to the participants’ memory (hippocampus) and emotional responses (amygdala) to the given environment. Further fMRI research has suggested that this relationship could be mediated by life experience and place preferences (Kim et al. Citation2010b) with increased activation in hippocampal and parahippocampal regions, associated with memory function (Vargha-Khadem et al. Citation1997, Tulving and Markowitsch Citation1998), as well as the amygdala, associated with emotional processing (Rasia-Filho et al. Citation2000, Phelps and LeDoux Citation2005), during viewing of urban scenes.
The restorative effect of environments has been directly assessed by fMRI (Martínez-Soto et al. Citation2013), showing that rural scenes induced increases in neural activation associated with bottom-up (i.e. exogenous/stimulus-driven) attentional processing while urban scenes induced increases associated with top-down (i.e. endogenous) attentional processing (Buschman and Miller Citation2007). There is a psychological benefit of bottom-up attentional processing as it relates to involuntary attention (Itti Citation2006), as defined in Attention Restoration Theory (Kaplan and Kaplan Citation1989, Kaplan Citation1995) as effortless attention which leads to reduction in fatigue, while greater mental effort and subsequent fatigue is associated with top-down processing, defined as directed attention (Berto et al. Citation2010, Mulckhuyse and Theeuwes Citation2010).
Recent research has suggested that a 90-min walk in nature (grassland and trees) leads to decreases in levels of rumination (associated with risk of depression), and decreases in activity in the subgenual prefrontal cortex compared with a 90-minwalk in a busy urban environment where these decreases were not seen (Bratman et al. Citation2015). However, these fMRI studies are limited to providing information obtained in a laboratory setting, either using 2D photographic imagery, or post in-situ real-world experience of environmental stimuli.
Electroencephalography (EEG) has been used to assess environmental effects on neural activity. Research has shown that passively viewing static rural images can induce increases in alpha (8–13 Hz) activity (Ulrich Citation1981, Chang et al. Citation2007) associated with decreased cortical activation likened to relaxation (Kubitz and Pothakos Citation1997) and resting cortical states (Sauseng et al. Citation2005). Decreases in alpha activity, with subsequent increases in higher frequencies such as beta (13–30 Hz), are indicative of higher stress and increased alertness/vigilance (Bonnet and Arand Citation2001). Increased beta activity has been correlated with visual attention and modulation (Wrobel Citation2000, Buschman and Miller Citation2007) and shown to increase in situations requiring high levels of attention, such as driving in an urban environment (Dehzangi and Williams Citation2015). There is evidence to suggest that viewing vegetation in the form of grass, plants and shrubs (landscape plants) can reduce levels of beta activity associated with traffic noise (Yang et al. Citation2011), suggesting that even minor green space interventions can have an impact on brain activity.
Exercise, in general, has also been associated with neural activity change, with increased levels of alpha activity post-exercise (Schneider et al. Citation2009) as measured from frontal EEG sites. Physical effort also leads to increased levels of lower beta activity (in this example, 13–22 Hz), associated with vigilance, and reduced levels of higher beta (23–30 Hz) activity, associated with cognitive processing (Smit et al. Citation2005). In-situ exposure to natural and urban contexts has been assessed using mobile EEG (Chen et al. Citation2016) with results suggesting that exposure to nature leads to increased neural connectivity, associated with more efficient processing, supporting theories on the restorative effects of natural spaces on psychological well-being. Furthermore, this objective measure of activity correlated with participants’ subjective experience of nature, shown by increased levels of ‘coherent’ landscape extent experience measured by the Perceived Restorative Scale (PRS).
Mobile EEG is an emerging tool that may lead to further understanding of the effect of real-world environments on neural activity (Mavros et al. Citation2016). There have been numerous studies that have used the Emotiv (www.emotiv.com) headset in health and well-being research (Milosevic et al. Citation2013, Choo and May Citation2014, Aspinall et al. Citation2015, Menshawy et al. Citation2015, Neale et al. Citation2017) and have validated its appropriateness in both laboratory and outdoor settings (Debener et al. Citation2012, Badcock et al. Citation2013), although some research casts doubt over its appropriateness for clinical measurements or critical medical applications (Duvinage et al. Citation2013). Emotiv offer the proprietary Affectiv suite software which defines distinct brain activity patterns and allocates a label to each (‘frustration’, ‘excitement’, ‘engagement’, ‘meditation’ and ‘long term excitement’). At the time of writing, there are no published studies that attempt to directly correlate the Affectiv suite terms with specific EEG frequency bands. Laboratory data using the Emotiv headset showed green space scenes were associated with increased levels of ‘meditation’ and lower levels of ‘excitement’, and these physiological findings were matched by participants’ preference for green space over grey space using subjective scales (Roe et al. Citation2013a). Furthermore, the presence of green landscape scenes could be predicted by increases in levels of ‘meditation’ and lower levels of arousal. However, laboratory data may not reflect an individual’s actual experience in a given real-world given environment. To address this issue, in situ assessments of brain activity in young participants physically walking through different urban (quiet urban street, urban green space and busy commercial urban street) environments sequentially, wearing a mobile EEG headset, have been undertaken (Aspinall et al. Citation2015). The results showed decreased levels of ‘frustration’ (associated with stress and negative valence), ‘engagement’ (associated with immersion and interest) and ‘excitement’ (associated with increased arousal) moving from a quiet university building district into an urban green space, with increased levels of ‘engagement’ moving from the green space into a busy, urban space.
Such methodology, using EEG in real-world environments as well as in laboratory settings, has been used increasingly in recent years, reflecting interest in the emerging discipline of ‘neurourbanism’ (Adli et al. Citation2017). Research to date has focused almost exclusively on young people, with the vast majority of experiments undertaken with university students and/or people aged under 30 years (Chang et al. Citation2008, Chen et al. Citation2016). However, patterns of neural activity are age-related and this may be important in understanding environmental interactions for older populations (Neale et al. Citation2017). For example, while increased beta activity is associated with visual attention and modulation (Wrobel Citation2000, Buschman and Miller Citation2007) it is also prone to age-related change (Vysata et al. Citation2014), correlated with deficits in either alertness (activating attentional processes) or vigilance (sustaining attentional processes) in older people (Gola et al. Citation2012). It seems likely that these attentional deficits may be exacerbated by distracting environments, such as busy urban environments, and this may be a barrier to people remaining active in the community in older age, as well as increasing vulnerability to falls (a leading cause of hospitalisation in people aged 65 and over (Curl et al. Citation2016)). In the context of an increasingly urbanised and ageing population (United Nations, Citation2018; Rutherford, Citation2012), where by 2050 it is estimated that 34% of the European population will be over 60 years old (Rutherford, 2012) it is important to consider how urban environments impact on older people. It is this dearth of studies people aged 65 and over that the present study addresses. While both laboratory and ‘real world’ interpretations of EEG signals are currently published, there are no published studies, to the authors’ knowledge, that directly assess the raw EEG signal of older participants when walking between different types of urban environments. This study developed its hypotheses based on limited existing findings from laboratory-based studies or from use of Affectiv Suite to interpret EEG in the field, as well as drawing on theories on the restorative effects of natural environments versus busy, building-dominated urban environments.
