ABSTRACT
In the present study, we revisit the advertisement call of Scinax montivagus based on new recordings from Rio de Contas, state of Bahia, Brazil. The species’ call consists of a single type of multipulsed note of relatively short duration (mean = 192 ms), having two non-harmonically related emphasized frequency bands. The dominant frequency corresponds either to the lower or the higher band, or it may shift between both along call emissions. Herein, we assess and discuss call features that diverge from what is in the literature and also provide further details on the species call. We reinforce that detailed and standardized advertisement call descriptions of different Scinax species would contribute to acoustic diagnosis between them.
Introduction
The advertisement call is the main type of acoustic signal emitted by anuran males during the breeding season and functions in the attraction of conspecific females [Citation1,Citation2] and in among-males spacing [Citation3]. Since the advertisement call may act as a prezygotic reproductive isolation mechanism, it is widely used in taxonomic studies to help in species delimitation [Citation4], especially in cases of cryptic species [Citation5–Citation8]. Furthermore, advertisement calls can be useful to address questions on macroevolutionary patterns [Citation9], conservation status [Citation10], and acoustic landscape monitoring [Citation11,Citation12].
Currently, Scinax Wagler, 1830 comprises 73 species distributed in South and Central Americas, and in some Caribbean islands [Citation13]. The genus was formerly composed by two major clades, those of S. ruber and S. catharinae clades [Citation14,Citation15], but a recent taxonomic change based on molecular analysis [Citation16] reallocated species of the Scinax catharinae clade to Ololygon Fitzinger, 1843 and species of the S. uruguayus group (formerly group of S. ruber clade) to Julianus Duellman, Marion, and Hedges, 2016; therefore, Scinax is currently composed solely by species of the S. ruber clade, with the exception of species of the formerly S. uruguayus group. The genus and its component species have a long and sometimes problematic taxonomic history [Citation14], due to the morphological conservatism and the large diversity within it [Citation17,Citation18], being frequent the description of new species [Citation19–Citation22] and synonimizations [e.g. Citation15,Citation23,Citation24]. These facts point out to the need to use lines of evidence other than morphology, such as molecular and acoustics characterizations, to uncover cryptic diversity in Scinax [Citation17]. In this sense, several studies have been done in an endeavor to properly document interspecific variation of vocal patterns in the genus [Citation25–Citation34]. The advertisement call of Scinax species is usually consisted of a single multipulsed note with variable duration [Citation25,Citation31–Citation35] and sometimes with more than one emphasized frequency band [Citation25,Citation30,Citation36], what seems to be relevant in species-specific recognition [Citation25,Citation37].
Scinax montivagus Juncá, Napoli, Nunes, Mercês, and Abreu, 2015 is a recently described species apparently endemic to the Serra do Espinhaço Range in state of Bahia (BA), where it is known from some few sites around the type locality [Citation38]. Herein we revisit its advertisement call based on recordings from Rio de Contas (BA), aiming to (1) provide further details on the species’ advertisement call; (2) report on differences in a call trait regarding the literature; (3) reinterpret the species’ call frequency features.
Methods
Data collection and species identification
We recorded and collected specimens in Pico das Almas, municipality of Rio de Contas [13°29ʹ53.32ʹ’S; 41°57ʹ52.44ʹ’W (WGS84 datum), 1540 m above sea level], Espinhaço Range, state of Bahia, northeastern Brazil. We recorded six males with a Marantz PMD 671 digital recorder (sampling rate of 48.0 kHz and 16 bits resolution) coupled to a Sennheiser ME67-K6 directional microphone, which was positioned at a distance of approximately 2 to 3 m from the calling males. We made the recordings on 25 November 2016, between 19:00 and 20:00 h, and air temperature at 25°C. Call voucher specimens and recordings (see ) are housed in the Coleção de Anuros do Museu de Biodiversidade do Cerrado (AAG-UFU), Universidade Federal de Uberlândia, Uberlândia, Minas Gerais, Brazil.
