Abstract
Neotropical dry forests are among the most diverse and threatened ecosystems worldwide. The extent and knowledge of Neotropical dry forests are quite heterogeneous with forests located in the Ecuadorian province especially diverse, threatened and poorly studied. In this work, we review patterns and conservation status of biodiversity, ecosystem processes and human perception of tropical dry forest of the Ecuadorian province. We found that patterns of biodiversity, endemism and conservation status are generally poorly studied. Overall, these forests provide habitat for at least 900 species including trees, birds, mammals, amphibians and reptiles. On average 18% of these species (range 6–25%) are endemic to the region and 25% (3–50%) are recognized as threatened. Little is known about groups such as invertebrates, fish, fungi, or herbaceous plants, and as well as about processes generating and maintaining critical ecosystem functions. Available literature points out the importance of positive ecological interactions such us plant–frugivore and plant–plant facilitation interactions in maintaining the regeneration dynamics of these forests. Faced by the formative state of knowledge about basic biodiversity patterns and ecological functions, the implementation of ecosystem risk assessment under the IUCN criteria for the Red List of Ecosystems may offer constructive means to organize, integrate and advance existing knowledge and conservation priorities for dry forests of the Ecuadorian province. With examples of existing conflicts between people and protected areas, we emphasize the importance of consultation and involvement of local communities in the development of conservation measures including new protected areas. Lastly, we reflect on some encouraging examples where ecosystem goods and services provided by these forests may be used in a sustainable manner, contributing to local communities’ income and preserving biodiversity. In this regard, we highlight how the interaction between research and innovation together with local management may lead to sustainable development and, thus, encourage these sectors to work together for the conservation of dry forests of the Ecuadorian province.
Introduction
Tropical dry forests (TDFs), perhaps the most threatened terrestrial biome in the tropics [Citation1,2], sustain a high diversity of plants and animals and ecological functions that supply a multitude of ecosystem goods and services to local human populations [Citation3]. These forests encompass a variety of form and composition across three continents [Citation4] and have long resisted the imposition of a clear definition [Citation3,5]. However, two simple characteristics define TDFs globally: forests occurring in the tropics (temperatures above 20º, complete absence of frosts) that experience marked rainfall seasonality with several months of drought (5–8 months) [Citation6,7] generating a particular ecosystem structure and physiognomy that is shared among TDFs [Citation2,5]. For many of the same reasons, tropical dry forests have typically coincided with areas of dense human inhabitation and land conversion [Citation2], which now render this ecosystem extremely threatened [Citation8,9].
Despite its more acute exposure to anthropogenic threats, and great relevance for human settlements over millennia, TDF remain poorly studied, especially in comparison to their latitude neighbors’, tropical wet and rainforests [Citation3,8]. From 1945 to 2004, for each research paper published in the Web of Science portal about tropical dry forests, six were published on tropical wet forests [Citation10]. In a more recent review (1997–2014), this ratio has slightly increased to one research article on tropical dry forests for each 4.5 in rainforest, but still only 10% of the research work performed in the tropics is about dry ecosystems (Cayuela et al. unpublished results) Within those articles concerning dry forests from the Neotropical region, one also finds a stark imbalance. Two countries, México and Costa Rica have attracted ~70% of the scientific research (Figure ), while other areas in Central and South America are virtually unknown. For example, TDFs in Bolivia are the subject of less than 5% of research articles despite representing 25% of the overall extension of Neotropical dry forests [Citation10].
Figure 1. Number of studies published in the Web of Science in the period 1900–2015 (search performed on December 2015) using the words ‘Tropical dry forest’ and ‘country name*’ in the title, keywords and abstract. Numbers above each bar represent the percentage of studies in each country.
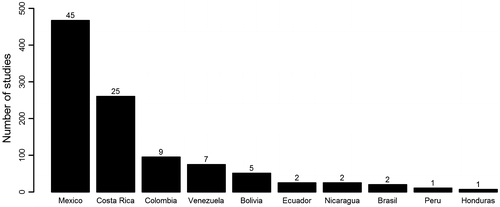
Around the border of Ecuador and Peru exists another highly diverse and threatened area of tropical dry forest, variously known as the Equatorial Pacific Region, Tumbesian region, or Tumbes-Piura [Citation11–15]. Despite the great importance of such forests in terms of biodiversity and endemism [Citation11,14], these have been largely neglected in the literature on Neotropical dry forests [Citation2,11]. According to the above-mentioned analysis, the area is represented by only 3% of tropical dry forest studies (Figure ). Part of this area is the focus of our review. According to the most recent update on Neotropical bioregionalization, the area target of this review has been denominated the Ecuadorian province [Citation16]. Hereafter, we will refer to the target area and ecosystem of this study as tropical dry forest of the Ecuadorian Province (EP-TDF).
