Abstract
The genus of direct-developing frogs Diasporus is currently composed of 11 species ranging from Honduras to Ecuador. Body size variability, advertisement call divergence, and polychromatism have all been reported at the interpopulation level, suggesting that the existence of some species may be masked within the current nomenclature of this group. This pattern highlights the need for integrative approaches to resolve the taxonomy of the genus and provide a robust delimitation of the species it contains. Here, we provide novel data on morphology, genetics, habitat use, and bioacoustics for D. tigrillo, the least known species in the genus. Then we use an integrative approach to assess the divergence between this species and D. citrinobapheus a recently described species. D. tigrillo was indicated as the most similar species to D. citrinobapheus by its authors; however, given the lack of available information for D. tigrillo this conclusion was based only on morphological data obtained from a few deteriorated specimens collected and preserved five decades ago. With our morphological redescription, molecular inference, ecological observations, and acoustic analysis, we found important differences between these two taxonomic entities. Our data, in addition to improving the knowledge of D. tigrillo, therefore provides robust evidence to support the validity of both species.
Molecular sequence data. GenBank Accession numbers are detailed in the text.
Phylogenetic data. Information related can be reviewed at this URL: http://purl.org/phylo/treebase/phylows/study/TB2:S18102
Introduction
On March 1964, the herpetologists Jay M. Savage and Norman J Scott Jr started an expedition to climb Cerro Utyum in southeastern Costa Rica.[Citation1] Their intention was to repeat the route originally traced by the geologist and pioneer collector William M. Gabb [Citation2] during his explorations of the Talamancan mountain range (Cordillera de Talamanca) from 1873 to 1874.[Citation3] Gabb’s collections provided the type specimens for the description of 15 new species of Costa Rican herpetofauna,[Citation4] which spurred Savage’s interest in replicating, rediscovering, and identifying the species at their respective type localities.[Citation1]
Their attempt to reach the high Talamanca peaks visited by Gabb was abruptly interrupted due to health issues during the expedition (Jay Savage, Personal Communication). Nevertheless, during their stay, they collected specimens that were later described as new taxa. Shortly after it was published the description of Duellmanohyla lithrodes, and elusive tree frog collected at the confluence of the Lari and Pare Rivers, about 21 km SW from Amubri, 440 m a.s.l.[Citation5]. From that same area, they collected other novelty, Diasporus tigrillo Savage 1997, which was only described over 33 years later because Savage suspected it may be synonymous with other species from South America.[Citation6] To date, with only the two specimens they collected at its type locality – Surayo peak, approximately 16 km SW from Amubri – this is the least known species in the genus.
The genus Diasporus is currently composed of 11 species distributed from eastern Honduras through Nicaragua, Costa Rica, Panama, and Colombia to northeastern Ecuador.[Citation7–11] However, body size variability, advertisement call divergence, and polychromatism within species of the genus suggest that species with high degree of morphological similarity may be masked under some nominal species.[Citation12] In order to address the issue of a potential underestimation of the real diversity of the genus, an integrative approach compiling different lines of evidence seems strictly necessary.[Citation13] A deep understanding of phylogenetic relationships and a detailed morphological and acoustic comparison between species are crucial in order to disentangle taxonomy, shed light on species boundaries, and quantify the real diversity contained in a taxon.[Citation14,15] Recently, Hertz et al. [Citation10] described Diasporus citrinobapheus, a new species of the genus from Cordillera Central in Western Panama, and according to them, a close relative of D. tigrillo. Considering the importance of advertisement calls as a mechanism of premating isolation and its consequent utility as taxonomic character,[Citation16,17] these authors also provided a preliminary acoustic analysis for some species of the genus. While in their description they highlighted D. tigrillo as the most similar species to D. citrinobapheus based on morphological characters, they were unable to include D. tigrillo in any phylogenetic or acoustic analyses, since the original description only provided an onomatopoeic description of its call, and neither tissues nor sequences were available. Padial et al. [Citation11] reinforces this dubious situation by qualifying as problematic the taxonomic status of D. citrinobapheus since no morphological data reported in the original description allow it to be distinguished from D. tigrillo which additionally lacks sequence data.
