Abstract
Ecuador’s territory harbors a unique set of species and ecosystems, many of them endemic to the countries’ territory and subject to different sources of threat of anthropogenic origin. Despite national and subnational conservation strategies developed in Ecuador to conserve its biodiversity in the long run, including the National System of Protected Areas (PANE) and the forest conservation incentive program SocioBosque (PSB), further actions are needed to mitigate and reverse the effects of threats for the persistence of biodiversity. This study was designed to identify the most important areas for biodiversity conservation in mainland Ecuador that can contribute to preserving key species (i.e. endemic, threatened) and ecosystems in the wider landscape, thus complementing current conservation efforts (i.e. PANE). Species distribution models and recent maps were used to identify a set of 744 species and 87 ecosystems as surrogates of the country’s biodiversity. Marxan, a systematic reserve selection algorithm was used to identify important biodiversity areas that could represent between 10% and 20% of the remnant distribution of the surrogates. The optimized solution generated by Marxan included 24% (3.64 million ha) of Ecuador′s remnant vegetation, of which 35% is within the current national protected area system and 13% (456 000 ha) are included within SocioBosque communal and private conservation agreements. Major conservation shortfalls of the PANE were concentrated in the Southern Andes, Central Amazonia, and the Central and Southern portions of the Coastal plain. The incidence of complementary criteria to prioritize conservation strategies, related to climate change, ecosystem conversion, carbon and accessibility, and population density change in relation to the important biodiversity areas was heterogeneous among regions. This confirms the need to implement differentiated conservation and sustainable landscape management strategies. Fourteen priority landscapes were identified based on these important biodiversity areas, including remnant ecosystems considered critical for maintaining large-scale connectivity among regions and preservation of restricted range and threatened species. Further work is needed to expand base information about distribution patterns of biodiversity, improve the representation of endemic and threatened species in conservation strategies, and to fully integrate conservation priorities among a wider set of goals in land use planning exercises at different scales.
1. Introduction
Ecuador is the smallest of the 17 megadiverse countries [Citation1] and harbors an astounding number of ecosystems and species, with many endemic species occurring in small geographic ranges [Citation2–4]. Unfortunately, the country has experienced a profound change of its natural habitats. For the year 2014, the country reports an estimated forest area of 12.75 million hectares, a 14% (i.e. 1.83 million ha) reduction with respect to 1990 [Citation5]. The annual deforestation rate for the period 2008–2014 was – 0.37%, equivalent to an average annual loss of 47,000 hectares [Citation5]. These figures, added to the historical changes occurred during the middle decades of the twentieth century (1940–1970), set up a scenario where only ~30% of the original natural vegetation remains in the Coastal plains, 60% in the Andean region, and 88% in the Amazon lowlands. Considering the accelerated pace of habitat transformation, it is important to identify important areas that require land management goals aligned to the preservation of biodiversity from both national and local authorities.
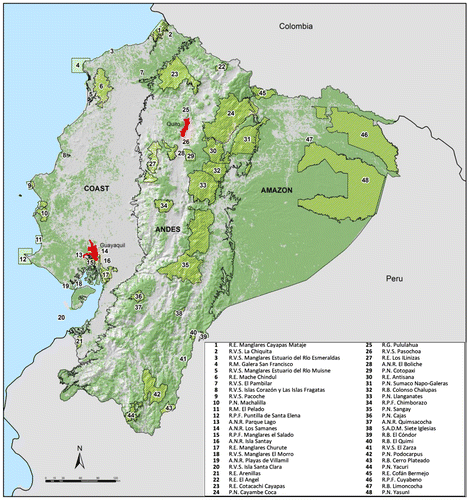
As with most countries that are members of the Convention on Biological Diversity (CBD), Ecuador′s major strategy to conserve its biodiversity has been the establishment of a network of protected areas. Ecuador′s efforts to preserve its natural heritage are noteworthy; currently, the national protected area system (PANE hereafter) is composed of 48 units,Footnote1 which cover nearly 20% of the country landmass (~ 4.3 million ha, Figure ). Establishing a representative protected areas network, in which biodiversity can persist in the long term, has also become a public policy goal [Citation6]. Additionally, the Ministry of the Environment has created a national incentive program of payments for biodiversity conservation (i.e. SocioBosque Program) involving both private and community lands [Citation7] that by the end of 2015 is conserving almost 1.5 million ha.
Figure 1. Study area in mainland Ecuador showing the distribution of remnant vegetation. The internal black lines limit the three major physiographic regions: (1) the Coastal plain, (2) the Andean highlands, and (3) the Amazon. Remnant native vegetation is shown in green, whereas converted areas are presented in gray. The red polygons are the two biggest cities in the country.
Nevertheless, important conservation gaps still remain for species, many of them endemics [Citation8,9], and ecosystems [Citation10]. In many cases, reserves were set up based on ad hoc approaches rather than biogeographic distributional information of the conservation targets. These ad hoc approaches have resulted in a bias in the distribution of the reserve network, with the majority of the reserves occurring at higher elevations, on steeper slopes, and in inaccessible lands of marginal interest for production [Citation11,12]. This leaves important areas for biodiversity conservation outside the PANE and in a context where the creation of new protected areas is less viable as pressure to allocate land for agriculture and other land uses increases [Citation13]. In this context, there is an urgent need to rethink biodiversity conservation strategies to mitigate the effects of climate change, land use – land cover change among other drivers, by maintaining and increasing the connectivity between reserves and regions (e.g. between the Andes and the Amazon) at local to national scales.
In the last decades, considerable advances have been made to develop science-based methodologies to select priority areas for biodiversity conservation [Citation14,15] and secure the provision of environmental services [Citation16–18]. These methods emerged from the field of systematic conservation planning, aimed at defining explicit conservation goals and objective rules for reserve selection in order to obtain maximized benefits (i.e. accomplish conservation goals in as little area as possible). Therefore, these methods can also be applied to identify complementary scenarios aimed at guiding land use planning exercises at local and national scales.
Previous studies focused on identifying priority areas for biodiversity conservation in mainland Ecuador used either species [Citation9] or ecosystems [Citation10,12] as biodiversity surrogates. Thus, complementary studies aimed at identifying areas that ensure both species and ecosystems conservation in mainland Ecuador are of utmost importance in order to inform policy formulation and definition of conservation strategies in the short term [Citation19].
Prioritization exercises are valuable tools for megadiverse countries that need to accommodate for multiple land management goals in heterogeneous landscapes [Citation13]. These exercises provide valuable information for land use planning, as priority areas for conservation can be compared and contrasted with areas important for other goals, and synergies and trade-offs can be made explicit spatially [Citation20]. Further, these important areas for conservation can be contrasted to different in situ conservation strategies already implemented in the country, and identify gaps where further actions, involving local stakeholders and authorities, could be promoted. With this context in mind, this paper addresses the following interrelated questions: (1) What are the most important areas for biodiversity conservation in mainland Ecuador? (2) How does different conservation strategies are contributing to conserving them? (3) Where should additional efforts be developed, considering multiple drivers of environmental change and distinctive land management objectives?
2. Study area
The study area covers the full extent of mainland Ecuador, which corresponds to nearly 25.3 million hectares, and it’s commonly classified into three major physiographic regions: (1) the Coastal plain, (2) the Andean highlands, and (3) the alluvial plains of the Amazon [Citation21,22] (Figure ). Ecuador is a country with high environmental heterogeneity due primarily to its latitudinal position (which determines its tropical condition), the existence of the Andean chain with active strato-volcanoes in its northern portion, the climatic influence of the trade winds of the Atlantic together with the effects of the warm current of the Pacific Ocean and the cold waters of the Humboldt current [Citation22–24]. These environmental setting have promoted the creation of a diverse array of ecological niches that have been filled with one of the most diverse sets of biological organisms on Earth. Ecuador’s biodiversity has been classified in 91 ecosystems [Citation25]and comprises ~17,300 vascular plant species of which nearly 26% are reported as endemic to the country [Citation8,26], 1659 bird species [Citation27], 416 mammals [Citation28], 558 amphibians [Citation29], 450 reptiles [Citation30], and 951 freshwater fishes [Citation31] (Table S1, SOM).
