Abstract
We describe a new species of climbing rat of the genus Rhipidomys based on cranial and external morphology, morphometrics, and phylogenetic analyses of cytochrome b gene. This taxon was compared with species of Rhipidomys present in Ecuador, principally R. latimanus, which is a closely related species based on molecular analysis, and with several species from Colombia and Peru. The new species is easily distinguished from congeneric species by the relatively small size of its body (average head–body length 123 mm) and its distinctive cranial morphology including: Interorbital region constricted; postorbital crest almost imperceptible in females and evident only slightly in males; braincase wide and round; nasals narrow in their posterior and gradually expand forward; anteromedian flexus is well defined and deep; m3 with hypoflexid large and deep; mesopterygoid fossa extends beyond the third molar and capsular process on mandible well developed, forming an evident projection. The new species is only known from the southeastern portion of Sangay National Park in Ecuador and is therefore likely endemic to the Cordillera Oriental of Ecuador. We also provide natural history and reproductive observations, vocalization analysis, habitat preference, and phylogenetic placement of this species.
Se describe una nueva especie de roedor arborícola del género Rhipidomys basado en morfología craneal, externa, datos morfométricos y en secuencias moleculares del citocromo b. Este taxón fue comparado con las especies del género Rhipidomys presentes en Ecuador principalmente con R. latimanus, con quien está más cercanamente relacionado basado en datos moleculares, y con algunas especies de Colombia y Perú. La nueva especie es fácilmente distinguida de especies congenéricas esencialmente por el tamaño corporal relativamente pequeño (promedio longitud cabeza-cuerpo 122.7 mm) y una morfología craneal distintiva, que incluye: región interorbitaria comprimida y en forma de reloj de arena; cresta postorbital apenas perceptible en la hembras, en los machos evidente; caja craneana ancha y redondeada; nasales delgadas en su región posterior y expandida anteriormente; flexo anteromediano bien definido y profundo; m3 con hypoflexido grande y profundo; la fosa mesopterigoidea va más allá de los alveolos del tercer molar. Mandíbula con proceso capsular del incisivo muy desarrollado. Esta especie nueva es endémica de la vertiente suroriental del Parque Nacional Sangay al este de Ecuador. También presentamos datos sobre la historia natural, reproducción, vocalizaciones, preferencias de hábitat, y posición filogenética.
Introduction
The genus of arboreal rats, Rhipidomys, includes 23 species. Individuals of this genus are considered to be among the most cryptic of Neotropical mammals [Citation1–6]. Members of this genus occur from Panama, Trinidad, and northern Venezuela to southeastern Brazil [Citation1,6–9] and Paraguay [Citation3]. In Ecuador, there are two known species of Rhipidomys; R. leucodactylus proposed by Tribe [Citation1] occupies the subtropical lowlands east and west of the Andes, and R. latimanus occurs along the western flank of the Andes [Citation10,11].
Several diagnostic characters distinguish members of this genus from other rodents in the subfamily Sigmodontinae: Wide feet with large plantar pads, dorsal surface of the feet with a dark patch, long thick tail covered in hair, generally with a tuft of longer hair at the end of the tail, short face with large eyes, and long mystacial vibrissae that pass the points of the ears reaching beyond the shoulders when angled toward the posterior [Citation1,6,11–15].
Based upon morphological characters and genetic analysis, we describe a new species of arboreal rat of the genus Rhipidomys from Sangay National Park (SNP), Ecuador. The park is a hotspot of diversity and endemism that has recently revealed multiple new species of vertebrates: Caenolestes sangay, Noblella personina, Pristimantis tinguichaca, Pristimantis latericius, and Pristimantis roni. This discovery is part of a series of inventory efforts that the authors began in SNP in 2010.
Methods
Study area
Sangay National Park encompasses a vast tract of forest on the Eastern Versant of the Andes in southern Ecuador (Figure ). Inventory efforts focused on a middle elevation site at 1800 m in Sardinayacu, a sector of the park characterized by subtropical rainforest with several lakes.
Field work
The collection effort took place over 12 nights consisting of three in October 2011 and April 2012 and 6 in June 2014. During each night we deployed 100 Sherman live traps (7.5 × 9 × 27 cm; H. B. Sherman Traps, Tallahassee, Florida), 20 Tomahawk live traps (14 × 14 × 40 cm; Tomahawk Live Trap Company, Tomahawk, Wisconsin), and using the technique of Voss et al. [Citation13] we deployed 10 pitfall traps distributed along a 80 m drift line. The total trap effort was 1540 trap nights. The traps were placed near runways, holes, and other signs of small mammal activity. Traps were baited with rolled oats mixed with vanilla and peanut butter. Handling followed care and use procedures recommend by the American Society of Mammalogists [Citation16]. Museum study skins and tissue samples preserved in 96% ethanol were deposited in División de Mastozoología del Museo Ecuatoriano de Ciencias Naturales (DMMECN), Instituto de Ciencias Biológicas de la Escuela Politécnica Nacional (MEPN), and the División de Mastozoología del Museo de Zoología de la Pontificia Universidad Católica del Ecuador (QCAZ) (Appendix 1).
Morphology
Cranial measures were taken with digital calipers (Buffalo Tools, New York) to ±0.01 mm. Each measurement was taken three times and averaged. Criteria of age, external, and cranial morphological characteristics are based on Voss [Citation17], Tribe [Citation1], Abdala & Días [Citation18] and Pacheco [Citation14]. Dental nomenclature is based on Reid [Citation19,20], Tribe [Citation1] and Carleton and Musser [Citation21]. Coloration is based on terminology in Smithe [Citation22] and Encycolorpedia [Citation23].
Vocalizations
Recordings were taken on 18 July 2014 in Sardinayacu from a trapped adult female (DMMECN 3791). Recordings were made using an Olympus WS-802 connected to a Sennheiser K6 Microphone System Powering Module with a Sennheiser ME 66 microphone head. Acoustic analysis was conducted with Adobe Audition CS6 at a sample frequency of 44.1 kHz and a digitalized 16-bit resolution using noise filters and temporal analysis. The program Raven 1.4 [Citation24] was used to generate graphics and for spectral analysis using a Hanning window at 85% superposition and a 512 bit resolution for the rapid transformation (FFT).