No scientific expedition has been conducted in the remote area sampled by Gabb since Savage and Scott 51 years ago. We recently endeavored to complete Gabb’s route 141 years later and, as part of our findings, we provide novel data on the poorly known D. tigrillo obtained in the vicinity of its remote type locality. In order to clarify the taxonomic validity of this species and D. citrinobapheus, we describe the advertisement call of D. tigrillo, its phylogenetic relationships with other representatives of the genus, and a detailed acoustic comparison with D. citrinobapheus. Furthermore, we present additional data on habitat use, distribution, and the first color photographs of the live specimen D. tigrillo. This information is especially relevant in the case of these poorly known species, which, at the same time, belongs to a genus with a complex and still unresolved taxonomic situation.[Citation18]
Methods
Species criterion and delimitation
Our view of species follows the general metapopulation lineage species concept.[Citation19] Because we adhere to this concept, we recognize a species when there is evidence of the separation of metapopulation lineages, preferably based on multiple lines of evidence following a consensus protocol for integrative taxonomy.[Citation20] We also emphasize that species should be diagnosable in taxonomic practice. This delimitation follows Nadler & Pérez-Ponce de León,[Citation21] who advocated that the delimitation of species with a high degree of morphological similarity must be based on hypothesis testing of species identity using a molecular approach. If the null hypothesis of a single species is rejected based on molecular data, then additional data and analyses are needed. We tested the validity of the species based on reciprocal monophyly and genetic distances using a fragment of mitochondrial 16S rRNA gene as well as evidence of divergence recovered from acoustic and morphological analyses.
Fieldwork
We conducted a herpetological expedition to the Caribbean foothills of Talamanca mountain range in March 2015. On 20 March, while returning to the small village of Amubri from Alto Lari, we heard a series of vocalizations while walking on a trail on the west bank of the Lari River (+9.45848, −83.03414, 273 m, 2 km NW of the type locality of D. tigrillo) at around 17:00 h (Figure ). Calls were single whistle-like notes, similar to those produced by some representatives of the genus Diasporus and were concentrated in a patch of old secondary growth dominated by ferns. After an intensive visual search, we collected a bright yellow male with small black spots, which fitted the original description of D. tigrillo.[Citation6] This identification was confirmed later by Savage based on our photographs (Figure (A) and (B)). We recorded two males of D. tigrillo using a shotgun microphone (Sennheiser ME66) and a solid-state digital recorder (Marantz PMD661; sampling rate: 44.1 kHz; accuracy: 24bit; file format: WAV) from an approximate distance of 1 m. In total, five males were collected, euthanized, fixed in 10% formalin, and stored in 70% alcohol. Tissue samples were stored in RNAlater. Vouchers were deposited in the herpetological collection of the Museo de Zoología, Universidad de Costa Rica (UCR).
Figure 1. Map showing the distribution of Diasporus tigrillo, D. citrinobapheus and D. aff. citrinobapheus in Costa Rica and Panama, as referred by Hertz et al. [Citation10].
![Figure 1. Map showing the distribution of Diasporus tigrillo, D. citrinobapheus and D. aff. citrinobapheus in Costa Rica and Panama, as referred by Hertz et al. [Citation10].](/cms/asset/fca8c02d-435e-41a0-b5b6-40522facc1f9/tneo_a_1168076_f0001_oc.gif)
Amplification and sequencing
We extracted and sequenced a fragment of mitochondrial 16S rRNA gene from the five specimens of D. tigrillo and two specimens classified as Diasporus vocator Taylor 1955. Total genomic DNA was extracted from RNAlater- or ethanol-preserved tissues (liver or muscle) with the Animal Genomic DNA Kit (BioBasic Canada Inc.). We used polymerase chain reaction (PCR) to amplify the mitochondrial 16S rRNA gene (16S), using the primers 16Sbr and 16Sar.[Citation22] PCR amplifications were performed in a total volume of 25 uL, containing 1 uL DNA template, 1 U Taq polymerase (Amplificasa®, Biotecnologias Moleculares), 1x PCR buffer with 1.5 mm MgCl2, 0.4 mM deoxynucleotide triphosphates (dNTPs), and 0.5 uM forward and reverse primers. PCR conditions consisted of an initial cycle of 94 °C (5 min), followed by 35 cycles with denaturalization at 94 °C (45 s), annealing at 50 or 55 °C (30 or 45 s), followed by an extension at 72 °C (45 or 120 s), and a final cycle at 72 °C (5 min). All PCR reactions contained a negative control. PCR products were quantified on either 1% TAE or TBE agarose gel and purified using shrimp alkaline phosphate and exonuclease I (ExoSap-IT, USB Corporation) to remove unincorporated dNTPs and primers. The fragments were sequenced on both directions, using the original amplification primers and BigDye termination reaction chemistry (Applied Biosystems). The sequencing reactions were performed in a total volume of 10uL, containing 2uL of purified PCR product, 0.25 uM primer, 0.5 uL BigDye Terminator v3.1 Ready Reaction Mix, and 5X Sequencing buffer. The cycle-sequencing products were column-purified with Sephadex G-50 (GE Healthcare) and run on an ABI PRISM 3100 DNA Analyzer (Applied Biosystems). Contiguous sequences for each individual were constructed using SEQUENCHER 5.3 (Genes Codes Corp.). The sequences are available under GenBank Accession numbers KT438502-KT438508.