Aims
This study aimed to understand the impact of walking through different urban environments (urban busy, urban green and urban quiet spaces) on neural activity using mobile EEG with a large sample of older participants. Much of the laboratory research to date has investigated the high contrast between natural and urban scenes, with increases of EEG alpha activity shown when viewing natural scenes compared with urban scenes. Previous research has shown increases in beta (including the lower and higher ends of the frequency) activity correlated with increased attentional demands and driving in urban spaces. Studies have previously shown changes in emotional parameters (based on the Affectiv suite) derived from EEG signals when transitioning between urban settings, including a quiet urban setting, but this study is novel in that it assesses raw EEG signals during a walking study.
Our research question was: what impact does transitioning between different urban environments have on brain activity as measured by alpha and beta waves? We further framed hypotheses to test answers to this general research question using the varying walking routes that participants undertook, as follows.
Urban Busy versus Urban Green. We expected to see increases in alpha activity when walking in urban green space, associated with increased relaxation, compared with an urban busy space., as well as increases in beta activity (associated with increased physical and mental effort) in the urban busy setting when compared with the urban green setting.
Urban Busy versus Urban Quiet and Urban Green versus Urban Quiet. We anticipated we would find that walking in a quiet urban setting would show increased levels of beta (associated with increased attentional capacity) relative to walking in green space, and increased levels of alpha relative (associated with increased relaxation) to the busy urban setting.
Methods
Participants
Participants were healthy adults aged over 65 years (N = 95, M age = 76.55 years, SD = 8.15, range = 65–92 years) and were recruited by purposive sampling methods to ensure they met the required inclusion criteria. Participants were recruited using various resources, such as online and print adverts, social media and research partner organisations’ mailing lists. Exclusion criteria for study participation included visual impairments, chronic mental illness and a history of epileptic or psychiatric disorders. All participants were required to be able to walk, unassisted by another person, for at least 15 min. Ethical approval for the study was provided by the University of Edinburgh, Edinburgh College of Art Ethics Committee. To account for brain hemispheric differences (Sperry Citation1968), all participants in this study were right-handed.
All participants scored above the threshold for inclusion based on Mini Mental State Exam (MMSE) scores. As described by Folstein et al. (Citation1975), scores above 24 indicate no cognitive impairment. The lowest score obtained in these results was 27, indicating that all participants had no indication of cognitive impairment as measured by the MMSE.
Experimental design and procedures
All participants were screened via a telephone conversation with the research team to ensure they fulfilled the inclusion criteria before being invited to undertake a practice session. This session served as an opportunity to demonstrate the EEG headset (described below) and the walking route they would take (described below) during the experimental session. This was achieved by showing participants a 15-min video of the route. Ensuring participants were aware of their assigned route was important as it meant participants did not have to refer to maps or consult the researchers for wayfinding and generally increased participants’ ease while walking, wearing the headset. Participants were shown a map pre-walk to remind them of their routes.
The experimental session was undertaken on a subsequent day when participants were equipped with a backpack (Dell Urban 2.0 Backpack, weighing 340.2 g) to store only the data acquisition computer (Dell Latitude E7240, weighing 1.31 kg, plus the Emotiv wireless USB dongle) as well as having the EEG headset calibrated prior to commencing the walk. Participants were instructed to walk on their assigned route at their own pace, understanding that a member of the research team was following approximately 10 m behind for safety purposes. shows an example of a participant wearing the headset for illustrative purposes.
The researchers logged events throughout the walk (including start and end times) using Fieldworker (Mavros and Skrompelou Citation2015). This data provided back up information to correctly segment the EEG data (indicating start and end times of each walking segment), as well as an opportunity to record unusual events (such as sirens or barking dogs). However, there were not enough data points for these events to allow for any meaningful analyses. On average, participants would complete the walks within 10–15 min. All experimental sessions were conducted during weekdays and in the morning in June, July and September, to ensure time of day and seasonal effects were kept to a minimum.
Routes
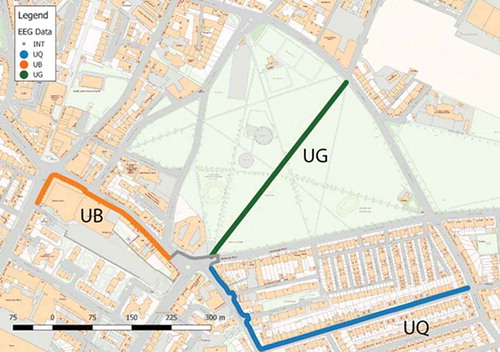
Participants walked through one of six walk scenarios, as indicated on the map in . The study site was based in Leith, an historic urban area in the City of Edinburgh, Scotland. It was selected due to the proximity of urban green space to busy, urban space, with a reasonably flat gradient on all study routes to ensure participants could undertake any route without excessive exertion. This ensured EEG responses were due to environmental change and not gradient variations (Bradford et al. Citation2015). We ensured that the routes were of close to equal length, ensuring that participants completed each section of their total route at approximately the same time. indicates an interchange zone in grey between the urban green (UG) space and the urban busy (UB) and quiet (UQ) spaces, where participants had to cross a busy road junction. The busy road crossing was not modelled in the analysis due to the unpredictable nature of the conditions around the pedestrian crossing.
Figure 2. Map of the walking routes undertaken by participants (walking in one of six possible scenarios). UQ – Urban quiet; UB – Urban busy; UG – Urban green.
These routes were paired to generate six walk scenarios;
Urban busy (UB) to urban green (UG)
Urban busy (UB) to urban quiet (UQ)
Urban green (UG) to urban busy (UB)
Urban green (UG) to urban quiet (UQ)
Urban quiet (UQ) to urban busy (UB)
Urban quiet (UQ) to urban green (UG)
) shows images of each of these environments. Using a between-subjects design, participants were randomly assigned to one of the six route scenarios and were required to walk sequentially between the two assigned environments. The urban green space is an area with a predominance of vegetated and non-built surfaces (including grass and trees). The urban busy space is characterised by mixed-use buildings, paved areas and a commercial street frontage that attracts a high footfall and vehicular traffic. The urban quiet space is largely residential with a predominance of residential buildings, with some front gardens and paved areas, but not attracting a high footfall or volume of vehicular traffic.