These parameters were analyzed: (1) Dominant frequency: frequency of highest energy determined by the Fourier calculation; (2) Frequency modulation: difference of frequencies between the initial and final points in an acoustic structure; (3) Minimum frequency: frequency of inharmonic partials of least visible value on the spectrum; (4) Maximum frequency: frequency of inharmonic partial of greatest visible value on the spectrum; (5) Number of notes per call: number of distinct acoustic signals recognizable in a call; (6) Call length: time from beginning to end of one call; (7) Interval between calls: time between distinct calls; (8) Duration of the notes; and (9) Intervals between notes. The acoustic parameters are adapted from Francescoli [Citation25], Tokumaru et al. [Citation26] and Brito & Batallas [Citation27].
DNA extraction, amplification, and sequencing
DNA was extracted from tissue samples of liver preserved in 96% ethanol (stored at −80 °C) following a guanidinium thiocyanate protocol [Citation28]. We amplified the mitochondral cytochrome b (Cyt b) using the primers MVZ05 (5′-CTT-GATATGAAAAACCATCGTTG-3′), MVZ14 (5′-TCTTCATCTYHGGYTTACAAGAC -3′), and MVZ16 (‘5 CTTGATATGAAAAACCATCGTTG-3′) [Citation28,29]. The amplification protocol was modified from Arellano et al. [Citation30] and consisted of denaturation at 94 °C for 2 min, then 35 denaturation cycles of 1 min, 1 min of annealing at 52 °C and 1 min of extension at 72 °C and a final stage of 5 min extension at 72 °C. PCR products were sent to Macrogen (Macrogen Inc., Seoul, Korea) for sequencing using the amplification primers. The DNA sequences were edited in Geneious 5.4.7 (Biomatters, 2005–2012) and aligned with the MUSCLE tool in the Mesquite 2.7.1 platform [Citation31] with sequences from outgroups Tylomys, Jucelinomys, Brucepattersonius, some Oryzomynes (Euryoryzomys and Nectomys), and the closest genus Thomasomys. DNA sequences are deposited in GenBank and are listed with museum catalog numbers in Appendix 2.
Phylogenetic analysis
Bayesian Inference (BI) analyses were conducted within the program Mr. Bayes 3.1 [Citation32]. The partition scheme and evolutionary models were obtained from the program PartitionFinder 1.1.1 [Citation33]. The model obtained for the first and third base was GTR + I + G while for the second base it was SYM + I + G. We ran four Markov chains 20,000 generations and we discarded 25% of the runs as burn-in before obtaining the consensus tree with support values expressed as Bayesian posterior probabilities. Corrected and uncorrected genetic p distances were obtained in the program MEGA 6.0 [Citation33].
Delimitation of species
The PTP model is based on the operational criteria of the coalescence of genes. This allows us to identify putative species depending on the number of substitutions that occur along the branches of the phylogeny. The analysis assumes that the number of substitutions between species is significantly higher than the number of substitutions within species. These differences are reflected in the branch lengths [Citation35]. We ran the PTP analysis using 100,000 MCMC generations, with a thinning value of 100, a burn-in of 0.1, and the outgroup was removed to improve species delimitation. Convergence of MCMC chain was confirmed visually as recommended [Citation35].
Results
Rhipidomys albujai sp. n
Rhipidomys sp. nov., Brito & Ojala-Barbour [Citation36].
Proposed common name in English
Albuja’s climbing rat
Proposed common name in Spanish
Rata trepadora de Albuja
Holotype
Adult female DMMECN 3719 (field number JBM 598); skin, skull, and complete skeleton, collected 16 June 2014 by Jorge Brito, Hernán Orellana and Germán Tenecota.
Paratopotypes
Two adult males. MEPN 12196 collected 19 April 2012 by Jorge Brito and Reed Ojala-Barbour; DMMECN 3790 collected 16 June 2014 by Jorge Brito, Hernán Orellana and Germán Tenecota.
Paratypes
One adult male, QCAZ 15214 collected 17 September 2015 in río Sardinayacu, Sangay National Park (02°05′53.67″ S, 78°09′20.37″ W, 1400 m), Sinaí, Morona, Morona Santiago, Ecuador.
Type locality
Sardinayacu, Sangay National Park (02°4′20.43″S, 78°13′1.16″W 1800 m), Sinaí, Morona, Morona Santiago, Ecuador (Figure ).
Etymology
The specific epithet albujai is patronymic in honor of Ecuadorean mammalogist Luis Albuja, who is a pioneering figure in mammalogy in Ecuador. He has worked with nearly all the mammalian groups found in Ecuador with research focusing on various arenas of population ecology, systematics, and taxonomy. His principal interest is in bats, primates, and marsupials.
Diagnosis
A small species of Rhipidomys (measurements in Table ) with yellowish brown-pelage and a white to yellowish ventral pelage that contrasts strongly with the dorsum. The tail is longer than the head–body length and ends in a tuft. The feet are wide with a dark dorsal patch in the medial region that extends to the base of the phalanges. Ears are medium in size and darker than the dorsal pelage. The skull is medium in size with shallow zygomatic notches when viewed from above. Nasals narrow in their posterior and gradually expand forward. The interorbital region is concave in the superior part with poorly developed supraorbital ridges; the interorbital region is in the form of an hourglass. The carotid circulation pattern is primitive (sensu Voss [Citation17]). The subsquamosal foramen is large and the hamular process is long with a slightly wider distal portion. Incisive foramen is elongated and narrow at margins with a slightly convergent posterior. Mesopterygoid fossa is M-shaped with a small palatal spine. M3 with a distinctive anteromedial flexus; m3 with deep a hypoflexid; mesopterygoid fossa extends beyond the third molar. Incisive capsular process of the mandible is well developed.
Table 1. Morphometric measures in millimeters of adults of Rhipidomys albujai sp. nov.
Description
Externally, R. albujai is small sized with yellowish to orangish-brown pelage with relatively short hairs; underfur 7–10 mm, guard hairs 9–13 mm. Ventral pelage is whitish with yellowish tones mostly present in the abdominal region that contrast strongly from the dorsum (Figure (A–C)), but these yellowish hues are only perceptible in some individuals. The pelage is soft and fine. The ends of the hairs often have a hue of orange. The pelage of the dorsum and venter has a basal gray band.
Figure 2. Holotype of Rhipidomys albujai sp. nov., viewed laterally (A); fronto-lateral view (B) and ventral view (C). DMMECN 3791 adult female. Head and body length 118 mm.