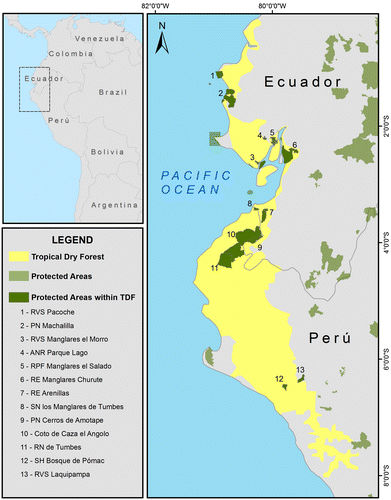
The EP-TDF conforms one the ecological sub-units of the Tumbes-Chocó-Magdalena hotspot [Citation17]. It covers a narrow strip of coastal hinterland delimited by the Pacific Ocean and the lower slopes of the Andes, extending over a distance of ~900 km from southeast Ecuador to northeast of Peru (Figure ). The TDF-EP experiences a profound influence of the cold and dry Humboldt current and it is intermittently influenced by the warm and wet El Niño current, both determining the strong seasonality encountered in this area [Citation18].
Figure 2. Location of dry forest of the Ecuadorian province in Peru and Ecuador, highlighting protected areas.
The extent of the EP-TDF has been vastly deforested to the present day, where only about 30% is thought to remain relatively undisturbed (25 and 5% in Ecuador and Peru respectively) and only 10% of it is under some kind of protective land designation. These historical facts, together with the current rate of deforestation, 5–10% for the period 2000–2012, [Citation9,19] suggest a bleak future for TDF-EP, unless significant changes to land use and development patterns actions are implemented. The future wellbeing of the EP-TDF is tightly bound to the livelihoods of the many people who live in and around them. To satisfy the needs of people living in EP-TDF is a necessary condition for the present and future conservation of this ecosystem; this may be achieved only if all stakeholders, people, managers and researches work together.
The aim of this review is to identify gaps in knowledge of biodiversity and ecosystem functioning of EP-TDF. We also consider how the nearby human populations, historically the principal degrading agent of the forests, currently perceive the forest and how they may contribute to a more sustainable future. We think the consideration of all this information together, may guide future efforts to generate the necessary knowledge to perform an ecosystem risk evaluation according to the IUCN criteria for the Red List of Ecosystems. The topics discussed herein resulted from a dynamic multidisciplinary workshop hosted by Universidad Técnica Particular de Loja (Ecuador) in November 2015. The workshop brought together researchers from various disciplines and conservationists, both concerned and enthusiastic about the future of the EP-TDF.
This review is articulated around several themes dealing first with the interaction of people and forests. EP-TDF has been occupied and utilized by various peoples for millennia [Citation3]. Now that only 30% of the former extent of EP-TDF still remains, the management of these interactions is more important than ever. Researchers should seek to generate the necessary knowledge to help overcome such challenges [Citation20]. More information about the biological diversity in EP-TDF should help identify and develop management options for conservation and sustainable use. We discuss key advances and gaps in knowledge about biodiversity patterns and ecological functions and processes, and also we tackle some possible synergies between ecological research and local management that may contribute to the long-term conservation of the EP-TDF.
Tropical dry forest of the Ecuadorian province, protected areas and people
Tropical dry forests of the Ecuadorian province are found mainly along the coastal hinterland (Figure ). Therein, the gentle topography, ease of access, fertile soils and moderate climate have favored the presence and settlement of human populations for around five to nine millennia [Citation21]. The expansion of the human settlements and activities such as natural resource extraction, agriculture and extensive grazing have combined to render EP-TDF one of the most threatened ecosystems in the world [Citation2]. Activities involving deforestation in particular, have fragmented extant habitat directly impacting species richness and biodiversity [Citation22,23].
Official protection of remnant EP-TDF habitat has progressed through a variety of regional, national and internationally recognized reserves. In Peru, ~10% of remnant dry forest (2337 km2) is protected by means of six protected areas (Figure ), most of them included in the UNESCO Northwest Biosphere Reserve (NBR), which covers an area of 231 km2. In Ecuador, dry forests occupy more than 6000 km2 [Citation9]. Until 2014, only 2–4% was protected in conservation reserves [Citation9,24]; however, this percentage recently rose to an estimated 8% of the extant tropical dry forests, following the declaration of Bosque Seco Biosphere Reserve (BSBR). The BSBR declaration was facilitated by the efforts of various municipal authorities to define zones within their jurisdiction critical for conservation and ecosystem connectivity. The Municipal Reserves that resulted from the zoning process today largely comprise the core area of the BSBR. The recognition of the conservation value and objectives, and the legal status established by the declaration were positive and necessary steps, and the establishment of protected areas has contributed to slow down deforestation rates. However, the designation of protected areas does not in itself assure long-term biodiversity conservation (reviewed in [Citation25]). The management of protected areas in developing countries is often complex because of poverty, population growth and the lack of resources available for effectively protecting those areas [Citation25].