EEG data acquisition
Brain electrical activity was recorded non-invasively from the scalp using the commercially available Emotiv EPOC+ EEG headset with 14 channels (AF3, AF4, F3, F4, F7, F8, FC5, FC6, T7, T8, P7, P8, O1 and O2), using Emotiv’s Xavier Testbench program. P3 acts as the Common Mode Sense (CMS) active electrode reference with P4 acting as the Driven Right Leg (DRL) passive electrode. All electrodes correspond to positions on the international 10–20 position system. Electrode impedances were checked for contact quality and signals are internally sampled at 1024 Hz before being filtered and down sampled to 128 Hz per channel (i.e. 128 samples per second) and sent via Bluetooth to the data acquisition computer. Optimal conductance was obtained by soaking the electrodes in saline solution before adding glycerine solution in order to prevent the sensors from drying out.
Pre-processing of the raw EEG data was undertaken in EEGLAB, a MATLAB toolbox. Individual participant data were imported, and the 14 channels were defined. Signals from the 14 channels were high-pass filtered with a 0.16 Hz cut-off and low-pass filtered with a 42 Hz cut-off before being re-referenced to the average reference in EEGLAB. Poor quality data (such as due to extreme head motion) were rejected by visual inspection before segmenting data sets from the continuous data file; these sections were dictated by the start and end times of the walking sessions (i.e. start/end times of an urban busy walk for example). An independent component analysis (ICA) was then performed on the data to filter out any potential remaining extreme signal artefacts from the data.
A fast Fourier transform (FFT) was then applied to the data, normalised by the length of recording, to determine which frequency components make up the raw signal. Of interest to this study is the alpha and beta band. Rather than simply calculating mean values for each walking segment, a single root mean square (RMS) value, as used previously in EEG studies to detect magnitude change in signal (Bhuvaneswari and Kumar Citation2015), was calculated using a MATLAB script for each walking segment for alpha (9–13 Hz), low beta (13–19 Hz) and high beta (21–27 Hz) activity. Descriptions of these frequencies are described in .
Table 1. Descriptions of the EEG signals of interest.
Data analysis
In order to analyse the data, the pre-calculated, single RMS value for each individual per walking segment was generated (e.g. a RMS value for the entire urban green space (UG) walking segment and a second value for the entire urban busy (UB) walk in a UG to UB walk) for each of the three raw EEG signals of interest; alpha, low beta and high beta. These RMS values were standardised by subtracting the group RMS from the raw RMS for each individual and dividing this by the standard deviation of the group RMS. This data set was subsequently analysed using a form of high dimensional correlated component regression (CCR) which has a wide range of applications, including being able to deal with smaller samples where p (number of predictors) is greater than n (number of cases), if required, as well as repeated measures, identification of suppressor variables and multicollinearity (Magidson Citation2010, Citation2013), making it preferable to standard regression analyses for this data set (Garver and Williams Citation2018, Rosero‐Vlasova et al. Citation2019). Furthermore, this method has been used consistently in a variety of health-related environmental studies with successful results (Aspinall et al. Citation2015, Curl et al. Citation2015, Ward Thompson et al. Citation2016, Neale et al. Citation2017). We assessed difference scores between environmental contexts for each of the walking routes (dependent variable; e.g. UB to UG and UG to UB), generated by a calculation of x = (Walk A – Walk B) at the participant level for each of the three EEG signals of interest (independent variables). Outliers were identified and amended using a criterion of z = 2.5 (i.e. high difference outliers which might be unduly influential were brought back to the highest value within 2.5 standard deviations (Osborne and Overbay Citation2004)). These final differences scores were used in the regression analysis. The CCR method generates a model (training) which is subsequently tested through multiple runs (n = 500).
Results
Comparison of walking between urban busy (UB) and urban green (UG) environments
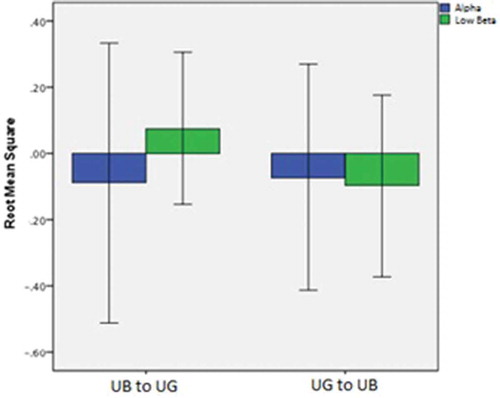
indicates that there were differences between UB and UG environments with respect to levels of alpha and low beta. There was no effect of the changing environment on levels of high beta in either route.
Table 2. Logistic CCR outputs for the UB and UG routes.
The ‘Model Fit’ section of shows the value of R2 from cross-validation along with its standard error (SE) showing a small to medium effect size. The standardised coefficients are shown (and interpreted as in any regression output), indicating the out of sample performance and the rank order of predictors in the model. The positive and negative signs of the standardised coefficients in associated with alpha and low beta levels are given context in . The figure shows the differences in alpha and low beta in going from the first to the second part of each walking scenario. A positive value above zero indicates levels for that parameter are greater in the first part of the walk, and a negative value below zero indicates levels for that parameter are greater in the second part of the walk. In , low beta is shown to be higher in UB than in UG, and this result is reversed in the UG to UB direction, meaning that low beta is still higher in the UB condition than UG. However, does not show any discrimination between the walks for alpha; to explore this further, a Spearman correlation was employed between the binary dependent (UB/UG) revealing the correlation to be non-significant (r = .03, p = .83). However, the Spearman correlation between the binary dependent variable (UB/UG) and low beta when controlling for alpha shows a significant correlation (r = – .301, p = .047) which is higher than that for the dependent and low beta alone (r = – .076, p = .67). This suggests that alpha, while not a predictor, is acting as a suppressor variable (Conger Citation1974, MacKinnon et al. Citation2000). The inclusion of alpha in the model strengthens the effect of low beta in relation to the route dependent variable by suppressing some of the irrelevant variance within low beta.
Comparison of walking between urban busy (UB) and urban quiet (UQ) environments
indicates that there is only one predictor that discriminates between walking between UB and UQ environments with respect to levels of alpha activity. There was no effect of the changing environment on levels of either low or high beta in either route.
Table 3. Logistic CCR outputs for the UB and UQ routes.