The ears are medium in size and extend beyond the pelage in an oval form. The exterior is covered in short blackish hair. The interior base of the ears is rosy flesh colored to pale in the medial region while the margins are gray. A small auricular patch of cream colored pelage is present behind the ears. The orbicular ring is black. The mystacial vibrissae are numerous, coarse, and long. Two superciliary and a genal vibrissae are much finer and shorter than the mystacial vibrissae. The forepaw is short and wide; five digits with claws. The first digit is greatly reduced with a short wide nail. The other claws are short and curved. Tufts of hair cover the claws of the forepaws and extend slightly beyond the points of the claws. The mesocarpal region is flesh colored with black hairs. The surfaces of the palms have 5 pads. The thenar pad is almost double the size of the hypothenar pad. The three interdigital pads are similar to the hypothenar pad. The ventral surface of each front foot is a pale flesh color. Relative length of the digits: Digit I substantially shorter than digit II; digit II is shorter than digit III; digit III slightly shorter than IV; digit V is shorter than digit IV. (Figure (A)).
Figure 3. Ventral and dorsal view (A); of the forepaw; ventral and dorsal view of the hind foot (B); posterolateral view (C) of Rhipidomys albujai sp. nov. holotype, DMMECN 3791. Abreviation are: I–V, digits; 1–4, interdigital tubercles; h, hypothenar; t, thenar; at, anal tube. Length of hind foot 26 mm.
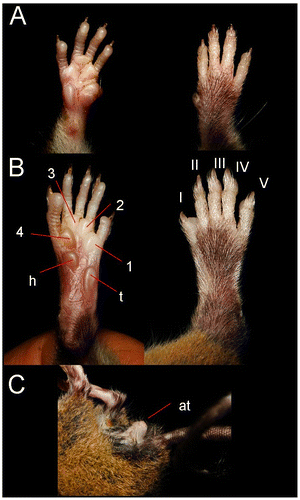
The foot is relatively short and wide with tufts of hair that cover the claws. The dorsal region of the foot is pale-rose colored with a patch of dark hairs covering the medial region of the foot and leaving white lateral fringes. The foot has six plantar pads. Thenar and hypothenar tubercles are well developed; the first being larger than the second. The hypothenar tubercle is located directly behind the interdigital pad of toe V and overlaps with the thenar tubercle and the interdigital cushion of toe I. The plantar surfaces are smooth. Ventral coloration of the foot is pale rose that extends to the middle of the foot and becomes whitish near pads 1–3. The toes are relatively short and thin: Toe I reaches the midpoint of toe II. Toe II is shorter than toe III, toe III slightly shorter than toe IV. Toe V is shorter than digit IV of the forepaw and the distal end of the second phalanx of toe IV (Figure (B)).
The tail is uniformly dark and covered in small hairs, but scales are visible from the base to the medial part of the tail. The posterior half of the tail has denser hairs that entirely hide the scales. On average, the tail length is 131% of head–body length and the last 5 mm of the tail ends in a tuft of hairs resembling a paintbrush 19 mm in length. The hairs along the anterior half of the axis extend beyond 3 layers of scales, and a centimeter covers 14 rings of the tail. The anus protrudes significantly (Figure (C)). Females have three pairs of nipples: an inguinal pair, abdominal pair, and a post-axial pair.
The skull is medium in size (average length 29.1 mm), and the braincase has a convex profile (Figure ). The rostrum is short and narrow with a reduced gnathic process. The nose is long and wedge-shaped, narrowing at the rear and widening toward the snout. The interorbital region is moderately narrow with the base of the molars visible in dorsal view. The postorbital crest is barely perceptible in females and slightly more evident in males. The braincase is broad and rounded. The zygomatic plate is slightly inclined anteriorly and narrow; of similar width as the length of M1. Zygomatic arches are rounded and narrow anteriorly. The lachrymal bones are medium, wide, with a slightly elevated posterior edge; frontal bone wide and with a depression in the medial region (most evident in the males). Nasals narrow in their posterior and gradually expand forward (Figure ). The region anterior to nasal is slightly globular; interparietal is large and diamond-shaped, longitudinally over half the length of the parietal. The incisive foramina are moderately long and narrow, with oval posterior margins, and extend slightly beyond the procingulum of M1. Diastema is slightly concave. M1 is located posterior to the base of the rear edge of the zygomatic plate. Palatine is short and wide without reaching the hypoflexid of M3 posteriorly. Posterolateral palatal pits are small and located at the end of the palatal bone. Mesopterygoid pit M-shaped (with a conspicuous palatal spine) wide and slightly tapered sides. Parapterygoid pit is shallow and without vacuities. Foramen ovale is medium. Postglenoid foramen is small but visible; subsquamosal fenestra wide and located posterodorsal to the postglenoid foramen; squamosal hamular process long and slightly wider in the distal area and in contact with the mastoid. Paraoccipital process is evident and not bifurcated. Auditory bullae are small and slightly globular. Stapedial foramen large, squamoso-alisphenoid groove visible but undeveloped, present and enlarged sphenofrontal foramen: carotid circulation pattern (1) as determined by Voss [Citation17]. The passage of the carotid canal is small and limited by the basioccipital tube.
Figure 4. Dorsal, ventral, and lateral views of the skull of the holotype R. albujai DMMECN 3719, adult female; and right holotype R. fulviventer BM 96.11.1.3 adult female (B). Scale bar = 4 mm.
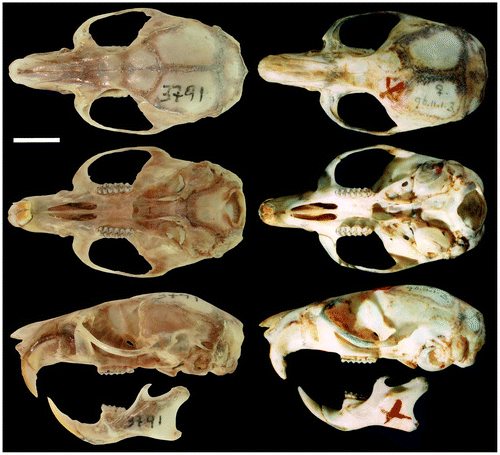
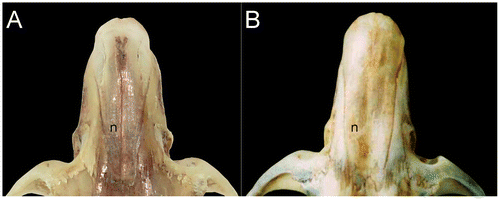
Figure 5. Dorsal views of the nasal of the holotype R. albujai DMMECN 3719 (A), and holotype R. fulviventer BM 96.11.1.3 (B).