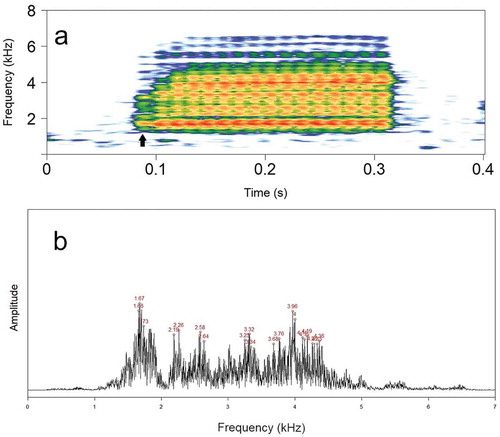
Our specimens (n = 6 males; ) match the original species diagnosis [Citation38] in the following features: (1) moderate adult size (25.9–26.8 mm SVL); (2) snout rounded in dorsal and lateral views; (3) straight and well-marked canthus rostralis; (4) dorsum background with a sparse and irregular dark-spotted pattern; two specimens (AAG-UFU 5625 and 5629) have fragmented brown blotches forming an inverted parenthesis in scapular region; (5) advertisement call duration of 142–239 ms, dominant frequencies ranging from 1.7 to 1.8 kHz (lower-frequency band) and 4.0–4.4 kHz (higher-frequency band), and 12–16 pulses per call.
Acoustic analysis
We analyzed 10 calls of each male (n = 60) using the Raven Pro 1.5 software [Citation39] with the following settings: window type Hann, window size of 256 samples, 3 dB filter bandwidth of 248 Hz, 90% overlap (locked), hop size of 0.590 ms, DFT size of 1024 samples and grid spacing at 43.1 Hz. We applied a filter at 200 Hz prior analysis to reduce background noise (wind). We manually measured temporal traits in the oscillogram and obtained dominant frequency values using the “Peak Frequency” function of Raven [Citation39]. We generated sound figures with the seewave package v. 1.7.6 [Citation40] in the R platform v. 3.1.1 [Citation41], using the following settings: window = Hanning, overlap = 90%, and FFT = 256 (for ) or 1024 (for ). Relative amplitude scale of 36 dB in spectrograms ( and ) is represented by colors (red being the maximum amplitude). We followed Bang and Giaretta [Citation29] for acoustic terminology and definitions, except for pulse rate and call rate which were measured according to Cocroft and Ryan [Citation9].
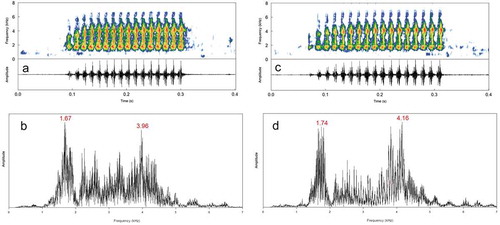
Figure 2. (a) Spectrogram (top) and its respective oscillogram (bottom), and (b) power spectrum of an advertisement call of Scinax montivagus depicting the lower frequency band as the dominant one (sound file = Scinax_montivagusRioContasBA4aAAGm671). (c) Spectrogram (top) and its respective oscillogram (bottom), and (d) power spectrum of a call depicting the higher frequency band as the dominant (sound file = Scinax_montivagusRioContasBA6aAAGm671). Recordings from Rio de Contas, state of Bahia, northeastern Brazil. Spectrograms with an FFT of 256. See further details on sound recordings in .
Results
The advertisement call (; ) consists of a single type of multipulsed note with duration of 142–239 ms (short call sensu Bilate and Lack [Citation32]), emitted at intervals of 1.4–4.8 s. Calls have 12–16 pulses that increase in amplitude from the first to the third or fourth pulse, until it reaches a plateau towards the end of the call. In some cases, pulses can raise gradually in amplitude until final portion of the call. Pulses have regular internal amplitude modulations (typically from 2 to 3), except for the first one that can be weakly modulated (i.e. the pulse has ill-defined amplitude modulation). Pulses are released at a rate of 66–81/s. The first pulses last 6–9 ms, mid-call pulses 7–10 ms, and last pulses 7–12 ms. Calls are emitted at rates of 11–38/min, and have two emphasized (i.e. most energetic) and not harmonic-related frequency bands ( and ). At FFT of 256, pulses have the shape of an “I” or an inverted “L” (). The lower-frequency band (LFB) ()) peaks at 1679–1851 Hz and the higher-frequency band (HFB) ()) at 3996–4431 Hz. Dominant frequency corresponded to the LFB in one individual and to the HFB in other four; in a male, it shifted between LFB and HFB along the call emission. At FFT of 1024, both LFB and HFB are embedded in several sidebands through the call’s bandwidth ()).
