Abstract
All animals have defenses against predators, but assessing the effectiveness of such traits is challenging. Neotropical katydids (Orthoptera: Tettigoniidae) are an abundant, ubiquitous, and diverse group of large insects eaten by a variety of predators, including substrate-gleaning bats. Gleaning bats capture food from surfaces and usually use prey-generated sounds to detect and locate prey. A number of Neotropical katydid signaling traits, such as the emission of ultrasonic frequencies, substrate vibration communication, infrequent calling, and ultrasound-evoked song cessation are thought to have evolved as defenses against substrate-gleaning bats. We collected insect remains from hairy big-eared bat (Micronycteris hirsuta) roosts in Panama. We identified insect remains to order, species, or genus and quantified the proportion of prey with defenses against predatory bats based on defenses described in the literature. Most remains were from katydids and half of those were from species with documented defenses against substrate-gleaning bats. Many culled remains were from insects that do not emit mate-calling songs (e.g. beetles, dragonflies, cockroaches, and female katydids), indicating that eavesdropping on prey signals is not the only prey-finding strategy used by this bat. Our results show that substrate-gleaning bats can occasionally overcome katydid defenses.
Introduction
Predation is one of the strongest agents of natural selection [Citation1], and in response, prey has evolved a spectacular diversity of anti-predator defenses [Citation2]; however, the efficacy of prey defenses is difficult to quantify in nature. In the Neotropics, katydids (Orthoptera: Tettigoniidae) are an abundant, ubiquitous, and diverse group of large insects that are eaten by a variety of predators including bats, monkeys, rodents, birds, lizards, and amphibians [Citation3,4]. Katydids exhibit an assortment of morphological and behavioral traits considered to be anti-predator defenses [Citation5].
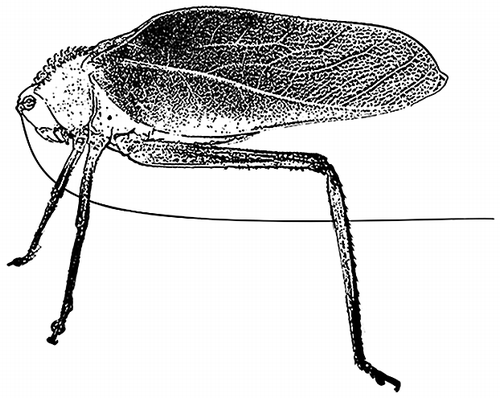
Katydid morphological defenses include crypsis, mimicry, chemical defenses, spines, and a strong bite. Many katydids are visually cryptic, having broad green wings to blend in with vegetation, or other forms of coloration to blend in with bark or lichen. Likewise, there are a number of spectacular forms of mimicry found in katydids, such as wasp (Scaphura spp. and Aganacris spp.) and leaf (Mimetica spp., Aegimia spp., Pycnopalpa bicordata) mimics [Citation6]. Some species have long, sharp spines on their legs or thorax (e.g. Steirodon spp., Figure ) and a strong bite owing to their large mandibles (e.g. Copiphora spp.). Behavioral defenses include well-hidden daytime roosts [Citation3,5], reduced activity during bright phases of the moon [Citation7], singing from protected perches [Citation8], and changes in acoustic behavior [Citation4]. Male katydids use their wings to produce calling songs that attract females, and have acoustic defenses such as song cessation at the approach of a potential predator and the production of loud sounds when touched that might startle a predator [Citation4]; however, the efficacy of these defenses in the wild remains unknown.
In the Neotropics, substrate-gleaning bats capture prey from surfaces and are known to exert top-down control on insect populations [Citation9,10]. In addition, many gleaning bats are significant predators of katydids specifically [Citation8,11–15, this study]. Many substrate-gleaning bats listen to the mate-calling songs of male katydids to detect and locate them as prey [Citation8,16,17]. Thus, some acoustic characteristics of Neotropical katydid songs are believed to be specific adaptations against gleaning bat predation [Citation3]. For example, sporadic/infrequent calling [Citation8,18,19], the emission of high ultrasonic frequencies [Citation20], and substrate vibration communication [Citation8,20,21] have all been proposed as defensive forms of communication to evade detection by eavesdropping bats. Katydids have ultrasound-sensitive ears and some will cease singing in response to ultrasonic pulses and bat echolocation calls [Citation22–25]. Cessation of singing can effectively thwart the gleaning attacks of temperate northern long-eared bats, Myotis septentrionalis [Citation26], but the efficacy of such an acoustic defense has not been tested against Neotropical gleaning bats. For example, the Neotropical common big-eared bat Micronycteris microtis, a congener of our study species, uses biosonar to locate silent, motionless arthropods resting on leaf surfaces [Citation27], suggesting that song cessation alone will not always prevent the predatory attacks of substrate-gleaning bats.