3. Methods
The methodology incorporates three main steps: (1) identification of important areas for biodiversity conservation, using tools from systematic conservation planning [Citation32,33], (2) the assessment of how current conservation efforts (e.g. PANE, SocioBosque) are effectively contributing to conserving important biodiversity areas in mainland Ecuador, and (3) comparing biodiversity priority areas with other areas relevant drivers of environmental change to assess patterns of spatial coincidence at the regional level.
3.1. Conservation surrogates
Two types of biodiversity surrogates were selected: (a) ecosystems, and (b) species. At the ecosystem level, 87 ecosystemsFootnote2 were included based on the recent ecosystem map published by the Ministry of Environment of Ecuador [Citation25]. The area of remnant ecosystems in the referred map comprises 61% of the country’s landmass (~15.2 million hectares); the remaining 39% (~9.65 million hectares) corresponds to transformed areas, mainly agricultural and pasture lands. Only seven ecosystems cover an area larger than 500,000 ha; at the other end, 19 out of 87 ecosystems occur in an area smaller to 10,000 ha (Appendix 1, SOM). At the species level, 744 species were chosen (Appendix 2, SOM), including vascular plants (n = 234), birds (n = 278), amphibians (n = 120), and reptiles (n = 112). Among these, 196 species (26%) are currently listed within the IUCN red list categories (Appendix 3, SOM).
3.1.1. Species distribution models (SDMs)
Species distribution information in the Tropics, including Ecuador, is fragmented and incomplete [Citation34], thus we used species distribution model techniques (SDMs) to project their geographic distribution at the country scale (see Appendix 3 in SOM for details). We applied an asymmetric modeling technique to build up the SDMs since true absence data were not available [Citation35]. SDMs were developed using Maxent, a machine learning algorithm based on the maximum entropy theory, which is used as a general-purpose method for making predictions or inferences from incomplete information [Citation36]. We chose Maxent over other algorithms as it has been tested extensively and has been found to suitably perform as an advanced modeling technique for predicting current and future species niche distributions [Citation37–39]. Maxent estimates species’ distributions by finding the distribution of maximum entropy (i.e. closest to uniform) based on species records and environmental niche variables [Citation40]. SDMs were developed with Maxent 3.3.3e following the parameters settings of [Citation41] and using eight environmental niche variables: Annual Precipitation, Precipitation Seasonality (Coefficient of Variation), Precipitation of Wettest Quarter, Precipitation of Driest Quarter, Temperature Seasonality (standard deviation), Ombrothermic index, Ombrothermic index of the driest two-months of the driest quarter, Terrain ruggedness index, and Topographic exposure index (see Appendix 3 in SOM for details). We modeled only species with at least five distinct locations, as a compromise between model quality and sufficient coverage of limited-range species [Citation42]; species with less than five records were used as an indicator of species distribution in Marxan (see below).
Probability distributions where reclassified to presence–absence data using the ‘prevalence threshold’ [Citation43]. This threshold is defined as the average probability over all input data points used to fit the model (i.e. training presence points). To obtain a better approximation of the species realized niche, the binary distributions were evaluated by comparing them with published literature [Citation29,30,44,45]. Finally, to avoid over-prediction we removed from the species distribution model all non-natural areas based on a recently produced land-cover map of Ecuador [Citation5].
3.2. Conservation goals
Conservation goals are related to the amount of area or number of biodiversity surrogates that should be protected, or in other words, the minimum representation of biodiversity features in protected areas [Citation46]. Goals should be defined based on a quantitative basis that translates concepts of representativeness and persistence in operational use. Additionally, goals should consider constraints related to costs and historical and geographic patterns of land use in a way that results are achievable [Citation47,48].
In this study, conservation goals were set using as a reference 10% of the species or ecosystem remnant distribution to be included in the site-selection algorithm within the selected portfolio. This proportion has been suggested as a conservation target by the Convention on Biological Diversity [Citation49,50] as well as in other conservation planning exercises meant to inform policy formulation [Citation51]. This proportion was adjusted up to 20% for species listed under the IUCN red list categories or for species with a restricted distribution range (<1,000,000 ha); the same proportion was applied to ecosystems with a remnant distribution below 20,000 hectares. This threshold was chosen considering that 30 out of 87 ecosystems (i.e. 35%) had a remnant distribution below 20,000 ha and can be considered the equivalent to a species with a restricted distribution range [Citation52].
The distribution of each species derived by the SDMs was contrasted with the national land cover map for the year 2008 to determine the remnant distribution and the rate of conversion. For ecosystems, a conversion rate was estimated for each one based on the deforestation maps produced by the Ministry of Environment for the period 1990–2008 [Citation5]. A relative low conservation goal was adopted, recognizing that a higher goal is too demanding in terms of the area that needs to be included. In regions with high levels of ecosystem conversion and high level of species richness such as the Ecuadorian coastal plains, a higher threshold would require conserving almost all the remnant vegetation and no prioritization would be required. The conservation targets defined here are lower than previous studies carried out in Ecuador (9, 10), but these represent a compromise between politically and socially viable scenarios to support policy-making at the national level and more robust scenarios to promote biodiversity conservation in the long term.
3.3. Selection of priority areas for biodiversity conservation
Reserve selection algorithms are generally based on the principles of complementarity, singularity, and persistence [Citation15,33,53]. For this study, we used the conservation planning software Marxan V. 1.8.2 [Citation14] which provides analytical support to a range of conservation planning problems, including biodiversity’s representativeness of current conservation schemes as well as the identification of important areas that should be managed with conservation goals.
Marxan aims to find reserve systems that meet biodiversity targets in the least number of planning units (PUs) in which all conservation targets are met, while minimizing cost and addressing spatial design objectives [Citation54]. This is achieved by minimizing the value of an objective function, which is a combination of the cost (usually area) of each planning unit (PU) included in the resulting reserve network, an additional cost related to the fragmentation of the network, and a penalty for unmet biodiversity targets:(1)
where equals the total cost of the reserve network;
is a cost related to the total reserve perimeter length multiplied by a boundary length modifier (BLM). The BLM factor defines the relative importance of the border, where higher values of BLM translate into solutions with lower perimeter (i.e. more aggregated);
is the costs imposed for not adequately representing conservation targets in the solution modified by a Species Penalty Factor (SPF) that increases the penalty for missing the goal for each species. Higher SPF values will influence the solution to increase the total cost up to the conservation goals are met [Citation55]. Following the Marxan good practice guidelines [Citation46], the boundary cost modifier (BLM) was set in 0.55 following a sensitivity analysis in which different BLM values were assessed against the resulting total cost of the solution (i.e. number of PUs) and the total border length. This value represents a good compromise on the compactness level of resulting areas at the national level taking into account differences in the spatial configuration of remnant vegetation in the country. The SPF parameter was set to 1.0 following the same procedure applied for the BLM (Appendix 3, SOM).
Ecuador′s mainland territory was divided into 50,683 hexagonal planning units of 500 ha each. This PU size was selected because it constitutes a reasonable landscape extent to harbor adequate representations of the selected biodiversity surrogates. Additionally, the same PU size was chosen in previous GAP assessments of Ecuador′s reserve network [Citation10]. Each planning unit was set to a standardized cost of 1 since all units have the same area, allowing each PU to have the same probability of being included in the final solution. Additionally, each boundary length was set to 1. Marxan runs were performed using the simulated annealing method, followed by iterative improvement, using 107 iterations and 100 runs. Two output results were generated: the best solution for all runs and the summed solution. The best solution is the solution with the lowest objective function value (i.e. the most efficient solution of the 100 runs) [Citation46]. The summed solution reports the number of times (i.e. frequency) a planning unit was selected as part of a good solution across the100 runs; consequently, it can be used as irreplaceability proxy [Citation46]. The solution generated by Marxan using these settings represents the important areas for biodiversity conservation in mainland Ecuador (the priority biodiversity areas hereafter).