Phylogenetic analyses
We compared the 7 sequences obtained here with 21 sequences available on GenBank for Diasporus quidditus Lynch 2001, D. vocator, D. citrinobapheus, and clades treated in the literature as affinities to Diasporus hylaeformis Cope 1875, Diasporus diastema Cope 1875 and D. citrinobapheus. Detailed molecular laboratory techniques and GenBank Accession numbers for these 21 sequences are provided in Hertz et al. [Citation10] We used an additional 16S sequence of Eleutherodactylus coqui Thomas 1966 as an outgroup following the relationships within Brachycephaloidea [Citation11] (GenBank Accession Number: EF107219). An alignment was performed in MUSCLE 3.7 [Citation23] and checked by eye. We used MrModelTest2.3 software [Citation24] and Akaike Information Criterion (AIC) scores to select an appropriate model of DNA sequence evolution; the GTR + G model was chosen. Individual gene trees were constructed using both maximum likelihood (ML) and Bayesian analyses (BA). ML analyses were performed in RAxML 8.1.11 [Citation25] and run on the CIPRES portal [Citation26]; one thousand bootstrap replicates were conducted to evaluate nodal support. Bayesian phylogenetic analysis was performed in MrBayes 3.2.2,[Citation27] and consisted of 10 MCMC chains sampled every 5000 generations for 50 million generations. We examined a plot of likelihood scores of the heated chain and checked the stationarity of such chains using the software Tracer 1.6.[Citation28] We discarded 25% of the trees as burn-in; the consensus tree and posterior probabilities for each node were estimated using the remaining trees. Estimates of evolutionary divergence over sequence pairs between and within groups were computed using MEGA6 [Citation29] and the Kimura two-parameter model,[Citation30] selected by the software as the best model of evolution among the models available based on our data. The rate of variation among sites was modeled with a gamma distribution, shape parameter = 4.
Acoustic analyses
We conducted a spectro-temporal analysis for a total of 58 D. tigrillo calls (28 from male 1 and 30 from male 2), using the software Raven 1.4 (Hann window; 256 kHz sampling and 50% overlap). For each call, we measured the following parameters: (1) Call duration, (2) Call rate, (3) Time between calls, (4) Lowest Frequency, (5) Highest Frequency, (5) Dominant Frequency, and (6) Average Entropy. We also analyzed call modulation by measuring changes in the dominant frequency within calls every 0.01 s and quantifying changes in peak frequency over the duration of the call.
In order to compare with D. citrinobapheus calls, we repeated all the previous analyses for 102 calls corresponding to 2 males of the type series recorded in Paredón, Panama and 2 males recorded at Cerro Willie Mazú, located halfway between the D. tigrillo and D. citrinobapheus type localities (Figure ). We generated all spectrograms, oscillograms, and power spectra presented here using the R [Citation31] package SEEWAVE.[Citation32] We used multivariate analysis of variance (MANOVA) on means of the two species for all call variables in order to identify the acoustic measures that differ between them.
Morphometry
We recorded the following morphological measurements as described by Savage [Citation12] and Duellman and Lehr [Citation33]: snout-vent length (SVL), head length (HL), head width (HW), eye diameter (ED), inter orbital distance (IOD), tympanum diameter (TY), width of upper eyelid (EW), tibia length (TL), eye–nostril distance (E–N), toe lengths (T1, T2, T3, T4, and T5), and fingers lengths (F1, F2, F3, and F4). Measurements were made using dial calipers, rounded to nearest 0.1 mm. All variables are shown in proportion to SVL. Additionally, the following proportions were calculated: EW/IOD, TY/ED, E-N/ED, ED/HL, IOD/HW, and T4/TL.