The goal of this study was to identify the katydid species eaten by the hairy big-eared bat M. hirsuta, a major gleaning bat predator, and to assess the proportion of katydid species with described anti-bat defenses in the diet. Previous studies have shown that M. hirsuta eats many katydids ([Citation11]: 25% by number, [Citation28]: 41% by number, 61.5% by weight); however, the relative number and sex of all katydid prey have never been reported. Detailed data about the dietary composition of bat predators can help address hypotheses about the efficacy of presumed katydid defenses.
Methods
The study was conducted at the Smithsonian Tropical Research Institute (STRI) field station on Barro Colorado Island (BCI) in Panama (9°10′ N, 79°51′ W). The 15.6 km2 island is covered with moist, semi-deciduous tropical forest composed of both young and old stands (90–600 years old; [Citation29]).
From November 2001 to January 2003, we collected arthropod remains from three hollow trees used as roosts by M. hirsuta. Bats were caught and identified to species when they emerged from the roost at night. We filmed the inside of one roost to confirm that the bats inside were responsible for dropping arthropod remains at night. This also demonstrated that bats frequently returned to the same roost to consume insects throughout the foraging period.
To collect culled arthropod parts, a plastic sheet was suspended off the ground inside the hollow tree roost to keep the prey remains dry and prevent them from being washed away by rainwater. From roost 1, collections were made 2–14 times/month covering all seasons except April and May (63 collections). Remains were also collected from two other M. hirsuta roosts (roost 2 collection dates: 27 September 2002, 21 November 2002 and 5 December 2002; roost 3 collection date: 29 November 2002). Cockroaches and ants were observed removing bat feces and, more rarely, insect remains, thus our samples likely underestimate the total number of prey items brought back to bat roosts. However, the purely chitinous remains that are important items for taxonomic classification, such as arthropod wings, legs, and ovipositors, were seldom removed.
Most insect parts were identified to order, and in case of katydids to the level of family, subfamily, genus, and species whenever possible using keys and information from [Citation4,30–32]. For all insect remains, we report the total number of body parts found as a measure of the maximum number of individuals that were captured for each taxonomic group. For katydids, we additionally examined forewings to classify them as male (having characteristic sound producing structures) or female (lacking these structures). Some wings could not be classified in this way because they were missing the area of the wing with sound-producing structures and these were included as individuals of unknown sex in the count. We also identified each forewing as a left or right wing. By matching the left and right wings of the same sex, we were able to calculate the minimum number of individual katydids at each roost.
We conducted a literature search to document defenses of Neotropical katydids believed to be effective against predatory bats. These defenses were noted for all species of katydid from Panama and are reported here for those species found as remains in bat roosts (Table ). A number of defenses are common to all Neotropical katydids and are not listed in Table : regurgitation of crop fluid (thought to be distasteful to predators; [Citation3,5]), holding tightly to a substrate when grabbed [Citation3], kicking [Citation5], and autotomy of the hind legs [Citation5]. Only defenses that vary by katydid species were documented, including large mandibles with a strong bite, large cuticular spines, defensive startle sounds, calling from protected perches, infrequent signaling, production of vibratory tremulation signals, and calling song cessation.
Table 1. Minimum number of individual katydids represented in remains collected from a Micronycteris hirsuta roost, and previously described anti-predator defenses specific to bats for each katydid species [sources in brackets].