Machalilla National Park, the only Ecuadorian national park that includes dry forests, exemplifies the conflicting interests of local communities and national park management objectives. The park was created in 1979 without much consultation with local communities. Consequently, illegal activities have continued to occur in the park in the face of deficient law enforcement [Citation26, Cervera et al. unpublished results]. Engaging local communities in environmental policies is vital for the success of conservation initiatives [Citation27,28]. A good example of consultation and involvement can be found in the remnant dry forests around Zapotillo county (Loja Province) in the south of Ecuador. In 2000, Nature and Culture International (NCI) began working to create a network of private ecological reserves in collaboration with local communities. In this area, owing to the remoteness and climate, the dry forests had not been cleared like in most other areas in the country. With virtually no infrastructure present, and a climate hostile to agricultural use, the area consisted of a few owners of vast haciendas, whose principal activity of extensive goat and cow farming depended on the dry forest ecosystem for stock feed and shelter. Two co-managed reserves of 18 km2 La Ceiba, and Cazaderos, were established and now form part of the BSBR. Importantly, local people contributed to the establishment of protection policies and regulations for forest use. Given that farmers make a living from goats and the animals in turn forage in the forest, farmers easily realized the forest needed to be protected to assure their livelihood [Nature and Culture International, https://natureandculture.org/]
The perceptions of people living nearby La Ceiba, one of the co-managed reserves in Zapotillo, were recently compared with those of people living near Arenillas Ecological Reserve [Citation29]. Arenillas Ecological Reserve (REA) was a military base and exclusion zone since the 1970s due to its strategic location on the Peru border. In 2001, responsibility for this area was transferred to the environmental ministry and the ecological reserve was established. The perception of the protected areas was usually positive in the case of La Ceiba (provision of ecosystem services, economic income by tourism) and negative in the case of Arenillas Ecological Reserve (restrictions of use, indifference) [Citation29]. Like Machalilla National Park, it seems that the people around REA lack of sense of attachment and ownership of the objectives of the protected area. Precisely these elements seem critical in the achievement of conservation and sustainable management goals. Given that dry forests are a highly threatened ecosystem in the region, and are under-represented in protected areas at the global scale, additional forest reserves may be required to establish a more robust conservation network, both from government and local initiative, such as the examples of the Zapotillo area and the Dry Forest Biosphere Reserve. However, given the tight relationship between human settlements, livelihoods and the dry forests, initiatives to identify and declare new reserves should follow a genuine process of consultation of local stakeholders, to assure that the parks may contribute to effective and lasting conservation of the ecosystem [Citation27,28].
Biodiversity patterns in the dry forests of the Ecuadorian province: what do we know?
To review the existing studies about biodiversity in the region, we performed a search in the Web of Science portal, with the key words ‘tropical dry forest’ AND Ecuador* OR Peru*. The search (performed 18 April 2016) yielded 30 records, of which most were about plants (60%), being almost 50% of those articles about woody plants. The remaining articles were about birds (17%), mammals (13%), insects (10%) and reptiles (3%). According to this evidence, further research on the biodiversity patterns in the EP-TDF region is required for all groups, but particularly for herpetofauna, fish, fungi and invertebrates, which would be expected to be the most species rich of all. Here, we provide some insights about the current knowledge on species numbers, endemism and conservation status for groups of woody plants, birds, mammals, reptiles and amphibians.
Overall, EP-TDF is home of at least ~900 species of trees, birds, mammals, amphibians and reptiles with an average of 18% being endemic (range 25–6%) as shown in Table . This is a high level of endemism similar to that reported for the whole Tumbes-Chocó-Magdalena region and other Neotropical biodiversity hotspots [Citation11]. Around 30% of these endemic species are under some threatened IUCN category (Table ). Amongst tree species, the richest taxonomic group of those well documented, 20% of the species are endemic to EP-TDF [Citation13,30]. There are 318 recorded tree species belonging to 180 genera and 54 families. Five families (Leguminosae, Malvaceae, Boraginaceae, Cactaceae and Moraceae), constitute around 40% of all recorded species; of those, the Leguminosae alone accounts for 22% of species. Most families, however, are represented by a few species and 13 families by a single species. Woody species richness, considering trees and shrubs (DBH ≥ 5 cm), is around 600 trees ha−1 (538–625), while when including individuals over 10 cm of DBH (thus probably excluding shrubs) density values become lower and much variable with a range of 22–524 trees ha−1 (Table ).
Table 1. Number of endemic species and their percentages.
Table 2. Diversity and density typical values of the dry forests of the Ecuadorian province. Note the minimum diameter threshold.
Birds are the second richest taxonomic group with an estimated 250–300 species. Tropical dry forest of the Ecuadorian Province (in the case of birds, better known as Tumbesian region) sustains one of the highest concentrations of species with restricted distribution in South America [Citation14] with 21% of species endemic to the region, and 30% under some IUCN threatened category [Citation24,31–33], (Table ). Although with much lower levels of endemism, the mammal community is represented by 200 species, with 12 considered endemic [Citation34,35]. Two squirrel species deserve special mention in this regard, the Guayaquil squirrel (Simosciurus stramineus), which is endemic to the coast of Ecuador and distributed from Esmeraldas to Guayas, and the white-napped squirrel (Simosciurus nebouxii), which is found in the dry forests of Arenillas, El Oro and north Piura, in Peru [Citation36].
Amphibians and reptiles are also important components of biodiversity in EP-TDF, contributing almost 100 species of which around 20% are endemic (see Table ). For reptiles, most species are snakes and lizards, one species of blind snake, Amphisbaena occidentalis, and one turtle (Rhinoclemmys annulata) [Citation37]. The conservation status of the herpetofauna, especially reptiles, remains uncertain as the taxonomy is not well resolved. For instance, the circumscription of several species of snakes and lizards has recently been revised following phylogenetic studies [Citation38]. This is the case of the species Lampropeltis micropholis, Erythrolamprus epinephelus, and Alsophis elegans [Citation39,40]. Taxonomic change is rather common for tropical dry forest species, not just in the case of the herpetofauna [Citation41], but also for other groups such as birds and mammals [Citation42,43].
