Abstract
Scinax exiguus was described from the Gran Sabana, southeastern Venezuela, and its distribution is associated with the one Amazonian savanna region in northern South America. Herein, we describe the aggressive call and redescribe the advertisement call of S. exiguus, including recordings from the type locality. Also, we report on intraspecific variation between Venezuelan and Brazilian populations and we make remarks on its distribution in Brazil. Advertisement calls from the type locality (Bolívar, Venezuela) mainly differ in temporal traits from those of the two Brazilian populations, especially in duration and pulse organization. In contrast, calls from both Brazilian populations had remarkable differences from each other in the dominant frequency. Body size also varied between the type series (Venezuela) and a Brazilian population. We suggest that the acoustic variation in S. exiguus might be explained partly by differences in temperature and varying degrees of motivational state of calling males. One of our study sites (Cantá, Roraima) now represents the southernmost record for the species in comparison to the previous record (Boa Vista). Additional research and conservation measures are required in the Guyanan savanna in northern South America for the protection of its endemics.
Introduction
Scinax exiguus (Duellman, 1986) was described in the mid-1980s from the Gran Sabana (Guyanan savanna) in southeastern Venezuela [Citation1]. In addition to that region, this species was reported from two regions of Amazonian savanna in the Brazilian state of Roraima (Boa Vista and Ilha de Maracá), northern Brazil [Citation2], which borders with Venezuela. During fieldwork in Roraima, we recorded and collected specimens that we assigned to S. exiguus based on the diagnostic characters provided in its original description [Citation1]. In this paper, we redescribe the advertisement call of S. exiguus from two populations in the Brazilian state of Roraima, including a reanalysis of the original files from the Gran Sabana (Bolívar, Venezuela) that were provided in its description, and describe for the first time its aggressive call. Additionally, we report on variation on its vocalization and body size, and comment on its distribution range in Brazil.
Methods
The fieldwork was conducted at two localities of the Brazilian state of Roraima: at Serra do Tepequém (3°45′57.63′′N, 61°45′10.29′′W; 621 m above sea level), municipality of Amajari, from 25 to 27 June 2016; and in the municipality of Cantá (2°45′59.51′′N, 60°36′32.14′′W; 70 m above sea level) from 20 to 28 July 2016. At Serra do Tepequém, males were observed calling early at night in an open area bordered by a forest remnant. They were perched up to 1.5 high on grassy vegetation or on a broad leaf on one occasion, calling in a vertical position. The area was also covered by small bushes. The soil was muddy and partly flooded, containing small puddles formed during rain. At Cantá, the recorded male was calling from dense grass tufts next to an abandoned and empty fish tank. Two other males were calling associated with grassy vegetation.
Specimens are housed in the Collection of Amphibians of Universidade Federal de Uberlândia (AAG-UFU), State of Minas Gerais, Brazil. Call vouchers are AAG-UFU 5503–5507; all collected at Serra do Tepequém. The recording from Cantá did not have a corresponding voucher, but these calls were assigned to S. exiguus because of the overall similarity in temporal and spectral structure with calls from other populations (Venezuela and Serra do Tepequém).
Vocalizations were recorded with ME67/K6 Sennheiser microphones and Marantz PMD 671 digital recorders. Recording settings were 48.0-kHz sampling rate and 16-bit resolution. The recordings were made under visualization and the microphone was positioned about 1.0–2.0 m from the voucher males at Serra do Tepequém. Calls were analyzed in Soundruler [Citation3] under the following settings: 1024 points resolution (FFT), 90% overlap, window type Hann. The sound figure was generated in R [Citation4] through seewave package [Citation5] using a FFT=256 points and 85% overlap. A high-band pass filter up to 200Hz was applied to the cuts from which the sound figures were generated. Each vocal emission, i.e. an advertisement call, was composed of a single note (call=note). The traits analyzed are as follows: temporal traits (note duration; note rate, defined as the number of notes minus 1 divided by the duration between the onset of first and last note of the recording or call bout; pulses per note; pulse rate, defined as the number of pulses minus 1 divided by the duration between the onset of first and last pulses); spectral traits (dominant frequency). The sound recordings made in Brazil are housed in the acoustic database of A. A. Giaretta Lab at Universidade Federal de Uberlndia (access request to AAG sound files at [email protected]; sound files will be sent to additional repositories once this study has been published); those made in Venezuela are housed at Biodiversity Institute at the University of Kansas (KU). See Appendix 1 for further information.
In addition to the advertisement call, we recorded a second type of call designated herein as an aggressive call, because we observed that males emitted this type of note only when they were closer to one another (ca. 1–2 m); males calling away from the chorus only emitted advertisement calls.