3.4. Assessment of conservation strategies in continental Ecuador
The biodiversity priority areas scenario was overlaid with the PANE to analyze the patterns of coincidence and differences between the two maps. Additionally, the areas under contracts belonging to the SocioBosque Program [Citation7] were overlayed to the biodiversity priority areas with similar purposes.
3.5. Identifying additional criteria to prioritize biodiversity conservation
The biodiversity priority areas scenario was contrasted with a set of maps that represent different drivers of social and environmental change. The purpose was to assess the degree of spatial coherence between the solution generated by Marxan and these maps that are relevant for different and complementary land management strategies and goals. Hence, four binary maps were generated using the same planning units (i.e. hexagons) used for the Marxan runs (Appendix 3 ‘Extended Methods’ in SOM).
The first map corresponds to areas of recent ecosystem conversion in the period 2008–2014 as represented by the maps of land use–land cover change at the national level generated by the Ministry of Environment [Citation5]. Priority areas were defined as those PUs where ecosystem conversion was greater than 40.5 ha (upper quartile of the national distribution of conversion per PU) and the PUs immediately adjacent to these. These areas are used as proxies of deforestation and forest conversion risk in the near future.
The second map corresponds to the turnover of species per PU calculated using the modeled distribution of 667 species of vascular plants, birds, amphibians, and reptiles using SDMs generated in Maxent for the reference period 1950–2000 and for 2050 for six models of the A1B emissions scenario [Citation56]. The turnover rate is a value between 0 (no change in the composition of species) and 100 (complete change between base and future conditions). The threshold used to generate the binary map corresponds to the upper quartile of the mean value per PU of the turnover index (Appendix 3 ‘Extended Methods’ in SOM). The resulting binary map represents areas where high impacts on the structure and composition of biotic communities are expected under novel bioclimatic conditions.
The third binary map depicts areas with high carbon contents in above ground biomass and with a high degree of accessibility. Carbon content was estimated for each PU as the mean value according to the map generated by Saatchi et al. [Citation57]. Accessibility was estimated as the mean travel time in hours to the closest province capital or municipality seat city per PU. Travel time was calculated using an adaptation of a cost weighted distance model developed by Jarvis et al. [Citation58]. Priority areas were identified using the lower quartile for accessibility and the upper quartile for mean carbon content for each of the three regions in continental Ecuador: coastal plains, Andes and Amazon (Appendix 3 ‘Extended Methods’ in SOM). By combining carbon and accessibility, the goal of this map is to represent areas of importance for the provision of ecosystem services (i.e. carbon storage) that could be sensitive to processes of ecosystem conversion or degradation.
Finally, the fourth binary map was generated using values of change in population density in the period 2001–2010 per parish using data from the two last national censuses (http://www.ecuadorencifras.gob.ec/sistema-integrado-de-consultas-redatam/). The percentage of change in population density in relation to the value in 2001 was calculated for each PU, and the priority areas were those with values above the threshold for the upper quartile at the national level (Appendix 3 ‘Extended Methods’ in SOM). This map represents an important underlying driver of ecosystem conversion associated to the increasing demand for ecosystem goods and services associated to increasing human population.
For each map, the percentage of the priority areas at the national level, for each region and in relation to the biodiversity priority areas were calculated. This allowed assessing coherence between areas where land management goals focus on different aspects of the persistence of biodiversity in the long term.
4. Results
4.1. Important areas for biodiversity conservation in mainland Ecuador
The Marxan best solution produced an optimized scenario in which nearly 4.14 million ha were included in the solution (Figure ), equivalent to almost 24% of Ecuador’s remnant vegetation (Table ). The solution includes a higher proportion of the remnant vegetation in the Coast (30%) and the Andes (26%) than in the Amazon region, where the selected PUs represents nearly 20% of the remnant vegetation (Table ). The areas selected in this scenario accomplished the conservation goals for nearly the entire set of biodiversity indicators (n = 840), except for two ecosystems and two species (Appendix 4 in SOM).
Figure 2. Important biodiversity areas in continental Ecuador selected from the best solution in Marxan. Numbers depicts 14 priority landscapes to improve the representation of the priority biodiversity areas within the National Protected Areas System.
Notes: Landscape 1: Mache-Chindul, Landscape 2: Chongon-Colonche mountain range, Landscape 3: Dry and mesic forest of Zapotillo, Landscape 4: Cotacachi-Cayapas, Landscape 5: Illinizas-Mindo-Nambillo mountain range, Landscape 6: Central Andes, Landscape 7: Cajas massif, Landscape 8: Sumaco-Napo Galeras, Landscape 9: Sangay- Siete Iglesias, Landscape 10: Condor Kutukú mountain ranges, Landscape 11: Southern Andes, Landscape 12: Cuyabeno-Pañacocha, Landscape 13: Yasuní, Landscape 14: Pastaza & Santiago Watershed.
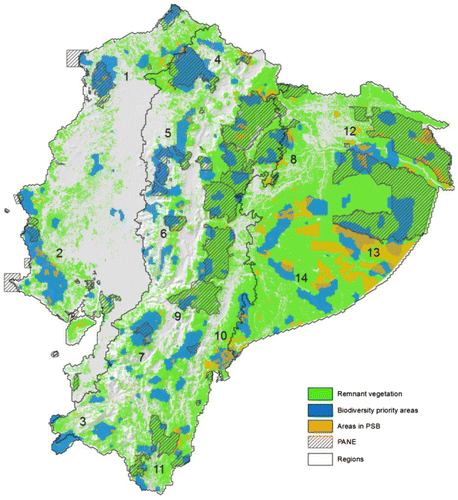
Table 1. Remnant vegetation included in the areas identified as biodiversity conservation priorities by Marxan solutions (blm 0.5; Spf 1.0) per region and at the national level.
Of the 4.14 million ha included in the solution, 3.64 million ha (88%) correspond to natural vegetation and the remaining ~ 500 thousand ha (12%) correspond to mosaics of agricultural uses and scattered fragments of natural vegetation. Almost 40% (0.2 million ha) of these cases are in the Coast, whereas only 8% are located within the Amazon. Thus, evidencing the high degree of fragmentation of the relic vegetation in the Ecuadorian coast (Table ).
4.2. Contribution of national conservation strategies in conserving important biodiversity areas
The planning units (PU) selected in the solution have a moderate spatial correspondence with the current reserve system. From the 3.64 million of hectares, 1.18 million ha (32%) are located inside of an existing reserve, being among the most important Yasuní et al. (Figure ). The remaining 2.36 million ha (65%) included in the Marxan solution are located outside the PANE, depicting a large proportion of important biodiversity areas beyond management of the national protected area system. The most critical areas that demand further conservation efforts are located in Southern Amazonia (Pastaza), the lowland (between the Cotacachi-Cayapas and the Pambilar reserves) and the piedmont forests (between the Illinizas and the Pululahua reserves) of the Chocó region, the xerophytic and mesic forests of the Coastal Mountain range, the mountain forests of the Southern Andes and the Cóndor-Kutukú Mountain ranges (Figure ).
The contribution of the PSB to conserve Ecuador′s mainland biodiversity is noteworthy. Currently, the PSB program protects almost 1.47 million ha of native vegetation of which almost one-third (0.46 million ha) has a spatial correspondence with the biodiversity priority areas (Table ). This is particularly evident in the Pastaza region (southern Amazonia), in the coastal mountain range and in the Cóndor-Kutukú ridges (Figure ).