Results
Species delimitation
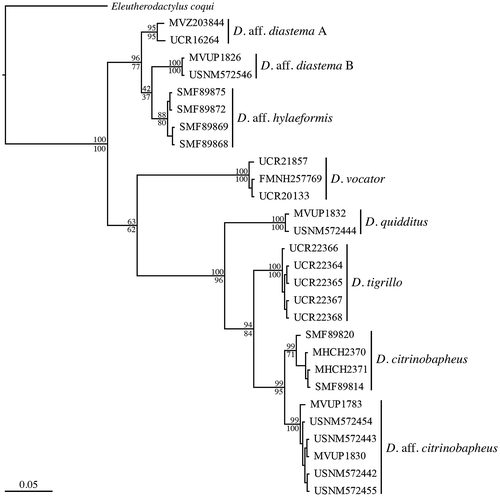
Our molecular analyses support the validity of D. citrinobapheus and D. tigrillo as two distinct species based on reciprocal monophyly and genetic distances of 4.4% in the 16S mitochondrial gene. Moreover, we detected a clear divergence between the structure of the advertisement call of these species both in the spectral and temporal dimensions. In addition, we found several morphological and ecological differences between these species.
Phylogenetic analyses
The resulting data matrix had a total length of 559 bp including gaps. Phylogenies generated by ML and BA were concordant in supporting the same clades within the genus Diasporus (Figure ). The five individuals classified as to D. tigrillo form a clade, supporting its status as a valid species. There was no genetic divergence between the five specimens of D. tigrillo. The clade D. tigrillo is sister to the clade D. citrinobapheus + the clade referred by Hertz et al. [Citation10] as D. aff. diastema orange. Here, we call this clade D. aff. citrinobapheus, considering its close relationship to this nominal species (mean genetic distance of 4.3%). The average genetic distances of D. tigrillo to D. vocator, D. citrinobapheus, and D. aff. citrinobapheus were 12.0, 4.4, and 4.3%, respectively.
Advertisement call
The advertisement call of D. tigrillo consists of a single note (Figure ) characterized by a mean duration of 0.131 ± 0.02 s and a call rate of 39 ± 7 calls min−1. These vocalizations have a mean peak frequency of 3314 Hz and range from 2911 to 3624 Hz. Mean values and standard deviations are shown in Table . In contrast to other representatives of the genus that produce pure tones – ‘dinks’ – [Citation8,12], the call of D. tigrillo has a modulated structure. The frequency increases progressively in each vocalization. This is perceived as a whistle rather than a ‘dink’ to the human ear. Compared to D. citrinobapheus, D. tigrillo produces calls of higher pitch and shorter duration. We found significant differences between these two species in call duration, lowest frequency, highest frequency, and peak frequency but not in average entropy (Table ).
Figure 4. Spectrogram and oscillogram of the advertisement call of (A) Diasporus citrinobapheus and (B) D. tigrillo.
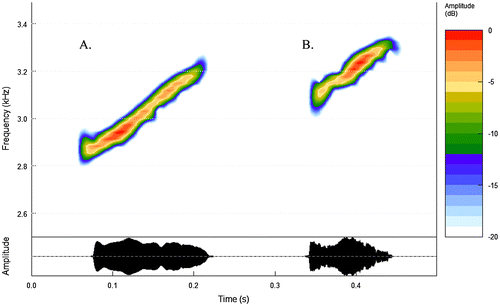
Table 1. Mean values of the spectro-temporal characteristics in the advertisement calls of D. tigrillo (n = 58 calls) and D. citrinobapheus (n = 102 calls).
Morphology
We present here an extension of the morphological description of D. tigrillo after Savage’s original paper.[Citation6] It is tiny species with the following characteristics: (1) low pustules on dorsum; venter shagreen, flanks are weakly areolate; sometimes with two postrictal tubercles; without lateral, occipital or discoidal folds; (2) tympanum distinct, round, with membrane undifferentiated, annulus partially evident through skin, (TY/ED = 27.78–37.50%); (3) head as long as it is wide; snout subovoid in dorsal view, rounded in profile; loreal region concave; canthus-rostralis indistinct; (4) eyes moderate, (ED/HL = 30.00–36.36%); eyelid smooth with low similar superciliar tubercles, (EW/IOD = 36.36–55.56%); cranial crests absent (5) vomerine teeth transverse; (6) single external subgular vocal sac and vocal slit in adult males; sometimes, males only with a vocal slit; (7) fingers lacking lateral fringes; thenar tubercle elongated and flattened; palmar tubercle rounded and flattened; palmar much smaller than thenar; supernumerary and accessory palmar tubercles absent; subarticular tubercles ovoid and flattened; (8) finger I shorter than finger II; with disk, symmetric, expanded in all digits; tips usually rounded; pads broadened; fingers without webbing (9) ulnar fold absent; (10) toes lacking lateral fringes; inner metatarsal tubercle elongate and flattened; outer rounded, flattened, much smaller than inner; supernumerary and plantar tubercles absent; subarticular tubercles ovoid, flattened and globular; (11) toe V larger than toe III, the tip of toe V reaching the distal subarticular tubercle on toe IV; disks expanded, symmetric; disk cover usually rounded, sometimes pointed on toes IV and V; disk pad broadened; toes without webbing; (12) heel smooth; (13) upper surfaces light yellow with dark dots or blotches formed by aggregation of dots; venter white, granules on venter with base grayish; throat cream; upper surfaces of thighs pinkish orange with dark dots; lower surface of thigh pinkish orange uniform; (14) SVL in males 17.20–19.30 mm. Morphometric measures are shown in Table .