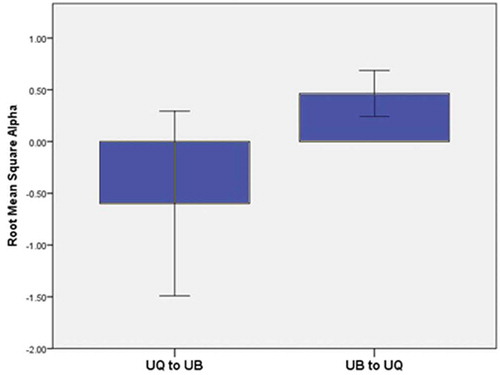
The ‘Model Fit’ section of shows the value of R2 from cross-validation along with its standard error (SE) showing a small to medium effect size. The positive and negative signs of the standardised coefficients in associated with alpha levels are given context in . In the first transition from UQ to UB, alpha activity is negative, meaning that alpha activity is lower in UQ compared to the UB section. However, for the walk in the reverse direction (UB to UQ), the results are reversed. Alpha is positive, meaning that it is higher in UB than UQ.
Figure 5. Alpha difference scores for each walking condition showing that alpha is greater in the UB setting than the UQ setting.
Note: A positive value above zero indicates levels for that parameter are greater in the first part of the walk and a negative value below zero indicates levels for that parameter are greater in the second part of the walk.
Comparison of walking between urban quiet (UQ) and urban green (UG) environments
indicates that there were no predictors that discriminate between UG and UQ environments across any of the predictors in the model.
Table 4. Logistic CCR outputs for the UG and UQ routes.
The ‘Model Fit’ section of shows the value of R2 from cross-validation along with its standard error (SE) showing a medium effect size. Despite this medium effect size, high area under curve (AUC) and model accuracy, there were no predictors that showed a statistically significant effect when walking between these two conditions.
Discussion
For the first time, we have shown, via raw EEG data analysis, different responses in alpha and low beta brain activity to walking in varying urban environments in a comparatively large sample of older people, aged 65 and over. In general, we found meaningful changes in brain activity only when urban busy (UB) was contrasted with other environments; the transition between urban quiet (UG) and urban green spaces showed no significant differences, whereas the routes that had an urban busy segment showed meaningful differences in brain activity between settings. This suggests that there are properties distinctive to the urban busy setting (a building-dominated and heavily trafficked urban environment) which perhaps play an important role in the models presented here.
Urban busy versus urban green
Results indicate that low beta (13–19 Hz) activity increased while walking in the urban busy setting in comparison with the other two settings in our study, as expected in hypothesis (a). Previous studies have shown that beta activity increases as a function of vigilance and visual attention (Wrobel Citation2000, Buschman and Miller Citation2007, Dehzangi and Williams Citation2015). The increased demands on attention and vigilance required to navigate a busy urban setting may explain the increase in low beta when walking in busy urban spaces compared with walking in urban green spaces. Recent research has shown a positive correlation between beta activity and associated arousal and attention when viewing streetscapes of varying complexity in a laboratory setting (Kacha et al. Citation2015). Given the higher levels of both pedestrian and vehicle traffic, a number of junctions and building size and visibility in the urban busy setting here, when compared with the green space, this increased complexity of the environment could be, in part, responsible for the increases in beta shown here. Interestingly, the effect on beta shown in the laboratory setting (Kacha et al. Citation2015) was across the entire beta band (13–30 Hz). The results we present here only show an effect for low beta and indicate no effect of environmental transitions on high beta (21–30 Hz) activity. The pattern of low beta activity found in this study follows the same pattern as ‘excitement’ levels that have previously been shown (Neale et al. Citation2017) when measured using the Affectiv suite software developed by Emotiv.
Of the three settings explored, the contrast in our outcome measures is greatest between the urban busy and the urban green sections, so the environmental contrast between these two settings is likely to account for the largest effect found in our analysis. Previous research, as discussed earlier, has shown that greenery such as small, ornamental trees and shrubs can reduce levels of beta activity (Yang et al. Citation2011). This increased exposure to vegetation may explain the reduced level of low beta seen in our study’s green space setting compared with the response to the absence of any planting in the urban busy setting.
The role of alpha (9–13 Hz), while not a predictor in the regression models shown here, is to have a suppressor effect, increasing the effect strength of low beta on the route-dependent variable. This is counter to our earlier prediction, where we expected alpha activity to increase in the green space when compared to the urban busy setting, as previously shown in laboratory studies (Ulrich Citation1981, Chang et al. Citation2007).
Urban busy versus urban quiet
In the urban busy versus urban green model, we did not see the expected increase in alpha activity in green space. However, when compared with the urban quiet setting, there was an increase in alpha activity in the urban busy setting, contrary to our framing of hypothesis (b), and restorative environment theory. We had hypothesised that the calmer environments of green and quiet urban settings would induce alpha activity associated with relaxed states, compared with the higher vigilance required for navigation of a busy urban setting. However, our findings suggest that, unlike in the urban busy versus urban green setting, alpha activity is not acting as a suppressor variable in the urban busy versus urban quiet setting.
Recent research suggests that, when viewing streetscapes, familiar environments are associated with increases in alpha activity (Kacha et al. Citation2015), an effect also shown in experts viewing familiar motor actions (Nota et al. Citation2017), and which may be physiologically modulated by levels of acetylcholine (Eckart et al. Citation2016). Potentially, the urban busy setting used here – a busy street with familiar shops (national supermarket) and landmarks (bus stops, street furniture, bins) – is one that is sufficiently familiar to participants, resulting in increases in alpha activity. The urban quiet setting, however, is likely to be less familiar to participants, being a much less-visited street which does not necessarily conform to expected urban street or urban park characteristics in the same way that the busy and green settings do here. If the urban quiet setting is less familiar, it may induce a level of ‘fascination’ that is a core component of the restorative experience highlighted in attention restoration theory (Kaplan and Kaplan Citation1989, Kaplan Citation1995). The familiarity of the urban busy setting compared with the relatively unknown urban quiet street may be responsible for the change in alpha shown in this model. It would be of interest to explore this further by assessing responses to familiar urban quiet settings (perhaps near the home of participants if appropriate) to establish if the increase in alpha is associated with familiarity versus ‘fascination’ or is associated with the particular features of the environmental contexts used here.
Urban green versus urban quiet
There were no differences shown in the model between alpha or beta activity in the green compared with quiet urban spaces. This could perhaps be because both settings are largely pleasant, quiet spaces, with some small but attractive front gardens, therefore the variation in experience between the two settings is not sufficient to generate a significant difference in effects as measured by alpha or beta.