Table 2. Contribution of the Patrimony of National Protected Areas of Ecuador (PANE by its Spanish acronym) and of the areas under ecosystem conservation agreements of the Socio Bosque Program (PSB by its Spanish acronym) to the protection of remnant vegetation in priority biodiversity areas.
Additionally, we assessed the achievement of ecosystems and species conservation goals by the current national protected areas. In general, the national reserve system had a better performance representing the geographic ranges of the species, while for ecosystems, the PANE revealed significant limitations (Appendix 6 SOM). Of the 87 ecosystems, 41 (47%) are well represented in the existing national protected areas (i.e. more than 20% of its remnant distribution); yet, 24 (28%) are not represented in the PANE, and 22 (25%) are underrepresented (i.e. less than 10% of its remnant distribution is within the reserve network). Most conservation gaps at the ecosystem level are in the southern Andes, including the Kutuku and Cóndor ridges, central Amazonia (Pastaza), and in particular, in the central and southern portions of the Coastal plain (Appendix 6 SOM).
At the species level, the current national protected areas system contains the majority of the species ranges (i.e. according to the SDMs). Nearly 60% of the target species (n = 445) meet the conservation targets used in the analysis (i.e. >20% of their remnant habitat) within the PANE (Appendix 6 SOM). Nevertheless, our results evidence that endemic and restricted-range species (e.g. Chaetocercus berlepschi, Eriocnemis nigrivestis, Pyrrhura orcesi) are not appropriately protected considering the current design and distribution of the national protected areas. From the 744 species selected for this study, 106 (14%) are endemic or have a restricted range (<5000 km2), of which only 4 are well represented in PANE. The remaining 102 endemic species are usually found outside (n = 10) the protected areas or are underrepresented (n = 92) (Appendix 6 SOM). A similar pattern was found for the threatened species. All of the species (n = 8) classified as critically endangered (CR) are underrepresented in PANE, being especially important the cases of Atelopus elegans, A. balios, A. guanujo, and Synallaxis maranonica. From the 25 species classified as Endangered (EN), only one is well represented in the national protected areas network, 21 are underrepresented and three are completely outside the reserve system (i.e. Anolis proboscis, Bothrops lojanus, Geonoma irena). In the same way, from the 36 species listed as Vulnerable (VU), 34 are underrepresented and one is out of the protected areas boundaries (i.e. Wetmorethraupis sterrhopteron).
4.3. Additional criteria to prioritize biodiversity conservation strategies
The binary maps for the four criteria selected (i.e. (a) land use change, (b) species turnover, (c) carbon stocks and accessibility, (d) changes in population density), and the spatial distribution of biodiversity priority areas are presented in Figure . As expected, the spatial patterns of the contextual factors varied markedly among factors and regions. This is related to the complex interactions of the underlying processes that drive potential impacts on the persistence of biodiversity across scales [Citation59]. For example, habitat conversion poses a continuous threat to biodiversity priority areas that distributed in areas with good accessibility (e.g. in the Coast and the southern Andes), whereas potential impacts from climate change related to species turnover reveals patterns associated to the global processes built into the GCMs used (Figure ).
Figure 3. Additional criteria to prioritize biodiversity conservation strategies: (a) recent ecosystem conversion for the period 2008–2014, (b) species turnover due to climate change for the year 2050 under A1B emissions scenario, (c) carbon/accessibility, (d) change in population density 2001–2010. See details in the supplementary material Tables S5 and S6.
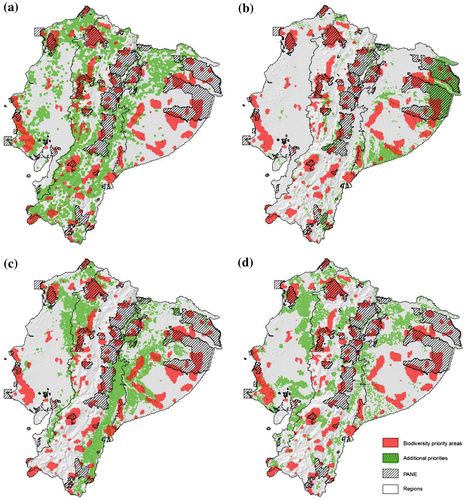
Combining the binary maps for the four criteria with the priority biodiversity areas reveals differentiated levels of spatial coherence (Table ). For example, areas with recent and potentially future ecosystem conversion are prevalent in the Andean region (44%). However, it is in the Coast where the overlap of these areas is highest with the biodiversity priority areas (47%), especially in the northern and southwestern portions of this region. In contrast, the overlap in the Amazon is the lowest (7%) and occurs mostly in the northern portion of this region (Figure ).
Table 3. Percent distribution of priority areas derived from drivers of environmental change in relation to the area of each region and to the distribution of priority biodiversity areas.
Critical areas due to the risk of high species turnover in scenarios of climate change are more prevalent in the Amazon region, especially in the eastern Amazon and represent 28% of the region (Figure ). The overlap with biodiversity priority areas is of ca. 31% in the Amazon. In the Andes, the overlap is 12% and it is concentrated in the central and western areas (Table ). In this last region, the areas of overlap are concentrated in the central Andes and in the external slopes of the western Andean range (Table ).
Areas of high carbon content and high accessibility are more prevalent in the Andes (20%) and the Amazon (18%) regions. The high percentage of these areas in the Andean region is related to the remnant distribution of montane forest ecosystems in the external flanks of the western and eastern Andean ranges (Table ). The highest overlap with biodiversity priority areas (16%) occurs in the Amazon region, especially in the central and western portions of this region (Figure ).
Finally, areas with high changes in population density during the period 2001–2010 were more concentrated in the Coast (22%). Overlaps with priority biodiversity areas likewise were concentrated in this region, whereas the incidence in the Amazon and Andean region is fairly low in both regions as well as at the national scale (Figure , Table ).
5. Discussion
5.1. The important areas for biodiversity conservation in mainland Ecuador
The important biodiversity areas identified in this study are a sound tool to guide biodiversity conservation efforts at national and subnational scales in mainland Ecuador based on the following reasons. Firstly, the use of a robust set of biodiversity surrogates (87 ecosystems and 840 species) allowed us to improve the identification of key biodiversity areas reported in the previous work of Cuesta et al. [Citation10] and Sierra et al. [Citation12]. The use of an important number of endemic and threatened species from different taxa, highlighted areas of specific conservation interest that were overlooked in the aforementioned studies (e.g. Illinizas, Cajas massif).
On the other hand, several of the selected areas outside the PANE by our findings converge with the results of Lessmannn et al. [Citation9], which is pertinent as it confirms the importance of the prioritized areas by two independent exercises carried out with a different set of biodiversity surrogates. Different conservation planning studies have argued that results using ecosystems as biodiversity surrogates may differ from the studies using species as the focal group [Citation60,61]. Previous studies focused on ecosystems in Ecuador [Citation10,12] diverge from a study in which priority areas were set up using species as the only biodiversity surrogate [Citation9]. Thus, our research is a step forward in selecting priority areas for biodiversity conservation, as it combines surrogates, species, and ecosystems. As pointed out by Lessmann et al. [Citation9], combining species- and ecosystem-based studies would improve the decision-makers’ solution portfolio, minimizing biases, and allowing better-informed decisions.
Secondly, not forcing the inclusion of protected areas in the solution allowed us to assess the effective contribution of the PANE and SocioBosque Program, as complementary national conservation efforts, to conserve important areas for terrestrial biodiversity in mainland Ecuador. It highlights priority biodiversity areas that are currently outside the national protected area system (Figure , Table ). These areas should be conceived as conservation priority as in many cases reflects areas with a high concentration of restricted range and threatened species. Thirdly, the use of reserve selection algorithms and its calibration together with the applied sensitivity tests (Appendix 3 ‘Extended Methods’ in SOM) on the results provided us with optimized solutions to identify key conservation areas in a rather small area (3,638,496 ha), particularly those outside the current reserve system (2,458,059 ha) (Table ). Although previous exercises to identify priority areas for biodiversity are not completely comparable, due to the underlying differences in the approach adopted in each case (e.g. conservation goals established), the amount of area prioritized in this study is almost a third lower than previous studies (Table ).