Results
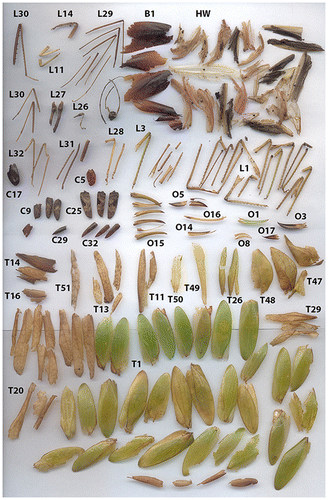
At three M. hirsuta roosts, we found a total of 1,931 culled arthropod parts, which were mostly insect wings, ovipositors, and legs (Figure ). Out of all the remains, 109 were Blattodea (6%), 296 were Coleoptera (15%), 1,443 were Orthoptera (75%), 25 were Odonata (1%), and 58 could not be identified to order (3%). Of the Orthoptera, 72 were from the family Gryllidae (crickets), 1,341 were from the family Tettigoniidae (katydids), and 30 could not be identified to family. Therefore, by far the most abundant group of the insect remains were the katydids, comprising 69% of all remains at the roosts.
Figure 2. Sample of culled katydid remains with identification numbers collected from a single Micronycteris hirsuta roost on one day. B: Blattodea wings, C: Coleoptera wings, T: Tettigoniidae (katydid) forewings, HW: hindwings, L: legs, O: ovipositors.
Based on wing morphology, we calculated the minimum number of katydid individuals as 784 (Table ; see methods). In agreement with Belwood [Citation28], the majority of katydids eaten by M. hirsuta were from the subfamily Pseudophyllinae (655, or 83% of katydids), with fewer individuals from the subfamilies Phaneropterinae (76, 10%) and Conocephalinae (53, 7%). More than half of the katydids (486, 62%) could be identified to genus or species (Table ). Six identified genera made up 56% of all katydid individuals collected from bat roosts: Docidocercus, Xestoptera, Idiarthron, Copiphora, Melanonotus, and Cocconotus. With the exception of Copiphora, these are all genera from the subfamily Pseudophyllinae.
Based on data from Orthoptera Species File Online [Citation32], there are approximately 130 katydid species in Panama. We found literature supporting defensive behavior for 38 of these species. For the katydid remains found in the bat roosts, we were able to classify katydids into 42 species or species groups, 12 of which had documented defensive behavior against bats (Table ).
Of the identified katydids, roughly 50% (390/784) came from 14 species with documented anti-predator defenses (Table ). Of these 14 species, two possess large mandibles with a strong bite, two have prominent cuticular spines, three produce startle sounds when touched, two sing from protected perches, five sing sporadically, nine use tremulations for substrate-borne vibratory communication, and four exhibit song cessation in response to playbacks of bat echolocation calls. We could identify the sex of the katydids for 52% of the individuals (408/784). Of those for which the sex could be identified, 36% were males and 64% were females. Similar percentages of males and females were also found for individual species with large sample sizes (e.g. Docidocercus sp. and Xestoptera cornea; Table ).
Discussion
Our results show that the effectiveness of many proposed defenses of katydids against bats may not be as effective as originally suggested in the literature. Given the high proportion of female katydids and silent prey in the remains, our results also suggest that M. hirsuta use sensory cues other than prey-generated acoustic signals to find their prey. Although we cannot quantify the number of prey taken relative to their availability or the reduction in predation provided by specific antipredator defenses, our data nevertheless show that tropical substrate-gleaning bats at least occasionally overcome katydid antipredator defenses. The detailed level of prey identification in our study provides an opportunity to assess specific predator-prey patterns and the prevalence of katydids with particular defenses in the diet of gleaning bats, something that is not possible when the identification of prey remains is limited to the level of order or family.
When searching the literature for examples of katydid defenses against bats, we considered both physical defenses (such as large mandibles, chemical defenses, and large spines), and behavioral defenses (such as acoustic startle sounds, calling from protected perches, infrequent calling, vibratory tremulation signals, and song cessation in response to bat echolocation calls). The presence among the prey remains of two katydid species (A. curvidens and C. brevirostris) with large mandibles and a strong bite suggests that biting does not guarantee safety from substrate-gleaning bats, perhaps because many gleaners disable prey by biting the back of the katydid’s thorax, thus effectively evading its mandibles [Citation28]. Chemical defenses are not well documented in katydids, but species in the genus Vestria are believed to produce a chemical deterrent when they are disturbed and it appears to be distasteful to monkeys [Citation5]. No individuals of this genus were found in katydid remains at M. hirsuta roosts.