Aside from taxonomic revisions, the estimated ranges of fauna from EP-TDF are also frequently modified as field studies continue to produce new records. Some of these recent examples are new records for the lizard Macropholidus ruthveni [Citation44] and the Tumbes tyrant Tumbezia salvini [Citation45] both recently confirmed in the Zapotillo area, Province of Loja in southeastern Ecuador. These examples highlight that more research is required to understand patterns of biodiversity in these forests, and to understand the effects of deforestation and anthropogenic activities on the distribution and species conservation status [Citation46]. As more work is conducted, often with improved techniques to study the distribution and abundance of species (especially cryptic and elusive ones), it is to be expected that changes in the conservation status will arise. A better understanding of both taxonomy and conservation status is required, considering that Neotropical dry forests constitute a hotspot of defaunation. A global phenomenon that is especially worrisome in the tropics [Citation47].
Ecosystem function and risk in tropical dry forests of the Ecuadorian province: a gap of knowledge
Given the immature state of knowledge of some individual taxa in EP-TDF, and the urgency of their conservation, a dual focus on improving species knowledge, and ecosystem level function and risk, should be pursued. Based on the policy success of the IUCN red list of species, an international movement to develop criteria for the recognition of globally threatened ecosystems was initiated in the early 2000s to create the IUCN Red List of Ecosystems [Citation48]. Importantly, an ecosystem level approach underlines the fundamental structural and engineering role that common species play in the maintenance of ecosystems and the functions that they generate [Citation49], as well as gathering essential information about the distribution and status of rare and threatened species. In recent years, the ecosystem risk assessment criteria and method of application have been improved [Citation50,51] and could now be considered for application in EP-TDF. These criteria include: rates of ecosystem loss and decline at the distribution range level, restricted distributions continuously declining or threatened, environmental degradation, degradation of biotic processes (e.g. mutualistic interactions) and a quantitative risk of ecosystem collapse as integrative indicator of all the above mentioned.
Recent work has established that from the processes involved in forest regeneration, those more vulnerable to human impact are pollination and seed dispersal mediated by mutualistic interactions between plants and animals [Citation52]. The disruption of mutualisms is considered as a key indicator of the IUCN criteria ‘biotic processes degradation’ [Citation50,51]. Accordingly, in this section we highlight some insights on key ecological interactions, such as endozoochorus seed dispersal and plant regeneration dynamics, that may aid in the development of a risk assessment approach for the ecosystem.
In spite of not being the most abundant dispersal syndrome, zoochorous species account for around 50% of woody plants in Neotropical dry forests [Citation53,54], especially in well preserved patches [Citation55]. This is also the case of EP-TDF, where up to 54% of woody species present a zoochorous dispersal syndrome [Citation54]. Following disturbance, zoochorous species tend to be the last ones to recover [Citation56], leading to impoverished plant communities and also reduced food resources for animals and less nutrient supply to soils [Citation57]. Despite this, relatively little is currently known about seed dispersal performed by animals in EP-TDF. A search of the subject performed in the Web of Science portal (on 19 November 2015) with the terms (‘frugivory’ AND ‘tropical dry forest’) OR (‘endozoochory’ AND ‘tropical dry forest’) OR (‘epizoochory’ AND ‘tropical dry forest’) lead to 18 publications. Most were about primates, birds and bats, with very few about reptiles or herbivorous mammals. Surprisingly, there were no studies dealing with carnivorous mammals as seed dispersers, despite the recognized importance of this group as seed dispersal agents (reviewed in [Citation58]). An investigation of the importance of the last herbivore-megafauna inhabiting EP-TDF, the white tailed deer (Odocoileus virginianus peruvianus) and also a mesocarnivore, the sechuran fox (Lycalopex sechurae Thomas, 1900) is currently underway (Jara-Guerrero et al., Escribano-Avila et al. unpublished data). These two mammals, seem to be important dispersers as they consume and deposit seeds of at least 15 woody species, most of which have been shown to be viable after passing through deer and fox gut. Birds, primates, carnivores and bats are known as relevant dispersers in mostly all kinds of ecosystems with fleshy fruited species. However, recent findings on dispersal patterns in other Neotropical dry forest areas highlight the relevance of non-typical dispersers, such as iguanas [Citation59,60] and small terrestrial mammals [Citation61,62]. Such work leads to the expectation that these groups of animals may be also relevant in the dispersal process of TDF-EC and thereby warrant further research.
Once a seed is dispersed, many processes act to influence germination, early recruitment and further stages until recruits reach reproductive adulthood [Citation63]. Plant-plant interactions may play a key role in determining the spatial structure and diversity of plant communities in the EP dry forests [Citation11]. Various authors propose that certain individuals or species may facilitate the recruitment of other species by modifying the surrounding micro-environmental conditions (e.g. [Citation64,65]). This hypothesis was tested in an Ecuadorian dry forest by means of evaluating the behavior of species in relation to their ability to attract or repel diversity, considered as a facilitation or competition effect respectively. At short distances (≤10 m), about 30% of species behaved as accumulators of diversity, or more still (over 50%), when the relationship was assessed between juveniles [Citation66]. Some ongoing work in dry scrublands of the Ecuadorian Province show higher recruitment success under the fringe of the canopy of the dominant shrub compared with open areas. Furthermore, the relationship was modified by environmental stress; seedlings were more dependent on facilitation in more stressful habitats (Quintana et al. unpublished data). Although this demonstration pertains to scrubland rather than forest, and should be further developed to test generality, these findings suggest that positive interactions between plants may be relevant in the maintenance of biodiversity and regeneration dynamics in EP-TDF. A greater understanding of these dynamics and interactions in the dry forest ecosystem should prove useful to guide management and strategic documents, such as restoration plans.