Table 4. Comparison of key elements used by published studies aimed at defining priority biodiversity areas in continental Ecuador.
5.2. How well represented is Ecuador’s ecosystem and species diversity within the current reserve system and the SocioBosque Program?
This study has shown that Ecuador’s protected areas system (PANE) offers protection to an important number of ecosystems and species. Half of the ecosystem diversity is well represented in the natural reserves as well as the majority of the analyzed species (~60%). A similar proportion was documented for the entire bird taxa of Ecuador [Citation62] and in a recent publication Fajardo et al. found a higher ratio in Peru in which 71% of the species are well represented in the current reserve system and using a conservation goal that varied between 5 and 50% of their current distribution. Globally, [Citation53] found that from 11,633 species analyzed, 12% (n = 1423) were considered to be a gap species of all protected areas analyzed. That figure increased to 25% if only protected areas higher than 1,000 ha were analyzed.
Nonetheless, we found the endemic and threatened species are poorly represented in the current national protected areas system. Only four species of the 106 endemic or restricted range species are contained in the reserve system. A similar result was reported by Lessmann et al. who found that nearly 75% of the threatened species were inadequately represented in Ecuador′s reserve system. A similar proportion was reported for the endemic plants of Ecuador [Citation8] evidencing a similar trend when all the species of a specific group are analyzed. Likewise, Fajardo et al. [Citation13] found that all the Critically Endangered, 86% of the Endangered, and 62% of the Vulnerable species are major conservation gaps of the Peruvian reserve system. Our findings are in line with the results of the global gap assessment of Rodrigues et al. [Citation53] who argued that on average ~36% of the 3896 threatened species are not protected by the global protected area network. In the same study, Rodrigues et al. [Citation53] reported that amphibians were the least represented taxon of all taxa in which 50% of the 1543 threatened amphibian species are outside the protected areas’ network. We found a similar pattern in which threatened birds and amphibians were the least protected groups, with 44 and 33% respectively.
Our findings have implications for the strategies needed to cover identified gaps, particularly those of endangered and endemic species which are a high priority [Citation63]. Covering these gaps is not related to a linear increase in areas under conservation since patterns of biodiversity distribution are not homogeneous in space [Citation64,65]. As such, restricted range species reflect aggregate patterns in specific regions resulting from current environmental conditions and past biogeographical and geological events [Citation66]. Thus, priority should be given to preserving the endemism centers where these species occur [Citation67].
The PSB program constitutes an important complementary strategy to secure the preservation of critical biodiversity areas. In the Coast, the PSB protects 8% of the selected priority areas of which the majority are located outside PANE. Especially important are the areas located in the Chongón-Colonche mountain ridge and between Pambilar and the Cotacachi-Cayapas reserve (Figures and ). On the Andes, the PSB program covers ~7% of the priority areas and, in different locations, these areas are located around the protected areas functioning as buffer zones and stepping stones between national protected areas (Figure ). Lastly, in the Amazon, the contribution of PSB increased up to nearly 22%. A great portion of these areas improves substantially the representation of the priority areas in conservation schemes (Figure ). Our findings have strong implications for the biodiversity national strategic planning. A priority action is to secure the long-term sustainability of the PSB incentive program. Of utmost important will be to secure the 500 thousand hectares directly linked to the priority areas for biodiversity conservation.
5.3. Conservation priorities and land use planning
The important biodiversity areas provide a spatially explicit framework to focus conservation efforts at the national level (Figure ). However, in a small country such as Ecuador, increasing competition among alternative land uses requires a flexible framework to formulate land management goals and identify key areas to attain these goals. For example, modeling the potential effects of climate change on the composition of biotic communities reveal that important biodiversity areas in the western and southern Ecuadorian Amazon (Figure ) are key to enhance the resilience of these ecosystems, and that maintaining habitat connectivity can be important to hold up the persistence of species tracking their climatic niches [Citation68].
In the short term, the prevalence of processes leading to ecosystem conversion and degradation highlights the need to protect threatened important biodiversity areas and the biotic communities these harbor. The most threatened ecosystem remnants from this perspective are the ones located in coastal Ecuador, where 47% of the important biodiversity areas identified in this study are close to recent (2008–2014) areas of habitat conversion due to deforestation (Table , Figure ). The risk of future ecosystem conversion is attached to a broader set of socioeconomic factors, such as demographic processes, markets fluctuations and infrastructure expansion [Citation69–71]. Recent growth (2001–2010) in population density reinforce the necessity of concentrating additional efforts in the Coast of Ecuador, where important demographic changes have occurred around important biodiversity areas (Table , Figure ).
Another important pattern is the concentration of ecosystems with high carbon content and potentially high level of threat (modeled by accessibility patterns) surrounding important biodiversity areas and PANE areas in the northern Ecuadorian coast and in the external slopes of the Andean ranges, particularly in the Eastern flank [Citation72] (Figure ). This supports the need to engage in conservation strategies that address drivers of habitat conversion in the context of rapid social and economic change. These areas can become ideal candidates for mechanisms such as REDD+, which target threatened ecosystems with high potential to mitigate the emission of GHG and, at the same time, generate additional benefits through the conservation of biodiversity. The important areas for biodiversity add an explicit link to these types of efforts by providing a specific geographic focus where biodiversity co-benefits can be optimized [Citation73].
Rather than having a unique framework to define a ranking of priority biodiversity areas, the four criteria used in this analysis, suggest that a more nuanced land use planning process is needed (Table , Figure ). Designing conservation strategies should provide opportunities to examine alternative priority scenarios under different assumptions and land use planning goals [Citation74]. For example, setting conservation priorities based on the likelihood of future habitat conversion will generate a different scenario if compared with setting priorities that minimize the potential impacts of climate change on the structure and composition of ecosystems. In this context, the important areas for biodiversity conservation identified in our study provide a common baseline to incorporate synergies and trade-offs into land use planning under different goals and assumptions [Citation75]. These factors are not meant to be exhaustive or representative of all the relevant processes related to the vulnerability of ecosystems. Rather, this recognizes the need to adopt and develop different conservation strategies adjusted to the importance of drivers of change at more local scales to qualify the results from the optimization exercise at the national level.