General discussion
One of the underlying strengths of the study design utilised here is the external validity of undertaking environmental research ‘in the wild’, but it also brings challenges. Castermans et al. (Citation2014) suggest that the EEG signal up to 15 Hz may become distorted when recorded at the same time as walking, albeit on a treadmill. This poses a methodological concern given that the alpha (8–13 Hz) and a section of the low beta signal (13–19 Hz) would be affected by this. However, not only were the data thoroughly pre-processed in order to clean any distorted data, but the changes we see between conditions appear to be context specific, suggesting that the findings presented here are not the result of excessive signal noise. Future researchers may wish to compare the conditions of this study, perhaps using virtual reality in a laboratory setting with a treadmill, to assess the neural effects of viewing the urban environment in order to distinguish laboratory from ‘real world’ effects. This could be important as our findings appear to contradict certain previous, laboratory-based EEG work, suggesting that it may be possible to identify distinctive neural signatures associated with viewing, as opposed to being immersed in, different urban environments. Research has begun into benchmarking EEG systems against sitting and walking conditions to assess data rejection rate, pre-stimulus noise per condition, signal-to-noise ratio and EEG amplitude (Oliveira et al. Citation2016), in order to assess differences between the two different data collection conditions. It would be valuable to replicate this method using the Emotiv Epoc+ system used in the present study.
One of the central principles of attention restoration theory is that, for an environment to be restorative, it must offer some level of effortless or ‘soft’ fascination (Herzog et al. Citation1997, Ouellette et al. Citation2005). The sites selected for this study were chosen on the basis that they were green, quiet and busy urban spaces close enough to each other to provide easily transitions for older people within a short walk period (). We deliberately chose a green space without water (known to influence landscape preferences), so that the sections of the route were as close to their descriptions of urban green, urban busy and urban quiet as possible. Given the methodological constraints placed on this site selection, it is possible that the green space chosen here was not sufficiently fascinating to induce a restorative experience or show a marked effect in EEG. However, the site is typical of many public parks that reflect everyday urban contexts in Great Britain, so the inferences from this study may be transferable to other local, accessible urban green spaces. Further research is recommended to demonstrate whether these results are reproducible in other urban contexts, both in Britain and internationally, with quiet, busy and green urban spaces. While the architectural style of buildings in Edinburgh is different to other parts of the world, it would be interesting to apply this method to other settings that retain the same broad urban forms (busy, quiet and green elements). Such work may help to elucidate the role that urban, architectural or landscape design plays in such results, as well as the influence of other people in the environment. For example, does the level of pedestrian flow through a space, or the percentage of route that is tree-lined, act as a key element in interpreting EEG responses?
Our study area was chosen partly due to it having comparatively level topography, in order to minimise any physical challenge to our participants associated with a route having steep gradients. There is recent research that suggests a unified canonical-correlation analysis for EEG data recorded while walking may be useful to remove any gradient artefacts that may come about from walking up hills (for example) that this study protocol avoided (Li et al. Citation2017). Therefore, future studies may be able to conduct mobile EEG experiments in more topographically varied and contrasting settings to assess different levels of fascination within or between environments that are not relatively flat.
A further consideration is that the ease of navigation of the study route may have contributed to the absence of change in high beta activity, in that there were perhaps no cognitively challenging aspects to the walk, which thus required lower levels of vigilance and attention (Wrobel Citation2000, Buschman and Miller Citation2007). The age of the cohort may also have contributed to the lack of response in high beta activity; it has been suggested that beta activity may be susceptible to change in older age (Gola et al. Citation2012), in particular at higher frequencies (Christov and Dushanova Citation2016). The notion of varying complexity in order to achieve cognitive-friendly environments (Cassarino and Setti Citation2016) that help people navigate in older age, despite some level of cognitive decline, suggests that a lack of environmental complexity may result in sub-optimal cognitive stimulation; this may explain the absence of high beta activity in any of the walking routes presented in the data here.
There are some limitations in the study, due to the nature of the experimental protocol. In particular, given the ‘real world’ settings used, the protocol was unable to control for real-world events resulting in potentially varying sensory experiences – such as sights and sounds – between participants that can be avoided with controlled laboratory studies. For such walking studies, future research may wish to isolate unusual events, such as increased traffic noise, and either remove the associated EEG data for this time period from the dataset, or analyse them separately. Research suggests brightness (Park et al. Citation2013) and ambient temperature (Lv et al. Citation2017) may affect EEG signals, so future research may wish to record these in real-world settings and covary for them in any subsequent mobile EEG analysis.
There are some concerns in the research community about the quality of the data that Emotiv headsets acquire, with studies suggesting that the quality of the data is not sufficient for clinical use (Duvinage et al. Citation2013). While the present study does not attempt to make clinical diagnoses, it would be advantageous to the research community working in environmental psychology if direct comparisons could be undertaken in future using this experimental protocol, comparing both medical grade and Emotiv data to assess neural activity, as has been previously done with other experimental protocols (Debener et al. Citation2012, Badcock et al. Citation2013).
In an increasingly urban and ageing population, it is important to have cities that encourage and support people in maintaining pleasurable walking into very old age. This ‘real-world’ research offers the opportunity to better understand how different urban contexts are experienced and responded to in ways that may be supportive of well-being or potentially hazardous. Issues relating to older people, in particular, include opportunities for relaxation away from distractions that demand attention, such as busy urban environments, where trips and falls may be more likely due to these demands. The study also suggests that there may be opportunities for using mobile EEG to better understand the ways in which some environments engage attention without being over-demanding, while others can quickly become exhausting.
We have shown, for the first time, changes in low beta activity related to differences between urban busy and urban green spaces. As previously discussed, beta activity is prone to age-related change (Gola et al. Citation2012, Vysata et al. Citation2014), so future studies should not only aim to assess if this effect is reproducible but also if there are differences between young and old populations in beta activity. Future research might assess the role of urban spaces on beta activity in older participants with particular, clinically recognised, states, such as those with dementia. Undertaking this research could perhaps lead to identification of neural signatures associated with the experience of different kinds of urban spaces by age and cognitive state, which in turn would be useful for designers of public spaces and residential environments, as well as for policy-makers and health practitioners to understand any beneficial or detrimental effects of urban spaces on an ageing population.
Conclusion
These results add to the growing evidence regarding neural change associated with viewing or experiencing changing urban environments. This is the first time, to the authors’ knowledge, that a mobile EEG project has been undertaken on a group of this size, and on older adults, to understand the effects of walking in the real urban environment using raw EEG data. We show clear changes in low beta activity between urban busy and urban green settings, perhaps linked with cognitive arousal and attention. Furthermore, we show differences in levels of alpha activity between urban busy and urban quiet settings, perhaps due to familiarity associated with the urban busy setting. While this investigation sheds some light on such effects, based on a simple design comparing different urban settings, further investigation is required to understand the exact neurological processes that underpin these changes.