The incisors are opisthodont with caudally oriented wear surface; molar cusps alternating slightly between upper and lower teeth; design and topography of the teeth are bunodont; hypoflexus and paraflexus of the molar are separated by the median mure (Figure (A)). Labial cingulum of the upper molars is well developed. Procingulum width of M1 is less than the width across the protocone and paracone. Anteromedian flexus is well defined and deep, which separates anterolingual condyle and anterolabial condyle in similar proportions. Anteroloph of M1 is present and perpendicular to the anterior wall; mesoloph is perpendicular to the median mure; hypoflexus is distinctive and oval; enterostyle and protostyle are undeveloped; paraloph developed and reaches mesoloph; and posterostyle oval. Protoflexus of M2 almost imperceptible, anteroloph and paraflexus are developed; paraloph of M2 oriented obliquely to the mure; hypoflexus and posteroflexus of M2 distinctive. M3 with a distinctive hypoflexus and without a visible metacone.
Figure 6. Right superior molar series (A), inferior right (B) of holotype R. albujai, adult female, DMMECN 3791; (C) and (D) respective orientation of holotype R. fulviventer BM 96.11.1.3, adult female. Abreviation are: anf, anteromedian flexus; hy, hypoflexid; prf, protoflexid. Scale = 0.5.
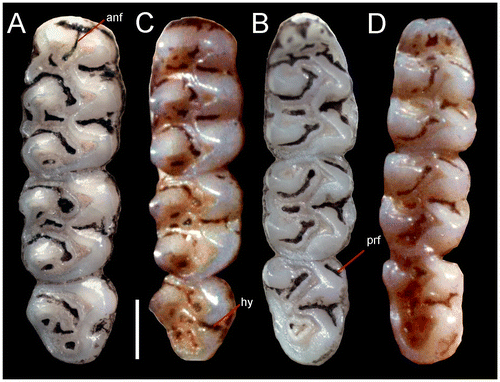
The procingulum of m1 is divided by the anteromedian flexid into two similarly sized conules (Figure (D)). m1 has a developed lingual cingulum; anteroflexid long and narrow; anterolophid and protolophid are present but underdeveloped; metacone rounded; entolophid and mesolophid are perpendicular to the medial mure; entoflexid broad and elongated; posterolophid and posteroflexid are long and narrow; ectolophid absent. The anterolabial cingulum of m2 is visible; protoflexid narrow; hypoflexid large and deep. The anterolabial cingulum of m3 is visible; protoflexid slightly wider; hypoflexid broad and deep; mesoflexid wide.
Masseteric crest level with the leading edge of m1; large mental foramen visible in side view and located ahead of the first molar; capsular process developed forming a well-evident projection; sigmoid notch closed (Figure ). Coronoid process well developed and directed toward the condylar process; condylar process rounded, robust and extends beyond the angular process. The angular notch is slightly concave. Mandibular foramen well developed and elongated transversely to the lunate notch.
Comparison
Comparisons are based on characters presented by Tribe [Citation1,6], Patton et al. [Citation12] and Pacheco and Peralta [Citation37]. Distinguishing between Peruvian species R. modicus and Colombian species R. caucensis, R. similis is based on Tribe [Citation1,6]. Additionally, we present new observations to distinguish between Ecuadorean species Rhipidomys latimanus, R. leucodactylus and skull photographs of the Colombian holotype R. fulviventer (BMNH 96.11.1.3). The characteristics of sister species used in comparison are in parentheses.
Rhipidomys albujai is distinguished from R. latimanus (section leucodactylus sensu Tribe, [Citation1]) by the yellowish brown to orange dorsal coat color (red, orange, yellow, or brown) and ventral white fur with pale yellow hues (creamy white); slightly shorter dorsal hairs 7−10 mm (9−10 mm). The skull of R. albujai is easily distinguished from R. latimanus by the hourglass shape of interorbital region (angular and narrow) and the palate with two to three posterolateral foramina (three to four pairs). Mandible with well-developed capsular process, forming a clear and well defined projection posteriorly to the coronoid process (low, with no high peak, and located anteromedial); the crescent notch is shallow and slightly convex (moderately deep and oval).
Rhipidomys albujai is distinguished externally from R. leucodactylus (section leucodactylus sensu Tribe, [Citation1]) by the yellow to orange-brown dorsal coat (dark brown); ventral white fur with pale yellow hues (white or slightly yellow); metatarsal patch coloring extends to the base of the phalanges of the foot (extending in its entirety); tail with a long tuft of terminal hairs 16–19 mm (15–40 mm); scales cover tail with 14 rows measuring 10 mm longitudinally (12 rows). The skull is broad and rounded (narrow and elongated); narrow face (broad and deep). The mesopterigoidea pit slightly convergent with side walls (parallel). Rhipidomys albujai has a much smaller skull: e. g. condylar-incisive length 28.8–30.1 (37–41), length of upper molar row 4.5–4.8 (6.3–7.1). R. albujai jaw has a well-developed capsular process forming a clear peak (faint peak).
Rhipidomys albujai differs from R. modicus (section leucodactylus sensu Tribe, [Citation1]) of the Andean valleys of northern and central Peru by their smaller size and in the following characteristics: smooth coat (coarser woolly texture); head–body length 118–130 mm (130–165 mm); foot length 21–26 mm (30–38 mm); upper molar row 4.5–4.8 mm (5.0–5.8 mm). Rhipidomys albujai differs from R. caucensis (section fulviventer sensu Tribe, [Citation1], southwest Colombia in Cauca and Huila for its moderately large, head–body length 118–130 mm (<100 mm).
Rhipidomys albujai is distinguished from R. fulviventer (section fulviventer sensu Tribe [Citation1]) of Colombia and Venezuela by the ventral white fur with cream shades (orange); nasals narrow in their posterior and gradually expand forward (wide at its base and expand only slightly); nasolacrimal capsule inflated (deflated); mesopterigoidea small and wide with slightly tapered sides (narrow and parallel sides) and auditory bulbs are slightly globular (enlarged and globular). Protostyle of M1 present (absent); anteromedian flexus is well defined and deep (very reduced); protoflexus of M2 present (absent); m3 with hypoflexid developed and deep (small and superficial). Incsive process of the mandible well developed (reduced).
Rhipidomys albujai is smaller than R. similis (section fulviventer sensu Tribe, [Citation1]) from southwest Colombia, head and body length of 118–130 mm (165 mm) tail length 146–162 mm (185 mm), the tail is covered in small hairs that do not hide the rows of scales on the proximal half of tail (covered with hair that almost hide the rows of scales).