Results
Species identification
S. exiguus shares overall morphology with species of the former and non-monophyletic Ololygon staufferi group (sensu Fouquette and Delahoussaye [Citation6], Faivoich et al. [Citation7]), such as S. fuscomarginatus (Lutz, 1925), S. squalirostris (Lutz, 1925), S. staufferi (Cope, 1865), S. wandae (Pyburn & Fouquette, 1971), which are relatively small in size and have acuminate snouts, longitudinal stripes/bands on the dorsum and vocalizations that sound like the buzzing of an insect.
The identification of S. exiguus was based on the following combination of traits from its original description [Citation1]: (1) basal webbing between fingers; (2) tibia length less than half of snout-vent length (SVL); (3) dorsum brown with dark brown dorsolateral and lateral stripes with intervening creamy tan stripe (Figure ). In addition to those diagnostic characters, some other traits were useful in the identification, such as (4) the small male SVL (up to 24 mm); and (5) a long, high-pitched, multipulsed advertisement call (Figure ). Specimens of S. exiguus from Serra do Tepequém have a larger size (SVL 21.2–24.2 mm, n = 7 males) in comparison with the species type series (SVL 18.0–20.8 mm, n = 25 males; [Citation1]), but the tibia/SVL ratio (0.45–0.48) is in agreement with the original description (0.44-0.50).
Vocalizations of S. exiguus from Serra do Tepequém (Roraima, Brazil)
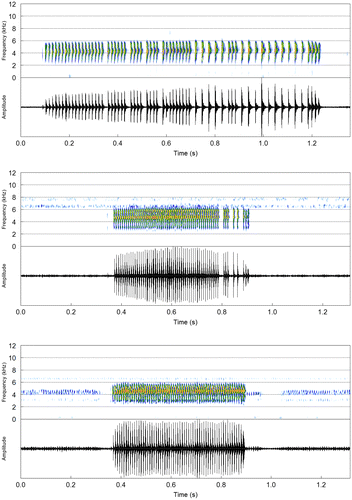
Descriptive statistics (mean ± SD) are presented in Table . The advertisement call (Figure ) is composed of a single type of pulsed note (n = 5 males, 89 notes), whose amplitude is relatively constant along the note and pronounced alterations in amplitude are restricted to the onset and end of each note. Notes lasted 316–624 ms and were emitted at rates of 52–74 notes per minute. Pulse number was 38–71; these were totally separated from each other along the note (i.e. complete between-pulse amplitude modulation), even though some notes had a more irregular pulse organization, especially in the last half, in cases that both isolated and partly separated pulses could be recognized. There were 102–125 pulses per second. The dominant frequency was contained in a wide bandwidth (ca. 1000-Hz frequency range under a 1024-point FFT), varying from 3811 to 4802 Hz.
Table 1. Advertisement call traits for three populations of S. exiguus: Gran Sabana, Bolívar, Venezuela (type locality); and Brazilian state of Roraima (Serra do Tepequém and Cantá). Values are presented as mean ± SD (minimum–maximum), n = recorded males (analyzed calls).
The aggressive call (Figure ) consists of a note (n = 1 male, 6 notes) with weaker and more irregular amplitude modulations, so pulse units could not be clearly recognized. Although this note type was heard several times in the field while we were recording their vocalizations, aggressive notes emitted by only one male were recorded. This note type was broadcast in-between advertisement notes, including a bout of five notes. Shifts in amplitude along the note were similar to the pattern described for advertisement notes. Note duration varied from 92 to 158 ms (121.0 ± 23.4). The dominant frequency varied from 3854 to 4285 Hz (4127.2 ± 148.5).
Advertisement call of S exiguus from Cantá (Roraima, Brazil)
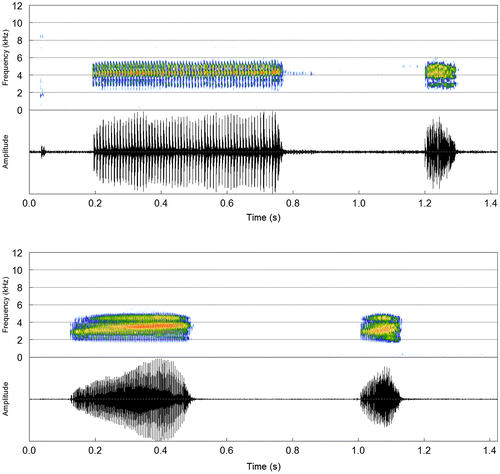
Descriptive statistics (mean ± SD) in Table . Despite a general structural similarity with calls from Serra do Tepequém (Table ), a striking difference exists in the dominant frequency, for which the range of values (5620–5879 Hz) does not even overlap with those from Serra do Tepequém (3811–4802 Hz) and Venezuela (4078–5016 Hz). Besides, the advertisement call from Cantá has a markedly shorter duration, especially the first and last notes in call bouts. The variation in pulse organization and between-pulse separation observed in calls from Serra do Tepequém was also present in this population (Figure ).