The presence of Steirodon stalii (Figure ) in prey remains at bat roosts demonstrates that a large body size and the presence of thoracic spines do not make katydids impervious to gleaning bat predation. In a captive feeding study, M. hirsuta successfully caught and ate large katydids of the genus Steirodon (wings ca. 0.1 m, body ca. 0.065 m, weight ca. 4 g, compared to the 14 g M. hirsuta; [Citation33,34]). However, we also noted that none of the katydids from Panama with exceptionally sharp and long spines (Steirodon careovirgulatum, Markia hystrix, Stilpnochlora acanthonotum, Panacanthus spinosus; [Citation4]) were found in prey remains, suggesting that long, sharp spines could potentially deter bat predation. When observed within a flight cage, the gleaning bats Trachops cirrhosus, Tonatia saurophila, Lophostoma silvicolum, M. microtis, and M. hirsuta often subdue heavily armored katydids, but only after a considerable struggle and the bats occasionally end up with holes in their flight membranes (R. A. Page, pers. obs.). Interestingly, many gleaning bats that we have captured in mistnets on BCI have scars and holes in their wing and tail membranes, although these injuries can have many different causes.
Katydid species with known behavioral defenses were also found in the roost remains. Three katydid species (Acanthodis curvidens, Pristonotus tuberosus, and Xestoptera cornea), including one of the most commonly found species in the remains, are known to emit very loud sounds when grabbed by humans. These sounds are thought to function to startle predators [Citation5]; however, whether the attack of a gleaning bat would trigger this behavior is unknown.
Much of the research on the interactions between katydids and substrate-gleaning bats has focused on the acoustic defenses of male katydids (e.g. use of sporadic/intermittent song). Micronycteris hirsuta are known to be attracted to prey-generated sounds like katydid mate-calling song [Citation8,35] and will glean singing katydids while ignoring silent ones in captivity [Citation35]. Some studies have shown that low duty cycle calls (duty cycle = call duration / signal period) or signaling with a low repetition rate reduces the probability of a predatory response by gleaning bats compared to high duty cycle calls or calling at a high repetition rate [Citation8,26]. Many low duty cycle katydid species, however, were present in prey remains found at M. hirsuta roosts. Neotropical katydids with high duty cycles that sing continuously tend to call from restricted locations generally inaccessible to gleaning bats, such as thorny plants (I. pulchripennis) or tall grasses (N. affinis). Although we found remains of these two species in the bat cullings, they were either female or the sex could not be determined, so singing from protected locations could also be effective against gleaning bats. Different species of gleaning bats might exhibit differential preferences for exploiting prey-generated signals and thus exert different degrees of selection pressure on katydid sensory-based defenses [Citation16,17]. In addition to passive acoustic defenses, such as low calling rates and calling from inaccessible locations, most katydids have acute ultrasonic hearing which allows them to detect the echolocation calls of passing bats and cease calling when they are in danger of an attack. Some katydids use this strategy, but others do not [Citation22–24].
Perhaps the most striking result of our study is that silent female katydids were more commonly represented in the remains found in M. hirsuta roosts than male katydids that sing. We also found species of silent insects that are not known to emit acoustic mate-calling signals, including dragonflies, cockroaches, and beetles. The presence of many silent prey species and katydid species with acoustic-based defenses against gleaning bat predation suggest that eavesdropping on insect acoustic signals cannot be the only strategy used by M. hirsuta to find prey. Alternative strategies include eavesdropping on other kinds of incidental prey sounds, using echolocation to locate silent and motionless prey on vegetation, and catching prey in flight, also called aerial-hawking.
Several bat species are known to use incidental noises generated by insects to detect and locate them as prey, such as wing beat or flight sounds [Citation36], landing and crawling sounds [Citation37] and rustling noises [Citation38,39]. Perhaps the general activity level of different prey species, including exposure time moving on various substrates, is an important risk factor governing gleaning bat predation. For example, during the day the katydid D. gigliotosi hides in plants on the forest floor and each night males make a long journey up into the canopy to sing and attract females [Citation40], potentially producing a number of incidental locomotory noises along the way. A tracking study of Phyllophilla ingens, a medium-sized katydid common to our study area, revealed much higher levels of activity during the day than anticipated. Although the diurnal altitudinal movements of P. ingens included moving closer to the ground in the evening, just prior to the main activity period of predatory bats, they usually stayed ca. 10 m above ground [Citation41]. Interestingly, P. ingens were not found in prey remains at M. hirsuta roosts even though they are readily captured and consumed by gleaning bats in captivity (33), and birds and rodents feed heavily upon them in the wild [Citation41]. Little is known about the natural behavior of Neotropical katydids, hence additional research is needed to determine the behaviors that represent the greatest risk for exploitation by substrate-gleaning bats.