Table 2. Morphometric measures to the new series of Diasporus tigrillo (n = 5).
Habitat and distribution
The individuals here analyzed were recorded and collected at the banks of the Lari River, 2 km NE of Surayo peak, the type locality. Although small in scale, this represents an extension of the distribution range documented for this poorly known species, as well as an increase in its altitudinal distribution (from 300 to 460 masl).
We observed this species calling only in secondary vegetation, composed of shrubs, wildcane, ferns, and many thorny plants (Figure (C) and (D)). The presence of this kind of vegetation in the area is the result of alterations caused by the dynamics of the river and of small streams in the riparian forest (Figure (C)). Human activities in the area (mainly by aboriginal people) may increase the suitable habitat for D. tigrillo. For example, Surayo peak, an ancient aboriginal sanctuary, is currently abandoned, but secondary vegetation is still evident, probably due to recent use for traditional agriculture, allowing the presence of the species as far as 1 km from the river (personal observation). It is possible that the species distribution runs along the course of the Lari River, from the Pare River to its confluence with the Telire River; however, future surveys are necessary in order to confirm the presence of D. tigrillo there. In previous surveys of other areas downstream, like Bribri and Gandoca, where the Lari River becomes the Sixaola River, this species has never been detected (G.C. unpublished data).
The behavior of D. tigrillo during recording and collection suggests that males call from perches 40–100 cm high approximately. When the males were disturbed, they escaped by jumping from one stalk to another lower stalk or directly to the ground. However, they showed high levels of tolerance to perturbation and only fled when we constantly moved the vegetation near their perch. Few minutes after fleeing, males usually came back to other nearby perches to continue calling. Calling activity of D. tigrillo is similar to other species of Diasporus, beginning late in the evening and continuing throughout the night.
Discussion
The integration of tools like molecular phylogenies [Citation34] and bioacoustics [Citation15,35] with traditional morphologic approaches has demonstrated to be useful for taxonomic purposes. This is especially helpful when dealing with groups where morphological evolution is conservative.[Citation13] This phenomenon seems to be widespread in many anurans. In the Neotropical direct-developing frogs (Brachycephaloidea: Günther, 1858), for example, marked intraspecific genetic divergence has been documented in groups with little (or poorly understood) morphological variation, such as the genera Craugastor,[Citation36] Pristimantis,[Citation37] Eleutherodactylus,[Citation38], and Ischnocnema,[Citation39] leading in some cases to the reconsideration of the taxonomic status of species previously and exclusively described based on morphological features.
Frogs in the genus Diasporus, another representative of direct-developing frogs, face this same issue. In this case, subtle differences in morphology as well as polychromatisms and call variation have been pointed out by some authors as evidence for the potential existence of a higher cryptic diversity in the genus.[Citation12,17,40] With novel data collected at the remote locality of the poorly known D. tigrillo, we complemented its original description [Citation6] and adopted a multisource approach to specifically clarify its relationship to the recently described D. citrinobapheus [Citation10] based on other potentially informative characters.
Our data provide multiple lines of evidence supporting the validity of both species. First, the structure of the advertisement call presented here for D. tigrillo reflects acoustic differences between this species and D. citrinobapheus. Although both are modulated calls showing a progressive increase in frequency, the calls produced by D. citrinobapheus are longer in duration and have lower peak frequencies than those produced by D. tigrillo. If, as in many anurans, body size is negatively correlated with dominant call frequency,[Citation41] then the higher frequencies produced by D. tigrillo could be the consequence of its slightly shorter average SVL compared to D. citrinobapheus. Given the central role that advertisement calls play in frog reproductive biology, these acoustic signals have species-specific features that facilitate recognition.[Citation41] Therefore, variation in these features is considered useful to help defining taxonomic boundaries between morphologically similar sister species.[Citation37,42,43]
Second, the BA and ML phylogenetic inferences are concordant in grouping our five individuals of D. tigrillo in a monophyletic group, separated from the D. citrinobapheus by a genetic distance of 4.4%. The 16S gene fragment used here has been suggested as a DNA barcode marker for amphibian diversity inventories [Citation44] to complement the standard COI-5’ marker used for animals in general.[Citation45] Although there is no consensus on the genetic distances that represent different species, Kim et al. [Citation46] showed that a divergence greater than 1.35% in the 16S gene represents different species in prokaryotes. Fouquet et al. [Citation47], based on 16S mtDNA sequences, suggested a pairwise genetic distance of 3% for flagging potential cryptic species in Neotropical frogs. In addition, based on the fact that Mendelson et al. [Citation48] showed that the 16S gene presents intermediate substitution rates among the six mitochondrial loci they studied, we consider that 4.5% of genetic divergence suggests different species.