5.4. Recommendations to conserve the priority biodiversity areas in the short term
The outcome of biodiversity priority areas and the overlay with the protected area system, depicts 14 priority landscapes, equivalent to nearly half (1,782,863 ha) of the area selected in the Marxan solution (Figure ). In the context of scarce resources and limited opportunities to set aside these areas from other land uses, these landscapes should be considered as a first priority due to their specific relevance in preserving Ecuador′s terrestrial biodiversity. In the last 10 years (2005–2014), 15 new areas have been created adding-up nearly 230,000 ha to the national reserve network; unfortunately, only 11% of these areas have a spatial correspondence with the priority areas defined here. It would be important to use these results to guide further protected areas design and declaration. The contrast of the priority landscapes for biodiversity conservation with the current reserve network highlights the following recommendations:
| (1) | Higher proportions (68%) of the priority biodiversity areas are located outside the PANE (Figure , Table ). Thus, a landscape approach is needed to effectively manage the areas located outside the national protected area system. Of primary importance is the contribution of the SocioBosque program to this aim. The areas included in this incentive program helps to protect an additional 13% (456,653 ha) of the priority biodiversity areas. | ||||
| (2) | The protected areas that better capture the spatial distribution of the biodiversity priority areas are Mache-Chindul, Machalilla, Cotacachi-Cayapas, Illinizas, Cajas, Sumaco Napo-Galeras, Yasuní, and Cuyabeno. These protected areas should be prioritized to strengthen their conservation effectiveness and investments allocations. | ||||
| (3) | From the 14 selected landscapes, three are entirely outside the PANE suggesting the urgent needs for designing sound-base actions to preserve these priority biodiversity areas (i.e. Zapotillo – Landscape 3; Condor-Kutukú – Landscape 10; and Pastaza-Santiago watersheds – Landscape 14). | ||||
| (4) | The majority of the reserves of the PANE (74%) are less than 100,000 ha and therefore too small to accomplish their conservation goals of sustaining viable populations and functional ecosystems [Citation76,77]. The majority of those small protected areas are located in the Coast and the Southern Andes (Figure ). In various cases, these reserves are isolated and embedded in an agricultural matrix, making them more prone to edge and fragmentation effects. We propose the expansion of many of the existing reserves together with the design of conservation corridors in which the existing reserves act as core areas. The priorities should be focused on the following landscapes: Mache-Chindul (Landscape 1), Chongón-Colonche mountain range (Landscape 2), Illinizas-Mindo-Nambillo mountain range (Landscape 5), Cajas Massif (Landscape 7), Sangay-Siete Iglesias (Landscape 9). | ||||
| (5) | In many of the prioritized landscapes, the possibility to create new reserves, or modify the limits of the existing ones is challenging. Land tenure conflicts, competing economic interests and local needs that trigger resource use pressure might hamper the suggested modifications [Citation78]. Consequently, placing environmental protection in the mainstream development agenda of local governments (i.e. municipalities and provinces) seems to be at the core of the solution to try to overcome the obstacles to policy implementation. In this context, establishing agreements for decentralizing protected areas is a promising path as it has been documented in the Brazilian state of Bahia [Citation79] among others [Citation80]. The rationale of this approach is to improve the delivery and impact of public sector services by increasing the role of local governments and rural communities in decision-making [Citation81]. In this sense, the conformation of a reserve system managed by local governments aimed at preserving essential environmental services for local dwellers is one of the major goals of the Ecuadorian Ministry of Environment [Citation6]. Complementarily, establishing conservation agreements through conservation incentives programs with private owners could also be an effective strategy, as it has been recently reported for the SocioBosque program [Citation82]. | ||||
| (6) | Since the formulation of the Convention on Biological Diversity (CBD) in 1992, the mission of protected areas has expanded from biodiversity conservation to improving human welfare. The result is a shift in favor of protected areas allowing local resource use [Citation83]. Nevertheless, in Ecuador, the different categories of protected areas still are managed as if their only goal is to preserve nature and preclude human use. In practice, the country has no sustainable-use management areas aimed at sustaining rural livelihoods while preserving biodiverse rich areas. A revision of the protected areas categories must be a priority for the PANE as a way to articulate biodiversity conservation and rural sustainable development. | ||||
| (7) | An effective articulation of the conservation priorities identified and land use planning processes pose important challenges, especially in the definition of sound conservation and sustainable land management strategies. Rather than embedding all the intervening variables in a rigid prioritization and feasibility analysis at a single scale (e.g. national level), a more productive effort should recognize the importance of different contexts at an optimal scale (e.g. landscapes, watersheds) to establish a set of measures that combine conservation of key areas with ecosystem restoration [Citation84] and other sustainable land management strategies in the surrounding landscapes [Citation85,86]. | ||||
Author contribution
Francisco Cuesta, Pi, designed the study, analyzed data, and wrote the paper.
Manuel Peralvo, Co-Pi, designed the study, analyzed data, and wrote the paper.
Andres Merino-Viteri was responsible for the generation and edition of SDMs models, consolidation of amphibians’ species database, and revision of priority areas based on Marxan results
Macarena Bustamante designed the study, revised the priority areas based on Marxan results, and wrote the paper.
Francis Baquero is the GIS analyst of Marxan scenarios, SDMs edition.
Juan F Freile consolidated birds’ database, SDMs edition, revised the priority areas based on Marxan results, and wrote the paper.
Priscilla Muriel consolidated the species’ database and consolidated vascular plants’ database, SDMs edition, and revised the priority areas based on Marxan results
Omar Torres-Carvajal consolidated reptiles’ database, SDMs edition and revised the priority areas based on Marxan results.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the German International Cooperation GIZ (GIZ, www.giz.de) through its Project GESOREN – Gestión Sostenible de los Recursos Naturales [grant number 83122981] and was coordinated with the Biodiversity Department of the Ministry of Environment of Ecuador.
Supplemental data
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/23766808.2017.1295705.
TNEO_1295705_Supplementary_material.zip
Download Zip (268.3 KB)Acknowledgments
This research has been developed thanks to the financial support of the German International Cooperation (GIZ, www.giz.de) through its Project GESOREN. FC and MP have also received additional funding to write the manuscript from the Andean Forest Program conducted by CONDESAN and Helvetas Swiss Intercooperation, and funded by the Swiss Agency for Development and Cooperation (SDC) and from the EcoAndes Project conducted by CONDESAN and funded by the Global Environment Facility (GEF) through the United Nations Environment Program. All the authors acknowledge the Pontifical Catholic University of Ecuador and its Biological Sciences School for their support and access to the biological collections stored there. All the arguments made in this article remain as the responsibility of the authors.
Associate Editor: Veerle Vanacke
Notes
1. The PANE at 2015 is composed of 48 continental reserves. In 2013, when the study was carried out, the PANE was composed of 47 units.
2. The version of the ecosystem map used in this study is from May 2012, date in which the map distinguished 87 ecosystems. The final version of the map, published in 2013, describes 91 units.
References
- Mittermeier RA, Myers N, Thomsen JB, et al. Biodiversity hotspots and major tropical wilderness areas: approaches to setting conservation priorities. Conserv Biol. 1998;12:516–520.10.1046/j.1523-1739.1998.012003516.x
- Bass MS, Finer M, Jenkins CN, et al. Global conservation significance of Ecuador’s Yasuní National Park. PLoS ONE. 2010;5:e8767.10.1371/journal.pone.0008767
- Brooks TM, Mittermeier RA, da Fonseca GAB, et al. Global biodiversity conservation priorities. Science. 2006;313:58–61.10.1126/science.1127609
- Brooks TM, Mittermeier RA, Mittermeier CG, et al. Habitat loss and extinction in the hotspots of biodiversity. Conserv Biol. 2002;16:909–923.10.1046/j.1523-1739.2002.00530.x
- MAE. Análisis de la deforestación en el Ecuador Continental 1990–2014. Quito, Ecuador: Ministerio del Ambiente del Ecuador; 2015.
- MAE. Estrategia Nacional de Biodiversidad del Ecuador y Plan de Acción (2015–2020). Quito, Ecuador: Ministerio del Ambiente del Ecuador; 2015.
- de Koning F, Aguiñaga M, Bravo M, et al. Bridging the gap between forest conservation and poverty alleviation: the Ecuadorian Socio Bosque program. Environ Sci Policy. 2011;14:531–542.10.1016/j.envsci.2011.04.007
- León-Yánez S, Valencia R, Pitman N, et al., editors. Libro rojo de las plantas endémicas del Ecuador [Red List of the endemic plants of Ecuador], 2a. ed. Quito: Pontificia Universidad Católica del Ecuador; 2011 .
- Lessmann J, Muñoz J, Bonaccorso E. Maximizing species conservation in continental Ecuador: a case of systematic conservation planning for biodiverse regions. Ecol Evol. 2014;4:2410–2422.10.1002/ece3.2014.4.issue-12
- Cuesta-Camacho F, Peralvo M, Ganzenmüller A et al. Identificación de vacíos y prioridades de conservación para la biodiversidad terrestre en el Ecuador Continental. Reporte Técnico. Fundación EcoCiencia. Quito, Ecuador: The Nature Conservancy, Conservación Internacional y Ministerio del Ambiente del Ecuador; 2006. p. 1–70.