Advertisement call of S. exiguus from the Gran Sabana (Bolívar, Venezuela)
Descriptive statistics (mean ± SD) in Table . Advertisement calls from Venezuela (n = 3 males, 49 notes) are like those from Serra do Tepequém and Cantá in temporal and spectral structure. In opposite, calls from the type locality had a much longer duration and lower pulse rate compared to the other two populations, which might reflect the irregular pulse organization along the note (Figure ).
Discussion
The advertisement and aggressive notes of S. exiguus have patterns shared with those already described for morphologically similar species, such as S. fuscomarginatus (Figure ). Acoustic variation in temporal and spectral traits was observed among the three populations studied. One main difference is the pulse organization, which is more regularly distributed along the note in calls from both Brazilian populations (Serra do Tepequém and Cantá), whereas they have an irregular pattern in calls from Venezuela, in which we observed the longest advertisement notes with the lowest pulse rate (Figure ). There is an expressive difference of temperatures between recordings made in Venezuela and Brazil (see Appendix 1). Possibly, this factor could explain in part the acoustic variation between Venezuelan and Brazilian populations [Citation8], which occur at different elevations, upland (≥1000 m a.s.l.) and lowland (70–600 m a.s.l.) regions, respectively [Citation1Citation9] (Figure ).
Figure 4. Distribution of S. exiguus in northern South America. Legends: 1 (star) – type locality (km 144 on the Eldorado-Santa Elena de Uairén Road in the Gran Sabana, Bolívar, Venezuela [Citation1]); squares (other localities): 2–4 (records by Gorzula and Señaris [Citation9]) – Piedra del Supamo, Yuruaní/Kukená confluence and Mapaurí, respectively (see these upland localities in their Gazetteer B), 5–9 (localities in Roraima, Brazil): 5 – Pacaraima [Citation11], 6 – Serra do Tepequém (municipality of Amajari [Citation11]), 7 – Estação Ecológica de Maracá (municipality of Amajari [Citation11]), 8 – Boa Vista [Citation2], 9 – Cantá. Source: The Author
![Figure 4. Distribution of S. exiguus in northern South America. Legends: 1 (star) – type locality (km 144 on the Eldorado-Santa Elena de Uairén Road in the Gran Sabana, Bolívar, Venezuela [Citation1]); squares (other localities): 2–4 (records by Gorzula and Señaris [Citation9]) – Piedra del Supamo, Yuruaní/Kukená confluence and Mapaurí, respectively (see these upland localities in their Gazetteer B), 5–9 (localities in Roraima, Brazil): 5 – Pacaraima [Citation11], 6 – Serra do Tepequém (municipality of Amajari [Citation11]), 7 – Estação Ecológica de Maracá (municipality of Amajari [Citation11]), 8 – Boa Vista [Citation2], 9 – Cantá. Source: The Author](/cms/asset/52b418a0-0ba8-4d3a-ae5b-99f3e17deb93/tneo_a_1375813_f0004_oc.gif)
The male recorded in Cantá, in contrast, emitted shorter and weaker notes at the beginning and the end of call bouts. This differs from those at Serra do Tepequém, where several males were embedded within a chorus and calling continuously without marked shifts in amplitude between vocal emissions. At Cantá, a few males were heard calling quite far from each other (hundreds of meters). This might reflect different motivational states during calling activity in isolated and chorusing males.
Vocalizations analyzed for the three populations that we assigned to S. exiguus have marked differences in both temporal and spectral traits (see Table ) and draw attention to the need for addressing instraspecific variation within this nominal species, as we cannot neglect the possibility of the existence of more than one taxonomic unit being contained within the nominal species, based mainly on such great acoustic differences to the extent some traits are even non-overlapping between populations (Table ) and also noticeable differences in SVL. For future studies, in addition to the increase in sample size for each population studied, physical factors, especially temperature, and social context should be taken into account, which may be of particular importance to better understand the sources of acoustic variability among populations assigned to S. exiguus in the Amazonian savannas of Venezuela and Brazil.