Third, the specimens here showed are extensively coincident with the description provided by Savage [Citation6,12]. Nevertheless, our specimens have the tips of fingers and toes rounded, sometimes pointed on toes IV and V, instead of spadate, as reported by Savage [Citation6]. Moreover, our specimens have the pads broadened, while Savage [Citation6] reported them as triangular. Savage [Citation6] indicated that subarticular tubercles on fingers and toe I are weakly bifid; however, none of the specimens we show here presented this characteristic. We consider these subtle differences might be the result of working with fresh material – as we did – in contrast to Savage’s measures based on specimens preserved 30 years before the description. The yellow dorsal coloration of D. tigrillo and D. citrinobapheus is very unusual in Costa Rican frogs from this genus; cream color with brown spots is more common in Costa Rican species.[Citation12]
The use of low elevation stalks as calling perches is also rare in other species of the genus present in Costa Rica. Diasporus hylaeformis and D. diastema (sensu lato) normally call from curled leaves or from small spaces between two or more leaves above 1.5 m (D. hylaeformis mean height perch = 1. 96 ± 0.54 m, n = 25 and D. diastema mean height perch = 1. 70 ± 0.44 m, n = 21 A.G-R, unpublished data). D. citrinobapheus and D. quidditus, both species very close to D. tigrillo, are reported to call from leaves at perches 1 to 3 m high [Citation10] and from epiphytes on trees, 2–4 m above the ground,[Citation49] respectively.
Many Costa Rican species have been described on the basis of just one or a few individuals. That is the case of D. tigrillo and several species described from the invaluable collection of herpetofauna that William Gabb gathered from remote areas of Talamanca. The description of new closely related species is particularly difficult due to the lack of complete information to facilitate robust comparisons. We consider it a necessary to conduct efforts to collect more data from the topotypes of this kind of species in order to improve the taxonomy and species delimitation of Costa Rican herpetofauna. This is extremely relevant considering the high levels of endemism documented for this country and the high degree of morphological similarity among several amphibian and reptile species.
Author contributions
AGR, EA, and GC conceived and designed the study. AGR wrote the first draft of the manuscript. EA and GC reviewed and improved the manuscript. AGR and EA analyzed the data. AGR, EA, and GC collected specimens and recorded calls.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
We are grateful to Justino Layan Gabb, Xavier Baltodano and Carlos Godínez, for their valuable assistance in the field. Andreas Hertz generously provided the recordings of D. citrinobapheus analyzed here. Adriana Fernández kindly reviewed the style of the early drafts of the manuscript. We thank Marcelo Gehara, Gabriel Costa and two anonymous reviewers for providing valuable comments that improved the content of this article and Jorge González, Edwin Cyrus and the Costa Rican Ministry of Environment and Energy (MINAE) for providing the corresponding scientific collecting permits for this expedition (SINAC-ACLAC-PIME-R-005-2015). The School of Biology at Universidad de Costa Rica provided supportive help in terms of logistics.
Editor: Juan M. Guayasamin.
Additional information
Funding
References
- Savage JM. On the trail of the golden frog : with Warszewicz and Gabb in Central America. Proc. Calif. Acad. Sci. 1970;38:273–287.
- Dall WH. Biographical memoir of William More Gabb 1839–1878. Biograph. Mem. Natl. Acad. Sci. USA. 1909;6:345–361.
- Chacón PD. Bonilla GJS. Contribución pionera de William M. Gabb a la geología y cartografía de Costa Rica. Anu. Estud. Centroam. 1999;25:103–138.
- Cope ED. On the Batrachia and Reptilia of Costa Rica. J. Acad. Nat. Sci. Philadelphia. 1875;8:93–154.