Acknowledgements
The authors would like to thank Agnès Patuano and Esther Rind for their assistance in collecting the experimental data and to Gary Bennett (The Stats People) for his assistance with the analysis software. Further thanks are given to all the participants who took part in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Chris Neale
Chris Neale is a researcher at the University of Virginia interested in understanding how urban and rural environments can impact health and wellbeing in a range of participants, but with particular interest in older adults. His background is in cognitive neuroscience, and he continues to use various neuroimaging methods to assess brain activity in research populations.
Peter Aspinall
Peter Aspinall is Emeritus Professor of Environmental Studies at Heriot-Watt University and an Honorary Fellow in the School of Clinical Sciences at the University of Edinburgh. His main research interests are in visual function and quality of life; environmental psychology and inclusive design, with a particular emphasis on quantitative methods. He has worked with Professor Catharine Ward Thompson on a number of projects focused on older people’s perceptions and experience of the outdoor environment.
Jenny Roe
Jenny Roe is an environmental psychologist who explores how our interactions with the world shape our health, wellbeing and behaviors. She specializes in understanding how access to restorative environments in our cities create and sustain our health and wellbeing. Her research aims to advance social justice by tackling health and environmental inequities relating to the built environment. She directs the Center for Design and Health at the University of Virginia.
Sara Tilley
Sara Tilley is a transport geographer, interested in exploring the links between mobility in the urban environment and health and wellbeing, especially in younger and older age. She is now Study Manager and Research Fellow on OPENspace’s NIHR-funded research into the effectiveness of the Forestry Commission Scotland woodland improvement programme, Woods In and Around Towns (WIAT).
Panagiotis Mavros
Panagiotis Mavros is a researcher at the Future Cities Laboratory (FCL) of the Singapore-ETH Centre, where he is the Project Coordinator of the project Cognition, Perception and Behaviour in Urban Environments. His research is focused in on wayfinding behaviours in complex multilevel environments, using psychophysiological methods to study the experience of urban space, and the translation of spatial cognition research into design and policy.
Steve Cinderby
Steve Cinderby is a Senior Researcher at the SEI centre in the University of York specialising in community resilience, wellbeing and participatory research methods. He has specialised in the development of communication approaches for improved environmental decision-making outcomes. These have been aimed at increasing knowledge sharing, improving the capacity for pro-environmental behavioural change, and boosting local community resilience and wellbeing.
Richard Coyne
Richard Coyne researches and teaches on the cultural, social and spatial implications of computers and pervasive digital technologies. He has published eleven books with Pitman, Addison-Wesley, Routledge, Bloomsbury and MIT Press on digital technologies. He recently published Network Nature: The place of Nature in the Digital Age (Bloomsbury Academic, 2018), and a book on the philosopher Charles Sanders Peirce: Peirce for Architects (Routledge, 2019).
Neil Thin
Neil Thin specialises in appreciative social planning, i.e. engaging multidisciplinary happiness and wellbeing scholarship in public policy and practice. He also has over 20 years of practical and policy experience working towards the reduction of poverty and promotion of justice and wellbeing in poorer countries, working at all levels from grassroots to governmental and international official agencies. He has frequently served as a social development adviser and trainer for international development agencies such as the UK Department for International Development, UN Agencies, the World Bank, and international NGOs.
Catharine Ward Thompson
Catharine Ward Thompson is Professor of Landscape Architecture at the Edinburgh School of Architecture and Landscape Architecture (ESALA), and Director of the OPENspace research centre. Her research focuses on inclusive access to outdoor environments, environment-behaviour interactions, landscape design for older people, children and teenagers, and salutogenic environments. Catharine also has expertise in the history and theory of urban park design and conservation, the history of landscape design, and landscape aesthetics and perception.
References
- Adli, M., et al., 2017. Neurourbanism: towards a new discipline. The Lancet Psychiatry, 4 (3), 183–185. doi:10.1016/S2215-0366(16)30371-6.
- Aspinall, P., et al., 2015. The urban brain: analysing outdoor physical activity with mobile EEG. British journal of sports medicine, 49 (4), 272–276. doi:10.1136/bjsports-2012-091877.
- Badcock, N.A., et al., 2013. Validation of the Emotiv EPOC® EEG gaming system for measuring research quality auditory ERPs. PeerJ, 1, e38. doi:10.7717/peerj.38
- Berman, M.G., et al., 2012. Interacting with nature improves cognition and affect for individuals with depression. Journal of affective disorders, 140 (3), 300–305. doi:10.1016/j.jad.2012.03.012.
- Berman, M.G., Jonides, J., and Kaplan, S., 2008. The cognitive benefits of interacting with nature. Psychological science, 19 (12), 1207–1212. doi:10.1111/j.1467-9280.2008.02225.x.
- Berto, R., et al., 2010. An exploratory study of the effect of high and low fascination environments on attentional fatigue. Journal of environmental psychology, 30 (4), 494–500. doi:10.1016/j.jenvp.2009.12.002.
- Bhuvaneswari, P. and Kumar, J.S., 2015. Influence of linear features in nonlinear Electroencephalography (EEG) signals. Procedia computer science, 47, 229–236. doi:10.1016/j.procs.2015.03.202
- Bonnet, M.H. and Arand, D.L., 2001. Impact of activity and arousal upon spectral EEG parameters. Physiology & behavior, 74 (3), 291–298. doi:10.1016/S0031-9384(01)00581-9.
- Bradford, J.C., Lukos, J.R., and Ferris, D.P., 2015. Electrocortical activity distinguishes between uphill and level walking in humans. Journal of neurophysiology, 115 (2), 958–966. doi:10.1152/jn.00089.2015.
- Bratman, G.N., et al., 2015. Nature experience reduces rumination and subgenual prefrontal cortex activation. Proceedings of the National Academy of Sciences, 112 (28), 8567–8572. doi:10.1073/pnas.1510459112.
- Buschman, T.J. and Miller, E.K., 2007. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science, 315 (5820), 1860–1862. doi:10.1126/science.1138071.
- Cassarino, M. and Setti, A., 2016. Complexity as key to designing cognitive-friendly environments for older people. Frontiers in psychology, 7, 1329. doi:10.3389/fpsyg.2016.01329
- Castermans, T., et al., 2014. About the cortical origin of the low-delta and high-gamma rhythms observed in EEG signals during treadmill walking. Neuroscience letters, 561, 166–170. doi:10.1016/j.neulet.2013.12.059
- Chang, C.-Y., et al. 2007. Psychophysiological responses and restorative values of wilderness environments. In: A. Watson, et al., eds. Science and Stewardship to Protect and Sustain Wilderness Values: Eighth World Wilderness Congress Symposium Proceedings RMRS-P-49. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, 479–484, 049. Available from: https://www.fs.usda.gov/treesearch/pubs/all/31072 September 30-October 6, 2005; Anchorage, AK.