Phylogenetic relationships
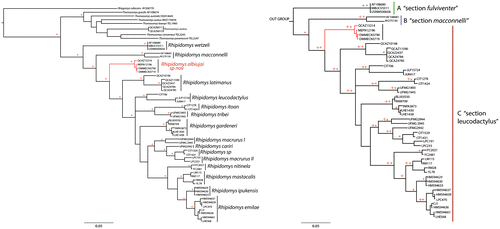
The genus Rhipidomys was recovered as a monophyletic group with high support values (Probabilities Posteiori – 0.99%). The samples of R. wetzeli and R. macconnelli from Venezuela (Figure ) formed two separate clades (A and B), both had a high value support (PP: 0.99), the genetic divergence between both were 16.73% ± 2.59 (in the study). The rest of samples formed a large clade (C) with a high support value (PP: 0.99), within this clade the 12 groups that are recognized species on basis of morphological characters. One group corresponded to the newly described species R. albujai. This specie was recovered as sister group of R. latimanus by a high support value (PP: 0.99) and genetic distance of 11.23% ± 2.21 (Figure ). These specimens were not included in the research of Costa et al. [Citation5], and de la Sancha et al. [Citation3], and are represented by specimens found in southeastern Ecuador in Sangay National Park, Morona Santiago. R. leucodactylus was recovered as a lone group (PP: 0.99) with a genetic distance from other species groups of between 11.32% and 15.47% (Figure ). Rhipidomys itoan and R. tribei were recovered as sister species (PP: 0.98), while R. macrurus turned out to be paraphyletic, with two clades identified as R. macrurus I and R. macrurus II set a high support (0PP: 0.95), while R. cariri was located between the two clades of R. macrurus, likewise specimens identified as Rhipidomys sp. clade turned out to be related to R. macrurus II. This relation presented a high support value (PP: 0.99) (Figure ). Rhipidomys mastacalis y R. nitinela was recovered as a separated groups with high value of support (PP: 0.99) and a genetic distance between them of 9.25% ± 1.26. R. ipukensis was recovered as sister group of R. emilieae (Figure ) with a low value of support (PP: 0.76) as genetic distance was 4.38 ± 0.73.
Figure 7. Left. Phylogeny of Rhipidomys. Tree based on Bayesian Inference, derived from the analysis of the cytochrome b. The * represent posterior probabilities values greater than 0.95. The new species (R. albujai) is red. Right. Expansion of the genus Rhipidomys corresponding section; three clades (A–C) are identified, each corresponding to the three sections which are grouped species of the genus Rhipidomys: A – fulviventer, B – macconnelli, C – leucodactylus.
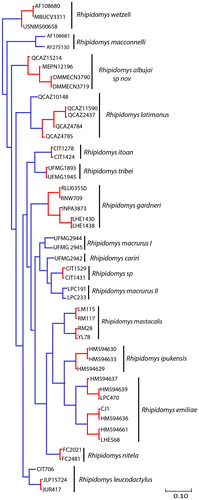
The PTP model identified a total of 23 putative species within the genus Rhipidomys (Figure ). R. albujai was identified as a putative species with a high support value (0.94). Two putative species were identified in the R. latimanus group, the first in northern Ecuador in Carchi province (QCAZ 10148) with high support (1.00), the second group present (QCAZ 2437, QCAZ 4784, QCAZ 4785, QCAZ 11590) a support value under 0.75. For other identified putative species, the support value was between 0.35 and 1 (Appendix 2).
Figure 8. Delimitation of the putative species model based on Poisson Process Tree. Bayesian inference phylogeny based on mitochondrial cytochrome b gene. Monophyletic groups in red indicate a single putative species and the terminal branches are in blue.
Distribution and Ecology. The species is only known from the type locality on the eastern flank of the Andes in Sangay National Park, Morona Santiago, Ecuador at an elevation of 1400–1800 m. The locality is located in the headwaters of the Río Volcán (Figure ) in the Subtropical Oriental ecosystem [Citation37] and was described as a premontane evergreen forest of the southern Cordillera Oriental of the Andes [Citation38]. It is characterized by abundant orchids and bromeliads. The tree canopy reaches 30 m high where the dominant species are romerillo (Prumnopitys montana), cedro (Cedrela montana), copal (Dacryodes peruviana), and royal palm (Dictyocaryum lamarckianum). Rhipidomys albujai was collected near the dominant understory species belonging to Araceae, Melastomataceae, Cyclanthaceae and Bromeliaceae [Citation39]. Two of the three specimens were collected at ground level and one was at 1.8 m above ground level. In June 2014, a female adult (DHMECN 3791) was lactating and a male adult (DHMECN 3790) had scrotal testicles. The adult female was held captive overnight and emitted rapid squeaking vocalizations that intensified with playback. The new species was found with sympatric species: Chilomys sp, Nephelomys auriventer, Oreoryzomys balneator, Hylaeamys tatei, Scolomys melanops, Neacomys spinosus and Rhipidomys sp. [Citation35]; and invasive Rattus rattus [Citation40].
Vocalizations
The call is composed of modulated and constant frequencies. The fundamental frequency is = 1.70 ± 0.24 kHz with a minimum frequency of
= 1.04 ± 0.11 kHz and a maximum frequency of
= 0.78 ± 0.74 s with intervals of
= 8.48 ± 9.78 s; composed of 1–5 notes with a duration of
= 96.12 ± 30.03 ms and intervals of
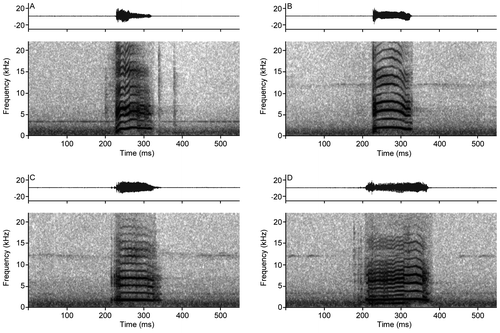
= 57.02 ± 10.27 ms (Table ). The temporal and spectral illustration is presented in Figure . The spectral structure of the calls is not uniform and is composed of ascending, descending, constant and irregular frequencies that are visible in the spectrum of 10–14 harmonics and various partial inharmonics that are more common with irregular modulations (Figure ).
Table 2. Summary of acoustic analysis of the vocalizations of Rhipidomys albujai sp. nov. The analyzed sample (n) corresponds to calls/notes/pulses. The abbreviations utilized in the parameters correspond to: kHz = kilohertz, s = seconds, and ms = miliseconds.
Figure 9. Acoustic parameters analyzed in the call of Rhipidomys albujai. Abbreviations correspond to: ND = Note Duration; IN = Interval between notes; CD = Call Duration; Min = Minimum Frequency; IH = Inharmonic partial; HR = Harmonics; Max = Maximum Frequency.