- Savage JM. A new red-eyed tree-frog (Family Hylidae) from Costa Rica, with a review of the Hyla uranochroa group. Bull. South Calif. Acad. Sci. 1968;67:1–20.
- Savage JM. A new species of rainfrog of the Eleutherodactylus diastema group from the Alta Talamanca Region of Costa Rica. Amphibia-Reptilia. 1997;18:241–247.10.1163/156853897X00125
- Hedges SB, Duellman WE, Heinicke MP. New world direct-developing frogs (Anura: Terrarana): molecular phylogeny, classification, biogeography, and conservation. Zootaxa. 2008;1737:1–182.
- Chaves G, García-Rodríguez A, Mora A, et al. A new species of dink frog (Anura: Eleutherodactylidae: Diasporus) from Cordillera de Talamanca, Costa Rica. Zootaxa. 2009;2088:1–14.
- Batista A, Ponce M, Hertz A. A new species of rainfrog of the genus Diasporus (Anura: Eleutherodactylidae) from Serranía de Tabasará, Panama. Zootaxa. 2012;3410:51–60.
- Hertz A, Hauenschild F, Lotzkat S, et al. A new golden frog species of the genus Diasporus (Amphibia, Eleutherodactylidae) from the Cordillera Central, western Panama. ZooKeys. 2012;196:23–46.10.3897/zookeys.196.2774
- Padial JM, Grant T, Frost DR. Molecular systematics of terraranas (Anura: Brachycephaloidea) with an assessment of the effects of alignment and optimality criteria. Zootaxa. 2014;3825:1–132.10.11646/zootaxa.3825.1
- Savage JM. The amphibians and reptiles of Costa Rica: a herpetofauna between two continents, between two seas. Chicago: The University of Chicago Press; 2002.
- Bickford D, Lohman DJ, Sodhi NS, et al. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007;22:148–155.10.1016/j.tree.2006.11.004
- Vieites DR, Wollenberg KC, Andreone F, et al. Vast underestimation of Madagascar’s biodiversity evidenced by an integrative amphibian inventory. Proc. Natl. Acad. Sci. USA. 2009;106:8267–8272.10.1073/pnas.0810821106
- Glaw F, Köhler J, De la Riva I, et al. Integrative taxonomy of Malagasy treefrogs: combination of molecular genetics, bioacoustics and comparative morphology reveals twelve additional species of Boophis. Zootaxa. 2010;2383:1–82.
- Narins PM, Smith SL. Clinal variation in anuran advertisement calls: basis for acoustic isolation? Behav. Ecol. Sociobiol. 1986;19:135–141.10.1007/BF00299948
- Padial JM, Köhler J, Muñoz A, et al. Assessing the taxonomic status of tropical frogs through bioacoustics: geographical variation in the advertisement calls in the Eleutherodactylus discoidalis species group (Anura). Zool. J. Linn. Soc. 2008;152:353–365.10.1111/zoj.2008.152.issue-2
- Ibáñez R, Rand AS, Jaramillo CA. Los anfibios del Monumento Natural Barro Colorado, Parque Nacional Soberanía y áreas adyacentes [The amphibians of Barro Colorado Nature Monument, Soberania National Park and adjacent areas]. Panama City: Editorial Mizrachi & Pujol; 1999.
- De Queiroz K. The general lineage concept of species, species criteria, and the process of speciation. A conceptual unification and terminological recommendations. In: Howard DJ, Berlocher SH, editors. Endless forms: species and speciation. Oxford: Oxford University Press; 1998. p. 57–75.
- Padial JM, Miralles A, De la Riva I, et al. The integrative future of taxonomy. Front. Zool. 2010;7:16.10.1186/1742-9994-7-16
- Nadler SA, Pérez-Ponce de León G. Integrating molecular and morphological approaches for characterizing parasite cryptic species: implications for parasitology. Parasitology. 2011;138:1688–1709.10.1017/S003118201000168X
- Palumbi S, Martin A, Romano S, et al. The simple fool’s guide to PCR, version 2.0. Hawaii: Special Publication, Department of Zoology and Kewalo Marine Laboratory, University of Hawaii; 1991.
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004;32:1792–1797.10.1093/nar/gkh340
- Nylander JAA. MrModeltest v2. Uppsala: Program distributed by the author. Evolutionary Biology Center, Uppsala University; 2004.
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313.10.1093/bioinformatics/btu033
- Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Paper presented at: Proceedings of the Gateway Computing Enviroments Workshop (GCE); 2010 November 14; Louisiana, USA. p. 1–8.