Another intriguing possibility is that M. hirsuta might use echolocation to find silent, motionless prey on surfaces, as has been demonstrated for the congeneric species M. microtis [Citation27]. In a study investigating the responses of gleaning bats to katydid calls, M. microtis also showed the least interest in katydid calls compared to three other gleaning bat species in Panama [Citation17], possibly because of greater reliance on echolocation for locating prey. Vegetation varies widely in its reflective properties [Citation42]. For bats using echolocation cues to detect silent, motionless prey, the leaves that katydids perch on at night – either exposed or covered, smooth or textured, large or small – will vary in their echoacoustic properties and this likely changes the conspicuousness of substrate-borne insects to gleaning bats that use echolocation to find prey regardless of whether the prey actively emits sound. Therefore, habitat use by katydids is also an important risk factor for avoiding bats that detect prey on surfaces using echolocation.
A final possibility is that M. hirsuta are more flexible in foraging than previously believed and facultatively glean and catch prey in flight. Because a number of bat species have been shown to use both aerial-hawking and substrate-gleaning foraging strategies (e.g. Cardioderma cor [Citation43], Myotis auriculus [Citation44], Nycteris grandis and N. thebaica [Citation45], Hipposideros ruber [Citation46], Megaderma lyra [Citation47], R. ferrumequinum and R. hipposideros [Citation48], M. emarginatus [Citation49], M. evotis [Citation50], M. lucifugus and M. septentrionalis [Citation51], Rhinolophus blasii, [Citation52], Otonycteris hemprichii [Citation53], Megaderma spasma [Citation54]), this serves as a reminder to remain cautious about assigning bats to a single foraging strategy.
Flight cage experiments show that, in captivity, M. hirsuta can also both glean and catch insects in flight (33), meaning that not all prey in our study were necessarily captured by substrate-gleaning. A study on M. spasma in India showed that although these bats sometimes approached male katydid calling song, they always attacked tethered flying katydids [Citation54]. In addition, the majority of katydid remains in the roosts of M. spasma were females, suggesting that the generally accepted idea that signaling males are at greater risk of predation than females does not hold true for katydids in this system [Citation54]. Our data support this conclusion for Neotropical katydids as well. Some katydid species are known to have a diving response to ultrasound in flight [Citation55,56], as do crickets [Citation57] and some beetles [Citation58,59]. It is interesting to note, however, that the majority of katydid remains in the roosts of M. hirsuta were from species in the subfamily Pseudophyllinae, which are not strong fliers (Lang and Römer, pers. obs.). In addition, there were a number of remains of dragonflies (Odonata), which are not active at night. Therefore, it is likely that M. hirsuta is using a variety of strategies to catch prey.
Taken together, our data indicate that the predator–prey relationship between Neotropical gleaning bats and katydids is more complicated than previously thought. Some katydid defenses, such as exceptionally long spines and calling from dense vegetation, might be successful in reducing the probability of being captured by some Neotropical gleaning bats, whereas others, such as large mandibles, sporadic calling, or even silence, do not guarantee safety. In addition, the large number of silent prey in the diet of M. hirsuta, including a very large proportion of female katydids, means that these bats must be using a variety of strategies to locate prey. More research is needed on the interactions and behavior of these animals to assess the many factors that potentially contribute to this predator–prey system.
Author contributions
DKND and SLV-H collected insect remains; HtH classified katydid wings to sex and side; all authors contributed to the analysis and writing of the manuscript.
Associate Editor: Nathan Muchhala
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
Funding was provided by a STRI Short-Term Fellowship and Dartmouth College to HtH, the Austrian Science Fund (FWF; Projects P14257 and P17986-B06) to HR, STRI and Human Frontier Science Program [grant number RGP0040/2013] to RAP, the Natural Sciences and Engineering Research Council (NSERC) of Canada to PAF, and the Roche Research Foundation and the ZUNIV-Fonds zur Förderung des Akademischen Nachwuchses (FAN) to DKND.
Acknowledgments
We are grateful to the staff of the Smithsonian Tropical Research Institute (STRI) on Barro Colorado Island and in Gamboa for logistical support. We thank Birgit Roenfeld for identification of prey remains at bat roosts, and Felix Fornoff and Yuanyuan Huang for making Figure 1. Sabine Spehn provided the insect remains from roosts 2 and 3.