Future challenges
Towards bi-national protection and management of the tropical dry forest of the Ecuadorian province
Tropical dry forests of the Ecuadorian Province extend over Ecuador and Peru. Whilst both countries have established protected areas to preserve the forest, better coordination could improve the connectivity of those reserves, and consider the management of strategic areas in the matrix between them. Both nations have declared their intent to establish a Bi-National UNESCO reserve that would protect 17000 km2 including dry forests and associated ecosystems (i.e. mangroves and wetlands). This will allow the coordination of conservation aims and management procedures. One of the proposed targets of this bi-national reserve is to support the development of a conservation culture, whereby local communities may generate economic activities, such as ecotourism (e.g. birdwatching, trekking or bicycle tours to enjoy the Guayacan (Tabebuia spp) blooming). These options will lead to widen the sources of economic income in the local region and, at the same time, promote the conservation of dry forests. This initiative is already supported by both countries and has been recently presented in the 4th World Congress of Biosphere Reserves-UNESCO, held in Lima, Peru on March 2016. We suggest that the work being performed in this regard may be useful and inspiring for both nations to collaborate towards an ecosystem risk assessment process for EP-TDF. The process of documentation, and analysis of risks and opportunities for the forests, should foster improved international coordination and collaboration in designing a sustainable future for the region and its people.
Joint projects of research, innovation and management may lead to achieve sustainable development in equatorial pacific dry forests
Protected areas are a pivotal instrument toward the conservation of EP-TDF, however human use of these forests, even in protected areas, is the rule rather than the exception. Thus, it is necessary to seek compromises that permit sustainable development of economic activities by local communities alongside biodiversity conservation. Research and innovation may be of great help in this regard. A good example is the Palo Santo project (this is the common name given in Ecuador to Bursera graveolens (Burseraceae)), which proposes to manage one of the most widespread and dominant trees in EP-TDF. A valuable essential oil is obtained from B. graveolens, typically from the trunk, and thus the exploitation of this economic resource is at the expense of forest conservation. The chemistry department of Universidad Técnica Particular de Loja (UTPL) developed a methodology to extract the essential oil from the fruits instead of the trunk; this was the first step of the Palo Santo project that brought together local communities, government institutions and local NGOs, such as the aforementioned NCI. The Palo Santo project resulted in the commercialization of B. graveolens essential oil to the Brazilian environmental enterprise Natura used in their natural cosmetic products. Most project management is carried out by the community association Bolívar Tello Cano. This is probably the most remarkable example of how the natural resources of dry forests may be used in a sustainable manner, in addition to create social networking and support local economies. Actually, in 2014 the Bolívar Tello Cano community association was awarded the United Nations Development Programme’s Equator Prize, in recognition of the Palo Santo project’s outstanding effectiveness in reducing poverty through biodiversity conservation and sustainable business practices [Citation67].
Researchers could seek to play a similarly creative and catalytic role in the development of sustainable initiatives from the products and management of stingless native melaponid bees (Melipona spp). Ancestral knowledge of the management of indigenous Melipona bees has almost been lost due to habitat deterioration and the introduction of the European bee (Apis melifera). However, there are still some areas in which meliponeculture still remains active, and these examples may serve as a base to recover and expand this ancestral knowledge and the resources that Melipona bees may provide. Researchers working together with local people could assist in recovering and codifying ancestral knowledge for its dissemination to other interested local communities, and at the same time increase scientific information on the biology and conservation of Melipona bees [Citation68].
Concluding remarks
Tropical dry forests of the Ecuadorian Region are home of at least 900 species of trees, birds, mammals, amphibians and reptiles. Around one in five of these species are endemic to the region, and many of them are threatened with extinction. These numbers, already startling, are likely to be conservative, as much remains to be learned about several groups such as mammals, fish, fungi and especially invertebrates, the most diverse living group in the world. It is possible that many species may go extinct before being discovered owing to the ongoing deforestation and fragmentation condition of EP-TDF. However, the growing number of protected areas with a strong focus on co-management with local people together with initiatives of sustainable development are encouraging and signaling the way forward. The IUCN process for the Red Listing of Ecosystems should serve as inspiration and direction for targeted research in EP-TDF. This research should address identified gaps in knowledge required for conservation, but also contribute to the development of sustainable options for local communities that are required to facilitate the long term conservation of EP-TDF.
Associate Editor: Elisa Bonaccorso.
Author contributions
Gema Escribano-Ávila designed, led the manuscript and performed most of scientific literature review; David Duncan provided guidance with the manuscript structure, content section and English language; Laura García Cervera contributed in art work. All authors actively collaborated in the literature revision of their field of expertise and manuscript writing (People-Forest interactions: Joahana Briceoño, Bruno Paladines; Mammal patterns: Laura García Cervera and Diego Lizcano; Bird patterns: Leonardo Ordoñez; Herpetofauna patterns: Luis Amador; Plant patterns and seed dispersal: Andrea Jara-Guerrero, Carlos Iván Espinosa, Gema Escribano-Ávila and Violeta Parés-Jimenez). All authors critically reviewed the manuscript.