The acoustic record for S. exiguus from Cantá extends the distribution approximately 65 km southward from the previous southernmost locality at Boa Vista [Citation2] (Figure ). Recently, this species was indirectly reported for three localities in the Brazilian state of Roraima (Boa Vista, and two new records: Pacaraima and Tepequém) in a list of examined specimens in the description of S. rossaferesae [Citation10]. Subsequently, one of the authors of the description of S. rossaferesae formalized those records in Araujo-Vieira [fCitation11] as being the first records of S. exiguus in Brazil. Actually, the first record of this species in Brazil was in the late 1990s by Martins [Citation2], as well as the southernmost record in Boa Vista. Based on this record, S. exiguus was included on the latest list of Brazilian amphibians [Citation12] prior to the records provided by Araujo-Vieira [Citation11]. Still, the contribution by Araujo-Vieira [Citation11] filled some gaps in the distribution in the Brazilian state of Roraima with the new records for Tepequém (one of our study sites), Estação Ecológica de Maracá, and Pacaraima on the border between Venezuela and Brazil (Figure ).
S. exiguus is apparently endemic to the wide region of savannas in southeastern Venezuela [Citation1Citation9] and northern Brazil (Figure ) and might have its southern distribution limited by the Amazon Rainforest in very southern Roraima. Amazonian savannas are of particular importance for conservation. These savannas shelter a unique flora and fauna and correspond to islands isolated by the Amazon Rainforest [Citation13]. The current anthropogenic threats and lack of studies on the biodiversity and dynamics in the Guyanan savanna may have severe consequences for the conservation of endemics to this region, e.g. S. exiguus, Anomaloglossus apiau and A. tepequem [Citation14], reinforcing the need for further research and conservation policies in this particular region in northern South America [Citation13].
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo [grant number 2012/15763-7]; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (BR) [grant number 1466776]; Conselho Nacional de Desenvolvimento Científico e Tecnológico (BR) [grant number 159817/2015-3].
Associate Editor: Juan Manuel Guayasamin.
Author contributions
TRC conceived and designed the study, and wrote the first draft of the manuscript. TRC, PAA, and AAG conducted fieldwork. WED and TRC identified the species. WED provided information on the type series (sound recordings and photographs). DLB conducted the acoustic analysis. WED, AAG, PAA, and DLB reviewed and contributed to the manuscript.
Acknowledgements
We thank Luciana Frazão Luiz for field companionship, Magno Segalla for making available a reference, and an anonymous reviewer for providing valuable comments. Collection permit: SISBIO/ICMBio #30059‒7.
Associate Editor: Juan Manuel Guayasamin.
References
- Duellman WE. Two new species of Ololygon (Anura: Hylidae) from the Venezuelan Guyana. Copeia. 1986;1986(4):864–870.10.2307/1445281
- Martins M. The frogs of the Ilha de Maracá. In: Milliken W, Ratter J, editors. Maracá: the biodiversity & environment of Amazonian Rainforest. Chichester (NY): Wiley; 1998. p. 285–306.
- Gridi-Papp M, editor. SoundRuler: acoustic analysis for research and teaching. 2007. Available from: http://soundruler.sourceforge.net
- R Development Core Team. R: a language and environment for statistical computing, version 3.2.2. Vienna; 2014. Available from: http://www.R-project.org
- Sueur J, Aubin T, Simonis C. Seewave, a free modular tool for sound analysis and synthesis. Bioacoustics. 2008;18:213–226.10.1080/09524622.2008.9753600
- Fouquette MJ, Delahoussaye AJ. Sperm morphology in the Hyla rubra group (Amphibia, Anura, Hylidae), and its bearing on generic status. J Herpetol. 1977;11(4):387–396.10.2307/1562720
- Faivovich J, Haddad CFB, Garcia PCA, et al. Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bull Am Mus Nat Hist. 2005;294:1–240.10.1206/0003-0090(2005)294[0001:SROTFF]2.0.CO;2
- Gerhardt HC, Huber F. Acoustic communication in insects and anurans: common problems and diverse solutions. Chicago (IL): The University of Chicago Press; 2002.
- Gorzula S, Señaris JC. Contribution to the herpetofauna of Venezuelan Guayana I: a data base. Caracas: Scientia Guaianae; 1999.
- Conte CE, Araujo-Vieira K, Crivellari LB, et al. A new species of Scinax Wagler (Anura: Hylidae) from Paraná, Southern Brazil. Zootaxa. 2016;4193(2):245–265.10.11646/zootaxa.4193.2
- Araujo-Vieira K. Geographic distribution: Scinax exiguus. Herpetol Rev. 2017;47:624.
- Segalla MV, Caramaschi U, Cruz CAG, et al. Brazilian Amphibians: list of species. Herpetologia Brasileira. 2016;5(2):34–46.
- Carvalho WD, Mustin K. The highly threatened and little known Amazonian savannahs. Nat Ecol Evol. 2017;1:1–3.
- Fouquet A, Souza SM, Nunes PMS, et al. Two new endangered species of Anomaloglossus (Anura: Aromobatidae) from Roraima State, northern Brazil. Zootaxa. 2015;3926(2):191–210.10.11646/zootaxa.3926.2