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755.10.1093/bioinformatics/17.8.754
- Rambaut A, Suchard MA, Xie W, et al. Tracer v1.6.1. 2014. Available from: http://beast.bio.ed.ac.uk/Tracer.
- Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729.10.1093/molbev/mst197
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120.10.1007/BF01731581
- R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available from: http://www.R-project.org.
- Sueur J, Aubin T, Simonis C. Equipment review. Bioacoustics. 2008;18:213–226.10.1080/09524622.2008.9753600
- Duellman WE, Lehr E. Terrestrial-breeding frogs (Strabomantidae) in Peru. Münster (Germany): Natur- und Tier-Verlag; 2009.
- Fouquet A, Vences M, Salducci MD, et al. Revealing cryptic diversity using molecular phylogenetics and phylogeography in frogs of the Scinax ruber and Rhinella margaritifera species groups. Mol. Phylogenet. Evol. 2007;43:567–582.10.1016/j.ympev.2006.12.006
- Vences M, Andreone F, Glos J, et al. Molecular and bioacoustic differentiation of Boophis occidentalis with description of a new treefrog from north-western Madagascar. Zootaxa. 2010;2544:54–68.
- Crawford AJ, Smith EN. Cenozoic biogeography and evolution in direct-developing frogs of Central America (Leptodactylidae : Eleutherodactylus) as inferred from a phylogenetic analysis of nuclear and mitochondrial genes. Mol. Phylogenet. Evol. 2005;35:536–555.10.1016/j.ympev.2005.03.006
- Padial JM, De La Riva I. Integrative taxonomy reveals cryptic Amazonian species of Pristimantis (Anura: Strabomantidae). Zool. J. Linn. Soc. 2009;155:97–122.10.1111/zoj.2008.155.issue-1
- Rodríguez A, Vences M, Nevado B, et al. Biogeographic origin and radiation of Cuban Eleutherodactylus frogs of the auriculatus species group, inferred from mitochondrial and nuclear gene sequences. Mol. Phylogenet. Evol. 2010;54:179–186.10.1016/j.ympev.2009.08.023
- Gehara M, Canedo C, Haddad CFB, et al. From widespread to microendemic: molecular and acoustic analyses show that Ischnocnema guentheri (Amphibia: Brachycephalidae) is endemic to Rio de Janeiro, Brazil. Conserv. Genet. 2013;14:973–982.10.1007/s10592-013-0488-5
- Lynch JD, Duellman WE. Frogs of the genus Eleutherodactylus (Leptodactylidae) in western Ecuador: systematics, ecology, and biogeography. Spec. Publ. Nat. Hist. Mus. Univ. Kansas. 1997;23:1–236.
- Wells KD. The ecology and behavior of amphibians. Chicago: The University of Chicago Press; 2008.
- Vences M, Wake DB. Species, species boundaries and phylogeography of amphibians. In: Heatwole H, Tyler M, editors. Amphibian biology. Vol. 7, Systematics. Chipping Norton (Australia): Surrey Beatty & Sons; 2007. p. 2613–2671.
- Goicoechea N, De La Riva I, Padial JM. Recovering phylogenetic signal from frog mating calls. Zool. Scr. 2010;39:141–154.10.1111/zsc.2010.39.issue-2
- Vences M, Thomas M, Bonett RM, et al. Deciphering amphibian diversity through DNA barcoding: chances and challenges. Philos. Trans. Roy. Soc. B. 2005;360:1859–1868.10.1098/rstb.2005.1717
- Smith MA, Poyarkov NA Jr, Hebert PDN. DNA BARCODING: CO1 DNA barcoding amphibians: take the chance, meet the challenge. Mol. Ecol. Resour. 2008;8:235–246.10.1111/j.1471-8286.2007.01964.x
- Kim M, Oh H-S, Park S-C, et al. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Micr. 2014;64:346–351.10.1099/ijs.0.059774-0
- Fouquet A, Gilles A, Vences M, et al. Underestimation of species richness in neotropical frogs revealed by mtDNA analyses. PLoS ONE. 2007;2:e1109.10.1371/journal.pone.0001109
- Mendelson JR III, Mulcahy DG, Williams TS, et al. A phylogeny and evolutionary natural history of mesoamerican toads (Anura: Bufonidae: Incilius) based on morphology, life history, and molecular data. Zootaxa. 2011;3138:1–34.
- Lynch JD. Three new rainfrogs of the Eleutherodactylus diastema group from Colombia and Panama. Rev. Acad. Colomb. Cienc. Exact. Fís. Nat. 2001;25:287–297.