Funding
This work was supported by the Research Program of Universidad Técnica Particular de Loja through the project CCNN-1054. Gema Escribano-Ávila was supported by the Prometeo Project of the Secretary for Higher Education, Science, Technology and Innovation of Ecuador (SENESCYT).
Disclosure Statement
No potential conflict of interest was reported by the authors.
References
- Hoekstra J, Boucher T, Ricketts T, et al. Confronting a biome crisis: global disparities of habitat loss and protection. Ecol Lett. 2005;8:23–29.
- Miles L, Newton AC, DeFries RS, et al. A global overview of the conservation status of tropical dry forests. J Biogeogr. 2006;33:491–505.10.1111/jbi.2006.33.issue-3
- Murphy PG, Lugo AE. Ecology of tropical dry forest. Annu Rev Ecol Evol Syst. 1986;17:67–88.10.1146/annurev.es.17.110186.000435
- Pennington RT, Lavin M, Oliveira-Filho A. Woody plant diversity, evolution, and ecology in the tropics: perspectives from seasonally dry tropical forests. Annu Rev Ecol Evol Syst. 2009; 40: 437–457.10.1146/annurev.ecolsys.110308.120327
- Dexter KG, Smart B, Baldauf C, et al. Floristics and biogeography of vegetation in seasonally dry tropical regions. Int For Rev. 2015;17(S2):10–32. doi:10.1505/146554815815834859.
- Mooney HA, Bullock SH, Medina E. Introduction. In: Bullock SH, Mooney HA, Medina E, editors. Seasonally dry tropical forests. Cambridge: Cambridge University Press; 1995. p. 1–8.10.1017/CBO9780511753398
- FAO. Global ecological zones for FAO forest reporting: 2010 update. Food and agriculture organization of the United Nations Forest resources assessment working paper 179. [cited 2016 Apr 20]. Available from: http://www.fao.org/docrep/017/ap861e/ap861e00.pdf
- Janzen DH. Management of Habitat fragments in a tropical dry forest: growth. Ann Mo Bot Gard. 1988;75:105–116.10.2307/2399468
- Portillo-Quintero CA, Sánchez-Azofeifa GA. Extent and conservation of tropical dry forests in the Americas. Biol Cons. 2010;143:144–155.10.1016/j.biocon.2009.09.020
- Sánchez-Azofeifa GA, Quesada M, Rodríguez JP, et al. Research priorities for Neotropical dry forests. Biotropica. 2005;37:477–485.
- Espinosa CI, De la Cruz M, Luzuriaga AL, et al. Bosques tropicales secos de la región Pacífico Ecuatorial: diversidad, estructura, funcionamiento e implicaciones para la conservación. Ecosistemas. 2012;21:167–179.
- Peralvo M, Sierra R, Young KR, et al. Identification of biodiversity conservation priorities using predictive modeling: an application for the Equatorial Pacific region of South America. Biodivers Conserv. 2007;16:2649–2675.10.1007/s10531-006-9077-y
- Linares-Palomino R, Kvist LP, Aguirre-Mendoza Z, et al. Diversity and endemism of woody plant species in the Equatorial Pacific seasonally dry forests. Biodivers Conserv. 2010;19:169–185.10.1007/s10531-009-9713-4
- Best B, Kessler M. Biodiversity and conservation in Tumbesian Ecuador and Peru (Vol. 218). Cambridge: BirdLife International; 1995.
- Dinerstein E, Olson DM, Gram DJ, et al. Una Evaluación del estado de conservación de las eco-regiones de America Latina y Caribe. Washington, DC: Banco Internacional de Reconstrucción y Fomento/Banco Mundial; 2011.
- Morrone JJ. Biogeographical regionalisation of the Neotropical region. Zootaxa. 2014;3782(1):1–110.10.11646/zootaxa.3782.1
- Mittermeier RA, Turner WR, Larsen FW, et al. Global biodiversity conservation: the critical role of hotspots in biodiversity hotspots. Berlin: Springer; 2011. p. 3–22.
- International Conservation. Biological diversity in Tumbes-Chocó-Magdalena. 2011. [cited 2015 Dec 4]. http://www.eoearth.org/view/article/150631/
- Hansen MC, Potapov PV, Moore R, et al. High-resolution global maps of 21st-century forest cover change. Science. 2013;342:850–853.10.1126/science.1244693
- Sanchez-Azofeifa GA, Kalacska M, Quesada M, et al. Need for integrated research for a sustainable future in tropical dry forests. Conserv Biol. 2005;19:285–286. DOI:10.1111/j.1523-1739.2005.s01_1.x
- Piperno DR, Dillehay TD. Starch grains on human teeth reveal early broad crop diet in northern Peru. Proc Natl Acad Sci USA. 2008;105:19622–19627.10.1073/pnas.0808752105
- Gillespie TW, O’Neill K, Keppel G, et al. Prioritizing conservation of tropical dry forests in the Pacific. Oryx. 2014;48:337–344.10.1017/S0030605313000264
- Tapia-Armijos MF, Homeier J, Espinosa CI, et al. Deforestation and forest fragmentation in south Ecuador since the 1970s – losing a hotspot of biodiversity. PLoS ONE. 2015;10(9):e0133701. DOI:10.1371/journal.pone.0133701
- Albuja L, Almendáriz R, Barriga LD, et al. Fauna de Vertebrados del Ecuador. Quito: Escuela Politécnica Nacional, Instituto de Ciencias Biológicas; 2012.