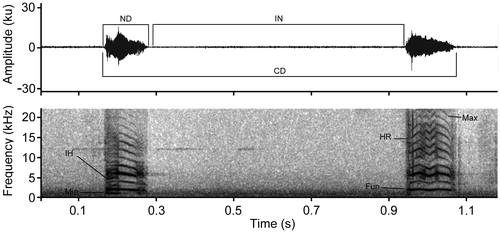
Figure 10. Oscilogram and spectrogram of the calls of Rhipidomys albujai are exemplifying frequency differences. (A) Frequency with modulation absent; (B) Frequency with descending modulation; (C) Constant frequency with modulation in the superior harmonics; (D) Irregular frequencies, with ascending and descending modulations.
Discussion
The phylogenetic analysis identified one new species in southeastern Ecuador, R. albujai, which are supported by a high support value and genetic divergence. The genetic distance from the R. latimanus is 11.23%. This distance is similar to the distance between R. macconnelli – R. wetzeli 16.73% reported in this study which is greater than the distance between R. tribei – R. ipukensis 8.27%, R. itoan – R. emiliae 5.1%, and R. ipukensis – R. nitinela 9.1% [Citation4], and among species of the genus Thomasomys such as T. taczanowskii – T. baeops 7.79%, T. vulcani – T. fumeus 9.13% [Citation42]. We will build on the level of divergence of Cytb proposed by Bardley and Baker [Citation43] and based on the PTP model for our interpretation that identified 23 putative species within the genus Rhipidomys (Figure ). In the phylogenetic tree, three clades can be seen clearly, clade A section macconnelli clade B section fulviventer, clade C section leucodactylus (Figure ). Based on phylogenetic reconstruction, R. albujai forms a sister clade to the group leucodactylus with a high support (0.99), so one might suggest that the species R. albujai is part of the leucodactylus section.
R. albujai has not been mentioned in previous studies of Rocha et al. [Citation4], Costa et al. [Citation5], de la Sancha et al. [Citation3], and García and Gonzales [Citation15]. This species is distributed in the Cordillera Oriental of southeastern Ecuador. Tribe [Citation6], mentions that R. latimanus may be on the eastern flank of the Andes in Ecuador and north eastern Peru based on the first two Ecuadorean specimens found in the province of Napo, near Archidona (BM 34.9.10.112) and a third collected in Peru, Department of Cajamarca, Perico (MCZ17043). This could be explained in two ways, the first specimens Tribe [Citation6], mentioned could actually be specimens of R. albujai that due to morphological complexity of the group were misidentified. The second explanation is that they are sympatric species, and because they are arboreal are difficult to collect. Additionally, there is no record of R. latimanus near R. albujai. In future analyzes, we plan to include more samples of the species of the sections fulviventer and macconnelli, to better understand intraspecific Rhipidomys relations.
Based on morphological characters, we tentatively assign Rhipidomys albujai to the fulviventer section, even though this and the other sections: leocodactylus and macconelli do not necessarily represent monophyletic groups (sensu Tribe [Citation6]). Rhipidomys albujai presents several diagnostic characters of the fulviventer section such as: smooth pelage, yellowish-brown to organish dorsal pelage, whiteish ventral coloration with pale yellow tones and a gray base; postorbital crest hardly perceptible; braincase wide and round; carotid circulation pattern is primitive; protostyle of M1 and protoflexus of M2 present are also generally present in members of the leucodactylus section [Citation6].
The distribution of Rhipidomys albujai is restricted to subtropical forests of Sangay National Park (1400 to 1800 m) in south eastern Ecuador, future collection efforts to the park’s north will help to clarify its range limits. The southern distribution could be limited to the Cordillera del Cóndor. Various specimens collected recently in the Cordillera del Cóndor, 200 km south of Sangay National Park, have similar sizes and coloration to Rhipidomys albujai but are genetically distinct and have cranial differences [Pinto et al. in prep].
The climbing rats of the genus Rhipidomys are considered to be arboreal [Citation6,43–45] associated primarily with tropical and subtropical forests. The well-developed plantar pads, wide feet and long tail are adaptive for an arboreal behavior. Regardless, two specimens were captured on the ground and a third was captured 1.8 m off the ground, which indicates that this species descends to ground level perhaps to forage. Other arboreal Sigmodontinae rodents have also been captured at ground level [Citation46,47]. It is possible that the arboreal habits of Rhipidomys have helped them to elude past collection efforts, furthermore, trapping efforts along the flanks of the Andes, particularly in the Cordillera Oriental have been very limited and have yielded few specimens [Citation48].
The acoustic analysis could help future efforts to understand the natural history of this species and make intraspecific comparison, as well as to better understand Neotropical rodent vocalizations in general.
Sangay National Park with its expansive and intact forest habitats has revealed multiple new species of vertebrates in recent years [e.g. Citation49–52] and novel natural history observations such as those of [Citation40,53–55]. Future studies in Sangay National Park, a UNESCO designated Natural World Heritage site, will likely continue to reveal new species.
Author contributions
JBM and ROB conceived and designed the study. JBM wrote the first draft of the manuscript. NT and DC analyzed the data. PMC and ROB reviewed and improved the manuscript. JBM and ROB collected specimens, calls and sequences.
Associate Editor: Miguel Pinto
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Fulbright Commission (Ecuador).
Acknowledgments
We thank the staff at Sangay National Park (Lower Zone), especially Victor León, for supporting field logistics. We acknowledge Glenda Pozo, Marcelo Sharup, Hernán Orellana, Patricia Bejarano and Germán Tenecota for assisting with field sampling. Reed Ojala-Barbour thanks the Fulbright Fellowship program for funding the field study. This research received institutional support from the Museo Ecuatoriano de Ciencias Naturales and Fundación Naturaleza Kakaram as part of the project ‘Diversity of small vertebrates in two areas of Sangay National Park.’ We thank James L. Patton who generously provided sequences of Rhipidomys from his collections. Santiago R. Ron facilitated laboratory; Santiago Burneo permited access to Rhipidomys latimanus at the mammalogy musem (QCAZ). Christopher Tribe so warmly facilitated and allowed us to use the photographs of the holotype of Rhipidomys fulviventer. Miguel Pinto, Ulyses Pardiñas, and William Teska provided valuable comments and suggestions that improved this manuscript. The Ministerio del Ambiente de Morona Santiago authorized our research permit N°. 05-2014-I-B-DPMS/MAE.
References
- Tribe CJ. The Neotropical rodent genus Rhipidomys (Cricetidae: Sigmodontinae) – a taxonomic revision [dissertation]. London: University College London; 1996.