References
- Darwin CR. On the origin of species by means of natural selection or the preservation of favoured races in the struggle for life. London: John Murray; 1859.
- Dawkins R, Krebs JR. Arms races between and within species. Proc Roy Soc London B. 1979;205:489–511.10.1098/rspb.1979.0081
- Belwood JJ. Anti-predator defences and ecology of Neotropical forest katydids, especially the Pseudophyllinae. In: Bailey WJ, Rentz DCF, editors. The Tettigoniidae. New York, NY: Springer-Verlag; 1990. p. 8–26.10.1007/978-3-662-02592-5
- Nickle DA. Katydids of Panama (Orthoptera: Tettigoniidae). In Quintero D, Aiello A, editors. Insects of Panama and Mesoamerica: selected studies. Oxford: Oxford University Press; 1992. p. 142–184.
- Nickle DA, Castner JL. Strategies utilized by katydids (Orthoptera: Tettigoniidae) against diurnal predators in rainforests of Northeastern Peru. J Orthop Res. 1995;4:75–88.10.2307/3503461
- Hogue CL. Latin American insects and entomology. Berkeley: University of California Press; 1993.
- Lang AB, Kalko EKV, Römer H, et al. Activity levels of bats and katydids in relation to the lunar cycle. Oecologia. 2006;146:659–666.10.1007/s00442-005-0131-3
- Belwood JJ, Morris GK. Bat predation and its influence on calling behavior in neotropical katydids. Science. 1987;238:64–67.10.1126/science.238.4823.64
- Kalka M, Smith AR, Kalko EKV. Bats limit arthropods and herbivory in a tropical forest. Science. 2008;320:71.10.1126/science.1153352
- Williams-Guillen K, Perfecto I, Vandermeer J. Bats limit insects in a neotropical agroforestry system. Science. 2008;320:70.10.1126/science.1152944
- Wilson DE. Food habits of Micronycteris hirsuta (Chiroptera: Phyllostomidae). Mammalia. 1971;35:107–111.
- LaVal RK, LaVal ML. Prey selection by a neotropical foliage-gleaning bat, Micronycteris megalotis. J Mammal. 1980;61:327–330.10.2307/1380057
- Giannini NP, Kalko EKV. The guild structure of animalivorous leaf-nosed bats of Barro Colorado Island, Panama, revisited. Acta Chiropt. 2005;7:131–146.10.3161/1733-5329(2005)7[131:TGSOAL]2.0.CO;2
- Kalka M, Kalko EKV. Gleaning bats as underestimated predators of herbivorous insects: diet of Micronycteris microtis (Phyllostomidae) in Panama. J Trop Ecol. 2006;22:1–10.10.1017/S0266467405002920
- Santana SE, Geipel I, Dumont ER, et al. All you can eat: high performance capacity and plasticity in the common big-eared bat, Mycronycteris microtis (Chiroptera: Phyllostomidae). PLoS One. 2011;6:e28584.10.1371/journal.pone.0028584
- Tuttle MD, Ryan MJ, Belwood JJ. Acoustical resource partitioning by two species of phyllostomid bats (Trachops cirrhosus and Tonatia sylvicola). Anim Behav. 1985;33:1369–1371.10.1016/S0003-3472(85)80204-9
- Falk JJ, ter Hofstede HM, Jones PL, et al. Sensory-based niche partitioning in a multiple predator-multiple prey community. Proc Roy Soc London B. 2015;282:20150520.10.1098/rspb.2015.0520
- Rentz DC. Two new katydids of the genus Melanonotus from Costa Rica with comments on their life history strategies (Tettigoniidae: Pseudophyllinae). Ent News. 1975;86:129–140.
- Morris GK, Beier M. Song structure and description of some Costa Rican katydids (Orthoptera: Tettigoniidae). Trans Am Ent Soc. 1982;108:287–314.