- Naughton-Treves L, Holland MB, Brandon K. The role of protected areas in conserving biodiversity and sustaining local livelihoods. Annu Rev Environ Resour. 2005;30:219–252.10.1146/annurev.energy.30.050504.164507
- Fiallo EA, Jacobson SK. Local communities and protected areas: attitudes of rural residents towards conservation and Machalilla National Park, Ecuador. Environ Conserv. 1995;22:241–249.10.1017/S037689290001064X
- Sheppard SR. Participatory decision support for sustainable forest management: a framework for planning with local communities at the landscape level in Canada. Can J For Res. 2005;35:1515–1526.10.1139/x05-084
- Brooks JS, Waylen KA, Mulder MB. How national context, project design, and local community characteristics influence success in community-based conservation projects. Proc Nat Acad Sci USA. 2012;109:21265–21270.10.1073/pnas.1207141110
- Briceño J, Iniguez-Gallador V, Ravera F. Factores que influyen en la apreciación de servicios eco-sistémicos de los bosques secos del sur del Ecuador. Ecosistemas. 2016;25(2):000–000.
- Leal-Pinedo JM, Linares-Palomino R. Los bosques secos de la Reserva de Biosfera del Noroeste (Perú): diversidad arbórea y estado de conservación. Caldasia. 2005;27(2):195–211.
- Freile JF, Carrión JM, Prieto-Albuja F, et al. La ornitología en Ecuador: un análisis del estado actual del conocimiento y sugerencias para prioridades de investigación. Ornitología Neotropical. 2006;17:183–202.
- Ridgely R, Greenfield P. Aves del Ecuador. Volumen II. Guía de Campo. Academia de Ciencias Naturales de Filadelfia. Fundación de Conservación. Quito: Jocotoco; 2006.
- BirdLife International. Endemic Bird Area factsheet: Tumbesian region. 2016. [cited 11/02/2016. Available from: http://www.birdlife.org
- Pacheco V, Cadenillas R, Salas E, et al. Diversidad y endemismo de los mamíferos del Perú. Revista peruana de biología. 2009;16:5–32.
- Albuja VLH. Lista de mamíferos actuales del Ecuador. Quito: Escuela Politécnica Nacional; 2011.
- de Vivo M, Carmignotto AP. Family Sciuridae G. Fischer, 1817. In: Patton JL, Pardiñas UFJ, D’Elía G, editors, Mammals of South America, Volume 2: Rodents Vol. 2. Chicago: University of Chicago Press; 2015.
- Venegas PJ. Herpetofauna del bosque seco ecuatorial de Perú: Taxonomía, Ecología y Biogeografía. Zona Áridas. 2005;9:9–26.
- Pyron RA, Burbrink FT, Wiens JJ. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol Biol. 2013;13:2–53. DOI:10.1186/1471-2148-13-93
- Ruane S, Bryson RW, Pyron RA, et al. Coalescent species delimitation in milksnakes (Genus Lampropeltis) and impacts on phylogenetic comparative analyses. Syst Biol. 2014;63:231–250.10.1093/sysbio/syt099
- Zaher H, Gobbi-Grazziotin F, Cadle JE, et al. Molecular phylogeny of advanced snakes (Serpentes, Caenophidia) with an emphasis on South American Xenodontines a revised classification and descriptions of new taxa. Papéis Avulsos de Zoologia. 2009;49:115–153.10.1590/S0031-10492009001100001
- Duellman WE, Barley AJ, Venegas PJ. Cryptic species diversity in marsupial frogs (Anura: Hemiphractidae: Gastrotheca) in the Andes of northern Peru. Zootaxa. 2014;3768:159–177.10.11646/zootaxa.3768.2
- Schulenberg TS, Stotz DF, Lane DF, et al. Birds of Peru. Revised edition. Princeton, NJ: Princeton University Press; 2010.
- Molina M, Molinari J. Taxonomy of Venezuelan white-tailed deer (Odocoileus, Cervidae, Mammalia), based on cranial and mandibular traits. Canadian J Zool. 1999;77:632–645.10.1139/z98-235
- Torres-Carvajal O, Gaona FP, Zaragoza C, et al. First record of Macropholidus ruthveni Noble 1921 (Squamata: Gymnophthalmidae) from Ecuador. Herpetol Notes. 1921;2015(8):25–26.
- Ordoñez-Delgado L, Tomas G, Espinosa CI. Nueva localidad del Tirano de Tumbes Tumbezia salvini (Aves: Tyrannidae) en el suroeste del Ecuador. Avances en Ciencias e Ingenierias. 2016;8:1–4.
- Loaiza CR. The Tumbesian center of endemism: biogeography, diversity and conservation. Biogeografía. 2013;6:4.