- Chang, C.-Y., et al., 2008. Psychophysiological responses and restorative values of natural environments in Taiwan. Landscape and urban planning, 85 (2), 79–84. doi:10.1016/j.landurbplan.2007.09.010.
- Chen, Z., He, Y., and Yu, Y., 2016. Enhanced functional connectivity properties of human brains during in-situ nature experience. PeerJ, 4, e2210. doi:10.7717/peerj.2210
- Choo, A. and May, A., 2014. Virtual mindfulness meditation: virtual reality and electroencephalography for health gamification. 2014 IEEE Games Media Entertainment, 1–3. doi:10.1109/GEM.2014.7048076.
- Christov, M. and Dushanova, J., 2016. Functional correlates of brain aging: beta and gamma frequency band responses to age-related cortical changes. Acta neurobiologiae experimentalis, 76 (2), 98–109. doi:10.21307/ane-2017-009.
- Conedera, M., et al., 2015. Residents’ preferences and use of urban and peri-urban green spaces in a Swiss mountainous region of the Southern Alps. Urban Forestry & Urban Greening, 14 (1), 139–147. doi:10.1016/j.ufug.2015.01.003.
- Conger, A.J., 1974. A revised definition for suppressor variables: a guide to their identification and interpretation. Educational and psychological measurement, 34 (1), 35–46. doi:10.1177/001316447403400105.
- Curl, A., et al., 2016. Developing an audit checklist to assess outdoor falls risk. Proceedings of the Institution of Civil Engineers. Urban Design and Planning, 169 (3), 138. doi:10.1680/udap.14.00056.
- Curl, A., Ward Thompson, C., and Aspinall, P., 2015. The effectiveness of ‘shared space’ residential street interventions on self-reported activity levels and quality of life for older people. Landscape and urban planning, 139, 117–125. doi:10.1016/j.landurbplan.2015.02.019
- Debener, S., et al., 2012. How about taking a low-cost, small, and wireless EEG for a walk? Psychophysiology, 49 (11), 1617–1621. doi:10.1111/j.1469-8986.2012.01471.x.
- Dehzangi, O. and Williams, C., 2015. Towards multi-modal wearable driver monitoring: impact of road condition on driver distraction. In: 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN), 1–6. doi:10.1109/BSN.2015.7299408.
- Duvinage, M., et al., 2013. Performance of the Emotiv Epoc headset for P300-based applications. Biomedical engineering online, 12 (1), 56. doi:10.1186/1475-925X-12-56.
- Eckart, C., et al., 2016. Acetylcholine modulates human working memory and subsequent familiarity based recognition via alpha oscillations. NeuroImage, 137, 61–69. doi:10.1016/j.neuroimage.2016.05.049
- Folstein, M.F., Folstein, S.E., and McHugh, P.R., 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research, 12 (3), 189–198. doi:10.1016/0022-3956(75)90026-6.
- Garver, M.S. and Williams, Z., 2018. Improving the validity of theory testing in logistics research using correlated components regression. International Journal of Logistics Research and Applications, 21 (4), 363–377. doi:10.1080/13675567.2017.1401054.
- Gidlow, C.J., et al., 2016. Where to put your best foot forward: psycho-physiological responses to walking in natural and urban environments. Journal of environmental psychology, 45, 22–29. doi:10.1016/j.jenvp.2015.11.003
- Gola, M., et al., 2012. Beta band oscillations as a correlate of alertness — changes in aging. International Journal of Psychophysiology, 85 (1), 62–67. doi:10.1016/j.ijpsycho.2011.09.001.
- Herzog, T.R., et al., 1997. Relfection and attentional recovery as distinctive benefits of restorative environments. Journal of environmental psychology, 17 (2), 165–170. doi:10.1006/jevp.1997.0051.
- Itti, L., 2006. Quantitative modelling of perceptual salience at human eye position. Visual cognition, 14 (4–8), 959–984. doi:10.1080/13506280500195672.
- Kacha, L., Matsumoto, N., and Mansouri, A., 2015. Electrophysiological evaluation of perceived complexity in streetscapes. Journal of Asian Architecture and Building Engineering, 14 (3), 585–592. doi:10.3130/jaabe.14.585.
- Kaplan, R. and Kaplan, S., 1989. The experience of nature: a psychological perspective. Cambridge, UK: CUP Archive.
- Kaplan, S., 1995. The restorative benefits of nature: toward an integrative framework. Journal of environmental psychology, 15 (3), 169–182. doi:10.1016/0272-4944(95)90001-2.
- Kaplan, S., 2001. Meditation, restoration, and the management of mental fatigue. Environment and behavior, 33 (4), 480–506. doi:10.1177/00139160121973106.
- Kim, G.-W., et al., 2010a. Functional neuroanatomy associated with natural and urban scenic views in the human brain: 3.0T functional MR imaging. Korean Journal of Radiology, 11 (5), 507–513. doi:10.3348/kjr.2010.11.5.507.
- Kim, T.-H., et al., 2010b. Human brain activation in response to visual stimulation with rural and urban scenery pictures: a functional magnetic resonance imaging study. The Science of the total environment, 408 (12), 2600–2607. doi:10.1016/j.scitotenv.2010.02.025.
- Kubitz, K.A. and Pothakos, K., 1997. Does aerobic exercise decrease brain activation? Journal of Sport and Exercise Psychology, 19 (3), 291–301. doi:10.1123/jsep.19.3.291.
- Li, J., et al., 2017. A unified canonical correlation analysis-based framework for removing gradient artifact in concurrent EEG/fMRI recording and motion artifact in walking recording from EEG signal. Medical & biological engineering & computing, 55 (9), 1669–1681. doi:10.1007/s11517-017-1620-3.
- Lv, B., et al., 2017. Effects of stimulus mode and ambient temperature on cerebral responses to local thermal stimulation: an EEG study. International Journal of Psychophysiology, 113, 17–22. doi:10.1016/j.ijpsycho.2017.01.003
- MacKinnon, D.P., Krull, J.L., and Lockwood, C.M., 2000. Equivalence of the mediation, confounding and suppression effect. Prevention Science, 1 (4), 173–181. doi:10.1023/A:1026595011371.
- Magidson, J., 2010. Correlated component regression: a prediction/classification methodology for possibly many features. American Statistical Association Proceedings, 1–19.
- Magidson, J., 2013. Correlated component regression: re-thinking regression in the presence of near collinearity. In: H. Abdi, et al., eds. New perspectives in partial least squares and related methods. New York: Springer, 65–78.
- Martínez-Soto, J., et al., 2013. Exploration of neural correlates of restorative environment exposure through functional magnetic resonance. Intelligent Buildings International, 5 (sup1), 10–28. doi:10.1080/17508975.2013.807765.