- Morris GK, Mason AC, Wall P, et al. High ultrasonic and tremulation signals in neotropical katydids (Orthoptera: Tettigoniidae). J Zool. 1994;233:129–163.10.1111/jzo.1994.233.issue-1
- Römer H, Lang A, Hartbauer M. The signaller's dilemma: a cost–benefit analysis of public and private communication. PLoS One. 2010;5:e13325.10.1371/journal.pone.0013325
- Faure PA, Hoy RR. The sounds of silence: cessation of singing and song pausing are ultrasound-induced acoustic startle behaviors in the katydid Neoconocephalus ensiger (Orthoptera; Tettigoniidae). J Comp Physiol A. 2000;186:129–142.10.1007/s003590050013
- ter Hofstede HM, Fullard JH. The neuroethology of song cessation in response to gleaning bat calls in two species of katydids, Neoconocephalus ensiger and Amblycorypha oblongifolia. J Exp Biol. 2008;211:2431–2441.10.1242/jeb.017285
- ter Hofstede HM, Kalko EKV, Fullard JH. Auditory-based defence against gleaning bats in neotropical katydids (Orthoptera: Tettigoniidae). J Comp Physiol A. 2010;196:349–358.10.1007/s00359-010-0518-4
- Hartbauer M, Ofner E, Grossauer V, et al. The cercal organ may provide singing tettigoniids a backup sensory system for the detection of eavesdropping bats. PLoS One. 2010;5:e12698.10.1371/journal.pone.0012698
- ter Hofstede HM, Ratcliffe JM, Fullard JH. The effectiveness of katydid (Neoconocephalus ensiger) song cessation as antipredator defence against the gleaning bat Myotis septentrionalis. Behav Ecol Sociobiol. 2008;63:217–226.10.1007/s00265-008-0652-y
- Geipel I, Jung K, Kalko EKV. Perception of silent and motionless prey on vegetation by echolocation in the gleaning bat Micronycteris microtis. Proc Roy Soc Lond B. 2013;280:20122830.10.1098/rspb.2012.2830
- Belwood JJ. The influence of bat predation on calling behavior in Neotropical forest katydids (Insecta: Orthoptera: Tettigoniidae) [dissertation]. Gainesville (FL): University of Florida; 1988a.
- Armstrong KN. Phylogeographic structure in Rhinonicteris aurantia (Chiroptera: Hipposideridae): implications for conservation. Acta Chiropt. 2006;8:63–81.10.3161/1733-5329(2006)8[63:PSIRAC]2.0.CO;2
- Naskrecki P. Katydids of Costa Rica, vol. 1, systematics and bioacoustics of the cone-head katydids. Philadelphia (PA): Orthopterists’ Society at the Academy of Natural Sciences of Philadelphia, Department of Entomology; 2000.
- Naskrecki P, Otte D. An illustrated catalog of Orthoptera: Tettigonioidea (katydids or bush-crickets). Philadelphia (PA): Orthopterists’ Society at the Academy of Natural Sciences of Philadelphia, Department of Entomology; 1999.
- Eades DC, Otte D, Cigliano MM, et al. Orthoptera species file. Version 5.0/5.0. http://Orthoptera.SpeciesFile.org; 2001.
- Spehn SE. Etho-ecology and sensory physiology of two gleaning insectivorous bats, Tonatia saurophila and Micronycteris hirsuta in Panamá [dissertation]. Ulm: University of Ulm; 2005.
- Santana SE, Dumont ER. Connecting behaviour and performance: the evolution of biting behaviour and bite performance in bats. J Evol Biol. 2009;22:2131–2145.10.1111/jeb.2009.22.issue-11
- Belwood JJ. Foraging behaviour, prey selection and echolocation in phyllostomine bats (Phyllostomidae). In: Nachtigall PE, Moore PWB, editors. Animal Sonar. New York, NY: Plenum Press; 1988b. p. 601–605.10.1007/978-1-4684-7493-0
- Faure PA, Barclay RMR. The sensory basis of prey detection by the long-eared bat, Myotis evotis, and the consequences for prey selection. Anim Behav. 1992;44:31–39.10.1016/S0003-3472(05)80751-1
- Fuzessery ZM, Buttenhoff P, Andrews B, et al. Passive sound localization of prey by the pallid bat (Antrozous p. pallidus). J Comp Physiol A. 1993;171:767–777.10.1007/BF00213073
- Siemers BM, Güttinger R. Prey conspicuousness can explain apparent prey selectivity. Curr Biol. 2006;16:R157–R159.10.1016/j.cub.2006.02.056
- Jones PL, Page RA, Hartbauer M, et al. Behavioral evidence for eavesdropping on prey song in two Palearctic sibling bat species. Behav Ecol Sociobiol. 2010;65:333–340.