- Dirzo R, Young HS, Galetti M, et al. Defaunation in the Anthropocene. Science. 2014;345:401–406.10.1126/science.1251817
- Rodriguez JP, Rodriguez-Clark K, David AK, et al. IUCN Red List of Ecosystems, S.A.P.I.EN.S [Online], 5.2 | 2012, Online since 2012 Aug 12. Available from: http://sapiens.revues.org/1286
- Keith DA. Assessing and managing risks to ecosystem biodiversity. Aust Ecol. 2015;40:337–346.10.1111/aec.2015.40.issue-4
- Keith DA, Rodríguez JP, Rodríguez-Clark KM, et al. Scientific foundations for an IUCN red list of ecosystems. PLoS ONE. 2013;8:e62111.10.1371/journal.pone.0062111
- Rodriguez JP, Keith DA, Rodriguez-Clark, KM, et al. A practical guide to the application of the IUCN red list of ecosystems criteria. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140003.10.1098/rstb.2014.0003
- Neuschulz EL, Mueller T, Schleuning M, et al. Pollination and seed dispersal are the most threatened processes of plant regeneration. Sci Rep. 2016;6:29839.10.1038/srep29839
- López-Martínez JO, Sanaphre-Villanueva L, Dupuy JM, et al. β-diversity of functional groups of Woody plants in a tropical dry forest in Yucatan. PLoS ONE. 2013;8:e73660.10.1371/journal.pone.0073660
- Hilje B, Calvo-Alvarado J, Jiménez-Rodríguez C, et al. Tree species composition, breeding systems, and pollination and dispersal syndromes in three forest successional stages in a tropical dry forest in Mesoamerica. Trop Conserv Science. 2015;8:76–94.10.1177/194008291500800109
- Jara-Guerrero A, De la Cruz M, Méndez M. Seed dispersal spectrum of woody species in south Ecuadorian dry forests: environmental correlates and the effect of considering species abundance. Biotropica. 2011;43:722–730.10.1111/btp.2011.43.issue-6
- Ceccon E, Hernández P. Seed rain dynamics following disturbance exclusion in a secondary tropical dry forest in Morelos, Mexico. Revista de Biología Tropical. 2009;57:257–269.
- Patricia L, Morellato C. Nutrient cycling in two south-east Brazilian forests. Litterfall and litter standing crop. Revista de Biología Tropical. 1992;8:205–215.
- Escribano-Ávila G, Pías Couso B, Escudero Alcántara A, et al. Ecological relevance of frugivorous mammals in the regeneration dynamics of old fields in Mediterranean environments. Ecosistemas. 2015;24:35–42.10.7818/ECOS
- Lasso E, Barrientos LS. Epizoochory in dry forest Green iguana: an overlooked seed dispersal mechanism? Colombia Forestal. 2015;18:151–159.
- Traveset A, Nogales M, Vargas P, et al. Galápagos land iguana (Conolophus subcristatus) as a seed disperser. Integr Zool. 2016;11:207–213.10.1111/inz2.2016.11.issue-3
- Lessa LG, Geise L, Costa FN. Effects of gut passage on the germination of seeds ingested by didelphid marsupials in a Neotropical savanna. Acta Botanica Brasilica. 2013;27:519–525.10.1590/S0102-33062013000300009
- Genrich CM, Mello MA, Silveira FA, et al. Duality of interaction outcomes in a plant-frugivore multilayer network. Oikos. 2016. DOI:10.1111/oik.03825
- Harper JL. Population biology of plants. London: Academic Press; 1977.
- Espinosa CI, Luzuriaga AL, de la Cruz M, et al. Climate and grazing control nurse effects in an Ecuadorian dry shrubby community. J Trop Ecol. 2013;30:23–32.
- Brooker RW, Maestre FT, Callaway RM, et al. Facilitation in plant communities: the past, the present, and the future. J Ecol. 2008;96:18–34.
- Espinosa CI, de la Cruz M, Jara-Guerrero A, et al. The effects of individual tree species on species diversity in a tropical dry forest change throughout ontogeny. Ecography. 2016;39:329–337. DOI:10.1111/ecog.01328
- Nature and Culture. 2014. [cited 2016 Apr 20]. https://natureandculture.org/places/ecuador/the-palo-santo-project
- Martínez-Fortún MS. Desarrollo sostenible y conservación etnoecológica a través de la meliponicultura, en el sur de Ecuador. Tesis de Maester. Universidad Internacional de Andalucía; 2015.
- Espinosa CI, Cabrera O, Luzuriaga AL, Escudero A. What Factors Affect Diversity and Species Composition of Endangered Tumbesian Dry Forests in Southern Ecuador? Biotropica. 2011;43(1):15–22. 2
- Josse C, Balslev H. The composition and structure of a dry, semideciduous forest in western Ecuador. Nordic Journal of Botany. 1994;14(4):425–434.
- Linares-Palomino R, Ponce-Alvarez SI. Tree community patterns in seasonally dry tropical forests in the Cerros de Amotape Cordillera, Tumbes. Peru. Forest Ecology and Management. 2005;209:261–272.
- Leal-Pinedo JM, Linares-Palomino R. Los bosques secos de la Reserva de Biosfera del Noroeste (Perú): Diversidad arbórea y estado de conservación. Caldasia. 2005;27(2):195–211.
- Jara-Guerrero A, De la Cruz M, Espinosa CI, Méndez M, Escudero A. Does spatial heterogeneity blur the signature of dispersal syndromes on spatial patterns of woody species? A test in a tropical dry forest. Oikos. 2015;124(10):1360–1366.