- Musser GM, Carleton MD. Superfamily Muroidea. In: Wilson DE, Reeder DM, editors. Mammal species of the world: a taxonomic and geographic reference. 3rd ed. Baltimore: Johns Hopkins University Press; 2005; 894–1531.
- de la Sancha N, D′Elía G, Tribe C, et al. Rhipidomys (Rodentia, Cricetidae) from Paraguay: noteworthy new records and identity of the Paraguayan species. Mammalia. 2011;75:269–276.
- Rocha RG, Ferreira E, Costa BMA, et al. Small mammals of the mid-Araguaia River in Central Brazil, with the description of a new species of climbing rat. Zootaxa. 2011;2789:1–34.
- Costa BMA, Geise L, Pereira LG, et al. Phylogeography of Rhipidomys (Rodentia: Cricetidae: Sigmodontinae) and the description of two new species from southeastern Brazil. J Mammal. 2011;92:945–962.10.1644/10-MAMM-A-249.1
- Tribe CJ. Genus Rhipidomys Tschudi, 1845. In: Patton JL, Pardiñas UFJ, D’Elía G, editors. Mammals of South America. Volume 2, Rodents. Chicago: The University of Chicago Press; 2015;583–617.
- López-Fuster MJ, Pérez-Hernández R, Ventura J. Variación craneométrica de Rhipidomys latimanus venezuelae (Muridae, Sigmodontinae) [Variation in cranial metrics of Rhipidomys latimanus venezuelae (Muridae, Sigmodontinae)]. Orsis. 2001;16:111–120.
- Tribe CJ. A new species of Rhipidomys (Rodentia, Muroidea) from North-estearn Brazil. Arq Mus Nac Rio de Janeiro. 2005;63:131–146.
- Sánchez-Hernández J, Lew D. Lista actualizada y comentada de los mamíferos de Venezuela [Current List of and Comments on the Mammals of Venezuela]. Mem Fund La Salle de Cienc Nat. 2012;173–238.
- Albuja L, Arcos R. Lista de mamíferos actuales del Ecuador [Current List of Mammals of Ecuador]. Rev Politéc. 2007;27:7–33.
- Tirira DG. Mamíferos del Ecuador. Guía de Campo [Mammals of Ecuad or: Field Guide]. Ediciones Murciélago Blanco. Publicación Especial de los Mamíferos del Ecuador 6. Quito; 2006.
- Patton JL, da Silva MNF, Malcolm JR. Mammals of the Rio Juruá and the evolutionary and ecological diversification of Amazonia. Bull Am Mus Nat Hist. 2000;244:1–306.10.1206/0003-0090(2000)244<0001:MOTRJA>2.0.CO;2
- Voss RS, Lunde DP, Simmons NB. Mammals of Paracou, French Guiana: a Neotropical lowland rainforest fauna. Part 2. Nonvolant species. Bull Am Mus Nat Hist. 2001;263:1–236.
- Pacheco V. Phylogenetic analyses of the Thomasomyini (Muroidea: Sigmodontinae) based on morphological data [dissertation]. New York, NYThe City University New York; 2003.
- García FJ, Sánchez-Gonzáles. Morfometría geométrica craneal en tres especies de roedores arborícolas neotropicales (Rodentia: Cricetidae: Rhipidomys) en Venezuela. Therya. 2013;4:157–178.
- Sikes RS, Gannon WL. The animal care and use committee of the American Society of Mammalogists. Guidelines for the American Society of Mammalogists for the use of wild mammals in research. J Mammal. 2011;92:235–253.
- Voss RS. Systematics and Ecology of Ichthyomyine rodents (Muroidea): patterns of morphological evolution in a small adaptive radiation. Bull Am Mus Nat Hist. 1988;188:259–493.
- Abdala F, Díaz MM. Anatomía craneana de Akodon albiventer (Rodentia, Muridae, Sigmodontinae). Iheringia, Série Zoologia. 2000;88:33–50.
- Reig OA. A proposed unified nomenclature for the enameled components of the molar teeth of the Cricetidae (Rodentia). J Zool. 1977;181:227–241.
- Reig OA. A new fossil genus of South American cricetid rodents allied to Wiedomys with an assessment of the Sigmodontinae. J Zool. 1980;192:257–281.
- Carleton MD, Musser GG. Systematic studies of oryzomyine rodents (Muridae: Sigmodontinae): Synopsis of Microryzomys. Bull Am Mus Nat Hist. 1989;191:1–83.
- Smithe FB. Naturalist’s color guide. New York, NY: American Museum of Natural History. EEUU; 1975.
- Encycolorpedia. Lista extendida de colores; 2015; [Acceso: 2015 de abril del 5]. Available from: http://encycolorpedia.es.
- Charif RA, Waack AM, Strickman LM. Raven Pro 1.4 User’s Manual. Ithaca, NY: Cornell Lab of Ornithology; 2010.
- Francescoli G. A preliminary report on the acoustic communication in uruguayan Ctenomys (Rodentia, Octodontidae): basic sound types. Bioacoustics. 1999;10:203–218.10.1080/09524622.1999.9753431
- Tokumaru RS, Ades C, Monticelli PF. Individual differences in infant guinea pig pups isolation whistles. Bioacoustics. 2004;14:197–208.10.1080/09524622.2004.9753525
- Brito J, Batallas D. Vocalizaciones de los ratones Reithrodontomys soderstromi y Thomasomys paramorum (Rodentia: Cricetidae) de la provincia de Carchi. Ecuador Avan Cien Ing. 2014;6:B13–B16.
- Bilton DT, Jaarola M. Isolation and purification of vertebrate DNAs. In: Clapp JP, editor. Species diagnostics protocols: PCR and other nucleic acid methods in molecular biology. Totowa, NJ: Humana Press; 1996. p. 25–37.
- Smith MF, Patton JL. The diversification of South American murid rodents: evidence from mitochondrial DNA sequence data for the akodontine tribe. Biol J Linn Soc. 1993;50:149–177.10.1111/bij.1993.50.issue-3
- Arellano E, González-Cozátl FX, Rogers DS. Molecular Systematics of Middle American Harvest mice Reithrodontomys (Muridae), estimated from mitochondrial cytochrome b gene sequences. Mol Biol Evol. 2005;37:529–540.
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. Version 3.02; 2015. Available from: http://mesquiteproject.org.