- Lang AB, Römer H. Roost site selection and site fidelity in the neotropical katydid Docidocercus gigliotosi (Tettigoniidae). Biotropica. 2008;40:183–189.10.1111/j.1744-7429.2007.00360.x
- Fornoff F, Dechmann D, Wikelski M. Observation of movement and activity via radio-telemetry reveals diurnal behavior of the Neotropical katydid Philophyllia ingens (Orthoptera: Tettigoniidae). Ecotropica. 2012;18:27–34.
- Simon R, Holderied MW, Koch CU, et al. Floral acoustics: conspicuous echoes of a dish-shaped leaf attract bat pollinators. Science. 2011;333:631–633.10.1126/science.1204210
- Vaughan TA. Nocturnal behavior of the African false vampire bat (Cardioderma cor). J Mammal. 1976;57:227–248.10.2307/1379685
- Fenton MB, Bell GP. Echolocation and feeding behaviour in four species of Myotis (Chiroptera). Can J Zool. 1979;57:1271–1277.10.1139/z79-163
- Fenton MB, Gaudet CL, Leonard ML. Feeding behaviour of the bats Nycteris grandis and Nycteris thebaica (Nycteridae) in captivity. J Zool. 1983;200:347–354.
- Bell GP, Fenton MB. The use of Doppler-shifted echoes as a flutter detection and clutter rejection system: the echolocation and feeding behavior of Hipposideros ruber (Chiroptera: Hipposideridae). Behav Ecol Sociobiol. 1984;15:109–114.10.1007/BF00299377
- Marimuthu G, Neuweiler G. The use of acoustical cues for prey detection by the Indian false vampire bat, Megaderma lyra. J Comp Physiol A. 1987;160:509–515.10.1007/BF00615084
- Jones G. Foraging behavior and echolocation of wild horseshoe bats Rhinolophus ferrumequinum and R. hipposideros (Chiroptera, Rhinolophidae). Behav Ecol Sociobiol. 1989;25:183–191.10.1007/BF00302917
- Krull D, Schumm A, Metzner W, et al. Foraging areas and foraging behavior in the notch-eared bat, Myotis emarginatus (Vespertilionidae). Behav Ecol Sociobiol. 1991;28:247–253.
- Faure PA, Barclay RMR. Substrate-gleaning versus aerial-hawking: plasticity in the foraging and echolocation behaviour of the long-eared bat, Myotis evotis. J Comp Physiol A. 1994;174:651–660.
- Ratcliffe JM, Dawson JW. Behavioural flexibility: the little brown bat, Myotis lucifugus, and the northern long-eared bat, M. septentrionalis, both glean and hawk prey. Anim Behav. 2003;66:847–856.10.1006/anbe.2003.2297
- Siemers BM, Ivanova T. Ground gleaning in horseshoe bats: comparative evidence from Rhinolophus blasii, R. Euryale, and R. mehelyi. Behav Ecol Sociobiol. 2004;56:464–471.
- Hackett TD, Korine C, Holderied MW. A whispering bat that screams: bimodal switch of foraging guild from gleaning to aerial hawking in the desert long-eared bat. J Exp Biol. 2014;217:3028–3032.10.1242/jeb.100362
- Raghuram H, Deb R, Nandi D, et al. Silent katydid females are at higher risk of bat predation than acoustically signalling katydid males. Proc Roy Soc London B. 2015;282:20142319.
- Libersat F, Hoy RR. Ultrasonic startle behavior in bushcrickets (Orthoptera: Tettigoniidae). J Comp Physiol A. 1991;169:507–514.
- Schulze W, Schul J. Ultrasound avoidance behaviour in the bushcricket Tettigonia viridissima (Orthoptera: Tettigoniidae). J Exp Biol. 2001;204:733–740.
- Wyttenbach RA, May ML, Hoy RR. Categorical perception of sound frequency by crickets. Science. 1996;273:1542–1544.10.1126/science.273.5281.1542
- Forrest TG, Read MP, Farris HE, et al. A tympanal hearing organ in scarab beetles. J Exp Biol. 1997;200:601–606.
- Yager DD, Cook AP, Pearson DL, et al. A comparative study of ultrasound-triggered behaviour in tiger beetles (Cicindelidae). J Zool. 2000;251:355–368.10.1111/jzo.2000.251.issue-3