- Mavros, P., Austwick, M.Z., and Smith, A.H., 2016. Geo-EEG: towards the use of EEG in the study of urban behaviour. Applied Spatial Analysis and Policy, 9 (2), 191–212. doi:10.1007/s12061-015-9181-z.
- Mavros, P. and Skrompelou, K., 2015. Fieldworker. Available from: github.com/pmavros/Fieldworker
- Menshawy, M.E., Benharref, A., and Serhani, M., 2015. An automatic mobile-health based approach for EEG epileptic seizures detection. Expert systems with applications, 42 (20), 7157–7174. doi:10.1016/j.eswa.2015.04.068.
- Milosevic, M., Milenkovic, A., and Jovanov, E., 2013. mHealth @ UAH: computing infrastructure for mobile health and wellness monitoring. XRDS, 20 (2), 43–49. doi:10.1145/2539269.
- Mulckhuyse, M. and Theeuwes, J., 2010. Unconscious attentional orienting to exogenous cues: A review of the literature. Acta psychologica, 134 (3), 299–309. doi:10.1016/j.actpsy.2010.03.002.
- Neale, C., et al., 2017. The aging urban brain: analyzing outdoor physical activity using the Emotiv Affectiv suite in older people. Journal of Urban Health, 94 (6), 869–880. doi:10.1007/s11524-017-0191-9.
- Nota, P.M., et al., 2017. Experience-dependent modulation of alpha and beta during action observation and motor imagery. BMC neuroscience, 18 (1), 28. doi:10.1186/s12868-017-0349-0.
- Oliveira, A.S., et al., 2016. Proposing metrics for Benchmarking Novel EEG technologies towards real-world measurements. Frontiers in human neuroscience. 10. doi:10.3389/fnhum.2016.00188.
- Osborne, J.W. and Overbay, A., 2004. The power of outliers (and why researchers should always check for them). Practical Assessment, Research & Evaluation, 9 (6), 1–8.
- Ouellette, P., Kaplan, R., and Kaplan, S., 2005. The monastery as a restorative environment. Journal of environmental psychology, 25 (2), 175–188. doi:10.1016/j.jenvp.2005.06.001.
- Park, J.Y., et al., 2013. Effects of color temperature and brightness on electroencephalogram alpha activity in a polychromatic light-emitting diode. Clinical Psychopharmacology and Neuroscience, 11 (3), 126–131. doi:10.9758/cpn.2013.11.3.126.
- Peschardt, K.K. and Stigsdotter, U.K., 2013. Associations between park characteristics and perceived restorativeness of small public urban green spaces. Landscape and urban planning, 112, 26–39. doi:10.1016/j.landurbplan.2012.12.013
- Phelps, E.A. and LeDoux, J.E., 2005. Contributions of the Amygdala to emotion processing: from animal models to human behavior. Neuron, 48 (2), 175–187. doi:10.1016/j.neuron.2005.09.025.
- Rasia-Filho, A.A., Londero, R.G., and Achaval, M., 2000. Functional activities of the amygdala: an overview. Journal of Psychiatry and Neuroscience, 25 (1), 14–23.
- Roe, J.J., et al., 2013a. Engaging the brain: the impact of natural versus urban scenes using novel EEG methods in an experimental setting. Environmental Sciences, 1, 93–104. doi:10.12988/es.2013.3109
- Roe, J.J., et al., 2013b. Green space and stress: evidence from cortisol measures in deprived urban communities. International journal of environmental research and public health, 10 (9), 4086–4103. doi:10.3390/ijerph10094086.
- Rosero‐Vlasova, O.A., et al., 2019. Soil organic matter and texture estimation from visible–near infrared–shortwave infrared spectra in areas of land cover changes using correlated component regression. Land Degradation & Development, 30 (5), 544–560. doi:10.1002/ldr.3250.
- Rutherford, T., 2012. Population ageing: statistics. Available from: House of Commons, UK website: https://researchbriefings.parliament.uk/ResearchBriefing/Summary/SN03228
- Sauseng, P., et al., 2005. A shift of visual spatial attention is selectively associated with human EEG alpha activity. European Journal of Neuroscience, 22 (11), 2917–2926. doi:10.1111/j.1460-9568.2005.04482.x.
- Schneider, S., et al., 2009. Changes in brain cortical activity measured by EEG are related to individual exercise preferences. Physiology & behavior, 98 (4), 447–452. doi:10.1016/j.physbeh.2009.07.010.
- Smit, A.S., et al., 2005. Mental and physical effort affect vigilance differently. International Journal of Psychophysiology, 57 (3), 211–217. doi:10.1016/j.ijpsycho.2005.02.001.
- Sperry, R.W., 1968. Hemisphere deconnection and unity in conscious awareness. American Psychologist, 23 (10), 723–733. doi:10.1037/h0026839.
- Takayama, N., et al., 2014. Emotional, restorative and vitalizing effects of forest and urban environments at four sites in Japan. International journal of environmental research and public health, 11 (7), 7207–7230. doi:10.3390/ijerph110707207.
- Tulving, E. and Markowitsch, H.J., 1998. Episodic and declarative memory: role of the hippocampus. Hippocampus, 8 (3), 198–204. doi:10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G.
- Ulrich, R.S., 1981. Natural versus urban scenes: some psychophysiological effects. Environment and behavior, 13 (5), 523–556. doi:10.1177/0013916581135001.
- United Nations, 2018. World urbanization prospects: the 2018 revision. Available from: http://www.un.org/esa/population/publications/wup2007/2007wup.htm
- Vargha-Khadem, F., et al., 1997. Differential effects of early hippocampal pathology on episodic and semantic memory. Science, 277 (5324), 376–380. doi:10.1126/science.277.5324.376.
- Velarde, M.D., Fry, G., and Tveit, M., 2007. Health effects of viewing landscapes – landscape types in environmental psychology. Urban Forestry & Urban Greening, 6 (4), 199–212. doi:10.1016/j.ufug.2007.07.001.
- Vysata, O., et al., 2014. Age-related changes in EEG coherence. Neurologia i neurochirurgia polska, 48 (1), 35–38. doi:10.1016/j.pjnns.2013.09.001.
- Ward Thompson, C., et al., 2016. Mitigating stress and supporting health in deprived urban communities: the importance of green space and the social environment. International journal of environmental research and public health, 13 (4), 440. doi:10.3390/ijerph13040440.
- Wrobel, A., 2000. Beta activity: a carrier for visual attention. Acta neurobiologiae experimentalis, 60 (2), 247–260.
- Yang, F., Bao, Z.Y., and Zhu, Z.J., 2011. An assessment of psychological noise reduction by landscape plants. International journal of environmental research and public health, 8 (4), 1032–1048. doi:10.3390/ijerph8041032.