- Campos F, Peralvo M, Cuesta F et al. Análisis de vacíos y áreas prioritarias para la conservación de la biodiversidad en el Ecuador Continental. Instituto Nazca de Investigaciones Marinas, EcoCiencia, Ministerio del Ambiente. The Nature Conservancy, Conservation International, Proyecto GEF-Ecuador: Sistema Nacional de Áreas Protegidas. Quito: Birdlife International y Aves & Conservation; 2007, p. 1–83.
- Sierra R, Campos F, Chamberlin J. Assessing biodiversity conservation priorities: ecosystem risk and representativeness in continental Ecuador. Landscape Urban Planning. 2002;59:95–110.
- Fajardo J, Lessmann J, Bonaccorso E, et al. Combined use of systematic conservation planning, species distribution modelling, and connectivity analysis reveals severe conservation gaps in a Megadiverse Country (Peru). PLoS ONE. 2014;9:e114367.10.1371/journal.pone.0114367
- Ball IR, Possingham HP, Watts M. Marxan and relatives: software for spatial conservation prioritisation. Spatial conservation prioritisation: quantitative methods and computational tools. Oxford, UK: Oxford University Press; 2009.
- Sarkar S, Pressey R, Faith DP, et al. Biodiversity conservation planning tools: present status and challenges for the future. Ann Rev Environ Res. 2006;31:123–159.10.1146/annurev.energy.31.042606.085844
- Chan KMA, Shaw MR, Cameron DR, et al. Conservation planning for ecosystem services. PLoS Biol. 2006;4:e379.10.1371/journal.pbio.0040379
- Nelson E, Mendoza G, Regetz J, et al. Modeling multiple ecosystem services, biodiversity conservation, commodity production, and tradeoffs at landscape scales. Front Ecol Environ. 2009;7:4–11.10.1890/080023
- Snäll T, Lehtomäki J, Arponen A, et al. Green infrastructure design based on spatial conservation prioritization and modeling of biodiversity features and ecosystem services. Environ Manage. 2016;57:251–256.10.1007/s00267-015-0613-y
- Groves CR, Jensen DB, Valutis LL, et al. Planning for biodiversity conservation: putting conservation science into practice. Bioscience. 2002;52:499–512.10.1641/0006-3568(2002)052[0499:PFBCPC]2.0.CO;2
- Raudsepp-Hearne C, Peterson GD, Bennett EM. Ecosystem service bundles for analyzing tradeoffs in diverse landscapes. Proc Nat Acad Sci. 2010;107:5242–5247.10.1073/pnas.0907284107
- Sauer W. El mapa geologico del Ecuador. Quito, Ecuador: Editorial Universitaria; 1957.
- Wolf T. Geografía y geología del Ecuador, publicada por orden del supremo gobierno de la República. Leipzig: F. A. Brockhaus; 1892.
- Borchsenius F. Patterns of plant species endemism in Ecuador. Biodivers Conserv. 1997;6:379–399.10.1023/A:1018312724137
- Skov F, Borchsenius F. Predicting plant species distribution patterns using simple climatic parameters: a case study of Ecuadorian palms. Ecography. 1997;20:347–355.10.1111/eco.1997.20.issue-4
- MAE. Sistema de clasificación de los ecosistemas del Ecuador continental. Quito, Ecuador: Ministerio del Ambiente del Ecuador; 2012. p. 1–136.
- Jørgensen PM, Ulloa CU, León B, et al. Regional patterns of vascular plant diversity and endemism. In: Herzog SK, Martínez R, Jørgensen PM, et al., editors. Climate change and biodiversity in the tropical Andes. Brasilia: IAI; 2011. p. 192–203.
- Santander T, Freile JF, Loor-Vela S, et al., editors. Important bird areas Americas – Priority sites for biodiversity conservation. Ecuador: BirdLife International Quito; 2009.
- Pinto CM, Nicolalde DA. MammaliaWebEcuador. Version 2015.0, P.U.C.d.E. Museo de Zoología, editor; [cited Sep 2015]. Available from: http://zoologia.puce.edu.ec/Vertebrados/mamiferos/MamiferosEcuador/
- Ron SRJM, Guayasamin MH, Yanez-Muñoz A, et al. AmphibiaWebEcuador. Version 2014.0. Museo de Zoología, Pontificia Universidad Católica del Ecuador; [cited Sep 2014]. Available from: http://zoologia.puce.edu.ec/Vertebrados/anfibios
- Torres-Carvajal O, Salazar-Valenzuela D, Merino-Viteri A. 2014. ReptiliaWebEcuador. Versión 2014.0. Museo de Zoología QCAZ, Pontificia Universidad Católica del Ecuador; [cited Sep 2015]. http://zoologia.puce.edu.ec/Vertebrados/reptiles/reptilesEcuador (September 2015).
- Barriga R. Lista de peces de agua dulce e intermareales del Ecuador. 2012;30:83–119.
- Kukkala AS, Moilanen A. Core concepts of spatial prioritisation in systematic conservation planning. Biol Rev. 2013;88:443–464.10.1111/brv.2013.88.issue-2
- Margules CR, Pressey RL. Systematic conservation planning. Nature. 2000;405:243–253.10.1038/35012251
- Feeley KJ, Silman MR. Keep collecting: accurate species distribution modelling requires more collections than previously thought. Divers Distrib. 2010;17:1132–1140.
- Thomassen HA, Fuller T, Buermann W, et al. Mapping evolutionary process: a multi-taxa approach to conservation prioritization. Evol Appl. 2011;4:397–413.10.1111/j.1752-4571.2010.00172.x
- Elith J, Phillips SJ, Hastie T, et al. A statistical explanation of MaxEnt for ecologists. Divers Distrib. 2011;17:43–57.10.1111/ddi.2010.17.issue-1
- Smith AB, Santos MJ, Koo MS, et al. Evaluation of species distribution models by resampling of sites surveyed a century ago by Joseph Grinnell. Ecography. 2013;36:1017–1031.10.1111/j.1600-0587.2013.00107.x
- Phillips SJ, Dudík M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008;31:161–175.10.1111/j.0906-7590.2008.5203.x
- Costa GC, Nogueira C, Machado RB, et al. Sampling bias and the use of ecological niche modeling in conservation planning: a field evaluation in a biodiversity hotspot. Biodivers Conserv. 2010;19:883–899.10.1007/s10531-009-9746-8
- Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006;190:231–259.10.1016/j.ecolmodel.2005.03.026
- Ramirez-Villegas J, Cuesta F, Devenish C, et al. Using species distributions models for designing conservation strategies of Tropical Andean biodiversity under climate change. J Nat Conserv. 2014;22:391–404.10.1016/j.jnc.2014.03.007
- Anderson RP, Martínez-Meyer E. Modeling species’ geographic distributions for preliminary conservation assessments: an implementation with the spiny pocket mice (Heteromys) of Ecuador. Biol Conserv. 2004;116:167–179.10.1016/S0006-3207(03)00187-3
- Liu C, White M, Newell G. Selecting thresholds for the prediction of species occurrence with presence-only data. J Biogeogr. 2013;40:778–789.10.1111/jbi.2013.40.issue-4
- IUCN. The IUCN red list of threatened species. Version 2015-3; [cited Oct 2015]. Available from: http://www.iucnredlist.org
- Ridgely RS, Greenfield PJ. The birds of Ecuador. Vol. I. Status, distribution and taxonomy. Vol. II. Field guide. Ithaca (NY): Cornell University Press; 2001.
- Ardron JA, Possingham HP, Klein CJ. Marxan good practices handbook. Vancouver: Pacific Marine Analysis and Research Association; 2008.