- Ronquist F, Huelsenbeck JP. MR.BAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574.10.1093/bioinformatics/btg180
- Lanfear R, Calcott B, Ho SYW, et al. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol. 2012;29:1695–1701.10.1093/molbev/mss020
- Zhang J, Kapli P, Pavlidis P, et al. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013;29:2869–2876.10.1093/bioinformatics/btt499
- Brito J, Ojala-Barbour R. Mamíferos no voladores del Parque Nacional Sangay [Non-volant Mammals of Sangay National Park]. Ecuador Pap Avulsos Zool. 2016;56:45–61.10.11606/0031-1049.2016.56.05
- Pacheco V, Peralta M. Rediscovery of Rhipidomys ochrogaster J.A. Allen, 1901 (Cricetidae: Sigmodontinae) with a redescription of the species. Zootaxa. 2011;3106:42–49.
- Albuja L, Almendáriz A, Montalvo LD, et al. Fauna de Vertebrados del Ecuador [Vertebrate Fauna of Ecuador]. Editorial Arial 12. Quito: Instituto de Ciencias Biológicas; Escuela Politécnica Nacional; 2012.
- Guevara J, Josse C. Bosque siempreverde piemontano del Sur de la Cordillera Oriental de los Andes. In: Sistema de Clasificación de los Ecosistemas del Ecuador Continental. Quito: Ministerio del Ambiente del Ecuador; 2013. Pg. 117–119.
- Cerón CE. Dos nuevas formaciones naturales del Ecuador. Rev Cinch. 2001;2:1–4.
- Brito J, Ojala-Barbour R. Presencia de la rata invasora Rattus rattus (Rodentia: Muridae) en el Parque Nacional Sangay, Ecuador. Therya. 2014;5:323–329.10.12933/therya
- Lee TE, Ritchie AR, Vaca-Puente S, et al. Small mammals of Guandera Biological Reserve, Carchi Province, Ecuador and comparative Andean small mammal ecology. Occas Pap Tex Tech Univ. 2015;334:1–20.
- Bradley RD, Baker RJ. A test of the genetic species concept: cytochrome- b sequences and mammals. J Mammal. 2001;82:960–973.10.1644/1545-1542(2001)082<0960:ATOTGS>2.0.CO;2
- Reig OA. Distribuição geográfica e história evolutiva dos roedores muroideos sulamericanos (Cricetidae: Sigmodontinae). Rev Bras Gen. 1984;2:333–365.
- Emmons LH, Feer F. Mamíferos de los bosques húmedos de América tropical. F.A.N., editor. Santa Cruz de la Sierra; 1999;298.
- Bonvicino CR, Oliveira CR, D’Andrea PS, et al. Guia dos Roedores do Brasil. Com chaves para gêneros baseadas em caracteres externos. Rio de Janeiro: Centro Pan Americano de Febre Aftosa – OPAS/OMS, 120 pp: il. ( Série de Manuais Técnicos, 11; 2008).
- Pinheiro P, Hartmann P, Geise L. New record of Rhagomys rufescens (Thomas 1886) (Rodentia: Muridae). Zootaxa. 2004;431:1–11.10.11646/zootaxa.431.1
- Brito J, Teska WR, Ojala-Barbour R. Descripción del nido de dos especies de Thomasomys (Cricetidae) en un bosque alto-andino en Ecuador. Therya. 2012;3:263–268.10.12933/therya-3_2
- Voss RS. A new species of Thomasomys (Rodentia: Muridae) from Eastern Ecuador, with remarks on mammalian diversity and biogeography in the Cordillera Oriental. Am Mus Novit. 2003;3421:1–47.10.1206/0003-0082(2003)421<0001:ANSOTR>2.0.CO;2
- Harvey M, Almendáriz A, Brito J, et al. A new species of Noblella (Anura: Craugastoridae) from the Amazonian Slopes of the Ecuadorian Andes with Comments on Noblella lochites (Lynch). Zootaxa. 2013;3635:1–14.10.11646/zootaxa.3635.1
- Ojala-Barbour R, Pinto CM, Brito J, et al. A new species of shrew-opossum (Paucituberculata: Caenolestidae) with a phylogeny of extant caenolestids. J Mammal. 2013;94:967–982.10.1644/13-MAMM-A-018.1
- Batallas D, Brito J. Nueva especie de rana del género Pristimantis del grupo lacrimosus (Amphibia: Craugastoridae) del Parque Nacional Sangay, Ecuador. Pap Avulsos Zool. 2014;54:51–62.10.1590/0031-1049.2014.54.05
- Yánez-Muñoz MH, Bejarano-Muñoz P, Brito J, et al. Ranas terrestres de los Andes Surorientales del Ecuador II: Una nueva especie de Pristimantis verde espinosa de los bosques montanos del Parque Nacional Sangay (Anura: Craugastoriadae). Avan Cien Ing. 2014;6:B63–B77.
- Lee Jr. TE, Boada-Terán C, Scott AM, et al. Small mammals of Sangay National Park, Chimborazo Province and Morona Santiago Province, Ecuador. Occas Pap Tex Tech Univ. 2011;305:1–14.
- Brito J, Orellana H, Tenecota G. Descripción del nido de Hylaeamys yunganus (Rodentia: Cricetidae) de los Andes del sureste de Ecuador. Avan Cien Ing. 2014;6:B10–12.
- Tirira DG, Camacho MA, Tinoco N, et al. Genus Glyphonycteris Thomas, 1896 (Mammalia: Chiroptera) in Ecuador: first confirmed record of G. sylvestris Thomas, 1896 and a geographical review to G. daviesi (Hill, 1965). Check List. 2016;12:1965.
Appendix 1. Specimens examined.
Rhipidomys latimanus (COLOMBIA): Antioquia, San Jerónimo: FMNH 70237, 88542. (ECUADOR): Cotopaxi, Las Pampas: QCAZ 2437, 4784-85; Pacayaku: QCAZ 11590.
Rhipidomys fulviventer (COLOMBIA): Cundinamarca, Agua Dulce: BM 96.11.1.3 (holotipo).
Rhipidomys leucodactylus. (ECUADOR): Orellana, Cuyabeno: DMMECN 45; Loreto, San José de Payamino: MEPN 6133-35, 6142. Napo, Zancudo Cocha: DMMECN 481, 484, 486; Loja, Zapotillo: DMMECN 2108, 2112-18, 2120-25, 2126-29. Sucumbios, río Cuyabeno: MEPN 6140. Pastaza, Mera: MEPN 6141. Loja, Catamayo: MEPN6137, 6139. Morona Santiago, Macuma, Wisui: MEPN 11960; Untsuants: MEPN 11645.
Appendix 2. Specimens and GeneBank accession numbers for sequences of cytochrome b used in this study. *Sequences generated for this study and **obtained from Dr. JL Patton, Museum of Vertebrate Zoology, UC Berkley.