- Pressey RL, Bottrill MC. Opportunism, threats, and the evolution of systematic conservation planning. Conserv Biol. 2008;22:1340–1345.10.1111/cbi.2008.22.issue-5
- Pressey RL, Possingham HP, Day JR. Effectiveness of alternative heuristic algorithms for identifying indicative minimum requirements for conservation reserves. Biol Conserv. 1997;80:207–219.10.1016/S0006-3207(96)00045-6
- Mace GM, Baillie JEM. The 2010 biodiversity indicators: challenges for science and policy. Conserv Biol. 2007;21:1406–1413.10.1111/j.1523-1739.2007.00830.x
- Nicholson E, Collen B, Barausse A, et al. Making robust policy decisions using global biodiversity indicators. PLoS ONE. 2012;7:e41128.10.1371/journal.pone.0041128
- Svancara LK, Brannon RJ, Scott M, et al. Policy-driven versus evidence-based conservation: a review of political targets and biological needs. Bioscience. 2005;55:989–995.10.1641/0006-3568(2005)055[0989:PVECAR]2.0.CO;2
- Rodríguez JP, Rodríguez-Clark KM, Baillie JEM, et al. Establishing IUCN red list criteria for threatened ecosystems. Conserv Biol. 2011;25:21–29.10.1111/j.1523-1739.2010.01598.x
- Rodrigues ASL, Akçakaya HR, Andelman SJ, et al. Global gap analysis: priority regions for expanding the global protected-area network. Bioscience. 2004;54:1092–1100.10.1641/0006-3568(2004)054[1092:GGAPRF]2.0.CO;2
- Ball IR, Possingham HP. MARXAN (V1. 8.2). Queensland: The University of Queensland; 2000.
- Game E, Grantham H. Marxan user manual: for Marxan version 1.8.10. Queensland, Australia: University of Queensland, St. Lucia; 2008.
- Cuesta F, Merino-Viteri A, Baquero F, et al. Escenarios de impacto del cambio climático en la Biodiversidad contenida en el sistema nacional de áreas protegidas del Ecuador. Quito, Ecuador: Condesan, PUCE; MAE; GIZ; 2015.
- Saatchi SS, Harris NL, Brown S, et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc Nat Acad Sci. 2011;108:9899–9904.10.1073/pnas.1019576108
- Jarvis A, Touval JL, Schmitz MC, et al. Assessment of threats to ecosystems in South America. J Nat Conserv. 2010;18:180–188.10.1016/j.jnc.2009.08.003
- Geist H, McConnell W, Lambin EF, et al. Causes and trajectories of land-use/cover change. In: Lambin EF, Geist H, editors. Land-use and land-cover change. Berlin Heidelberg: Springer; 2006. p. 41–70.10.1007/3-540-32202-7
- Larsen FW, Bladt J, Balmford A, et al. Birds as biodiversity surrogates: will supplementing birds with other taxa improve effectiveness? J Appl Ecol. 2012;49:349–356.
- Pressey RL. Conservation planning and biodiversity: assembling the best data for the job. Conserv Biol. 2004;18:1677–1681.10.1111/j.1523-1739.2004.00434.x
- Freile JF, Rodas F. Conservación de aves en Ecuador: ¿cómo estamos y qué necesitamos hacer? Cotinga. 2008;29:48–55.
- Stattersfield AJ, Crosby MJ, Long AJ, et al. Endemic bird areas of the world: priorities for biodiversity conservation. V 7. Cambridge, UK: Birdlife International; 2005.
- Gentry AH. Tropical forest biodiversity: distributional patterns and their conservational significance. Oikos. 1992;63:19–28.10.2307/3545512
- Kreft H, Sommer JH, Barthlott W. The significance of geographic range size for spatial diversity patterns in Neotropical palms. Ecography. 2006;29:21–30.10.1111/j.2005.0906-7590.04203.x
- Kreft H, Jetz W. Global patterns and determinants of vascular plant diversity. Proc Nat Acad Sci. 2007;104:5925–5930.10.1073/pnas.0608361104
- Venter O, Fuller RA, Segan DB, et al. Targeting global protected area expansion for imperiled biodiversity. PLoS Biol. 2014;12:e1001891.10.1371/journal.pbio.1001891
- Duque A, Stevenson PR, Feeley kJ. Thermophilization of adult and juvenile tree communities in the northern tropical Andes. Proc Nat Acad Sci. 2015;112:10744–10749.10.1073/pnas.1506570112
- Geist HJ, Lambin EF. Proximate causes and underlying driving forces of tropical deforestation. Bioscience. 2002;52:143–150.10.1641/0006-3568(2002)052[0143:PCAUDF]2.0.CO;2
- Balthazar V, Vanacker V, Molina A, et al. Impacts of forest cover change on ecosystem services in high Andean mountains. Ecol Ind. 2015;48:63–75.10.1016/j.ecolind.2014.07.043
- Vanacker V, Govers G, Barros S, et al. The effect of short-term socio-economic and demographic change on landuse dynamics and its corresponding geomorphic response with relation to water erosion in a tropical mountainous catchment, Ecuador. Landscape Ecol. 2003;18:1–15.10.1023/A:1022902914221
- Baynard CW, Ellis JM, Davis H. Roads, petroleum and accessibility: the case of eastern Ecuador. GeoJournal. 2013;78:675–695.
- Panfil SN, Harvey CA. REDD+ and biodiversity conservation: a review of the biodiversity goals, monitoring methods, and impacts of 80 REDD+ projects. Conserv Lett. 2016;9:143–150.10.1111/conl.2016.9.issue-2
- Pierce SM, Cowling RM, Knight AT, et al. Systematic conservation planning products for land-use planning: Interpretation for implementation. Biol Conserv. 2005;125:441–458.10.1016/j.biocon.2005.04.019
- Phelps J, Friess DA, Webb EL. Win–win REDD+ approaches belie carbon–biodiversity trade-offs. Biol Conserv. 2012;154:53–60.10.1016/j.biocon.2011.12.031
- Fahrig L. How much habitat is enough? Biol Conserv. 2001;100:65–74.10.1016/S0006-3207(00)00208-1
- Noss RF, Carroll C, Vance-Borland K, et al. A multicriteria assessment of the irreplaceability and vulnerability of sites in the greater Yellowstone ecosystem. Conserv Biol. 2002;16:895–908.10.1046/j.1523-1739.2002.01405.x
- Messina JP, Walsh SJ, Mena CF, et al. Land tenure and deforestation patterns in the Ecuadorian Amazon: Conflicts in land conservation in frontier settings. Appl Geogr. 2006;26:113–128.10.1016/j.apgeog.2005.11.003
- de Oliveira JAP. Implementing environmental policies in developing countries through decentralization: the case of protected areas in Bahia, Brazil. World Dev. 2002;30:1713–1736.
- Lane MB. Affirming new directions in planning theory: comanagement of protected areas. Soc Nat Res. 2001;14:657–671.10.1080/08941920118212
- Lutz E, Caldecott JO. Decentralization and biodiversity conservation. Washington: World Bank Publications; 1996.
- Jones KW, Holland MB, Naughton-Treves L, et al. Forest conservation incentives and deforestation in the Ecuadorian Amazon. Environ Conserv. 2016;44:1–10.
- Naughton-Treves L, Holland MB, Brandon K. The role of protected areas in conserving biodiversity and sustaining local livelihoods. Ann Rev Environ Res. 2005;30:219–252.10.1146/annurev.energy.30.050504.164507
- Lamb D, Erskine PD, Parrotta JA. Restoration of degraded tropical forest landscapes. Science. 2005;310:1628–1632.10.1126/science.1111773
- Scherr SJ, McNeely JA. Biodiversity conservation and agricultural sustainability: towards a new paradigm of 'ecoagriculture' landscapes. Philos Trans R Soc B Biol Sci. 2008;363:477–494.10.1098/rstb.2007.2165
- Harvey CA, Komar O, Chazdon R, et al. Integrating agricultural landscapes with biodiversity conservation in the Mesoamerican hotspot. Conserv Biol. 2008;22:8–15.10.1111/j.1523-1739.2007.00863.x