Table 1. Advertisement call features of Scinax montivagus from Rio de Contas (present study), topotypes (Miguel Calmón [Citation38]), and from Mucugê, all in the Espinhaço range, state of Bahia, northeastern Brazil. Data are presented as mean ± SD (minimum-maximum). HFB = Higher-frequency band; LFB = Lower-frequency band.
Discussion
Juncá et al. [Citation38] recognized the advertisement call of Scinax montivagus as being composed of two harmonic bands, but as demonstrated herein, this relationship does not seem to exist. A harmonic series is a set of component frequencies that are all integer multiples of a fundamental frequency (f0, 2f0, 3f0, and so on; see Bradbury and Vehrencamp [Citation42]). However, it is noticeable (FFT = 1024; ) that these frequency bands are embedded in several peaks representing sidebands resulting from amplitude modulations [Citation43]. For a harmonic series, the next peak following the LFB (fundamental frequency = 1679–1851 Hz) should be its double, but instead, it is at approximately 2300 Hz ()). Furthermore, the first pulse, which is weakly amplitude modulated, does not have the HFB apparent in its frequency range ()).
We found slight differences in pulse rate (66–81 pulses/s, n = 60 calls) when compared to the values reported by Juncá et al. [Citation38] (53–70 pulses/s, n = 337 calls, n = 7 males), possibly due to random effects related to small sample or interpopulational variation. Also, call variations may be heavily influenced by extrinsic factors, such as temperature variations (our values were higher than those of Juncá et al. [Citation38]; see ). Therefore, only through a statistical analysis encompassing larger samples, standardized call descriptions from other populations, as well as considering environmental variables (e.g. temperature, humidity), that we could address a putative intraspecific variability in Scinax montivagus advertisement call.
We assert that deeper acoustic analyses and a standardized methodology (as intended by Köhler et al. [Citation4]) could raise a more comparable acoustic dataset in which diagnostic call features might be revealed. Once this dataset is raised, it could serve as a framework to understand macroevolutionary patterns through comparative methods [Citation44–Citation46], as well as to aid in conservation efforts involving acoustic monitoring [Citation12]. Furthermore, considering that several Scinax species have advertisement calls composed of two non-harmonically related main emphasized frequency bands [Citation25,Citation28,Citation30], studies on biomechanical/physiological process could elucidate how these species produce such complex calls, as well as how they are able to shift the dominance between these frequency bands along call emissions.
Authors’ contribution
AAG conceived and designed this study. AAG recorded and collected the specimens. AGL conducted the acoustic analysis. AGL and DLB interpreted the results and wrote the first drafts of this manuscript. The final version was reviewed by all authors.
Acknowledgments
Bernardo F. V. Teixeira and Felipe S. Andrade for field companionship. ICMBio/SISBIO provided collection permits (#24954-2; #02015.008064/02-51). The Cornell Lab of Ornithology (Bioacoustics Research Program) for providing free licenses of Raven Pro software.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Duellman WE, Trueb L. Biology of amphibians. New York (NY): McGraw-Hill; 1986.
- Blair WF. Mating call in the speciation of anuran amphibians. Am Nat. 1958;92(862):27–51.
- Narins PM, Feng AS, Fay RR, et al. Hearing and sound communication in amphibians: prologue and prognostication. New York (NY): Springer; 2007.
- Köhler J, Jansen M, Rodríguez A, et al. The use of bioacoustics in anuran taxonomy: theory, terminology, methods and recommendations for best practice. Zootaxa. 2017;4251(1):1–124.
- Padial JM, De la Riva I. Integrative taxonomy reveals cryptic Amazonian species of Pristimantis (Anura: Strabomantidae). Zool J Linn Soc. 2009;155(1):97–122.
- Carvalho TR, Giaretta AA, Magrini LA. New species of the Bokermannohyla circumdata group (Anura: Hylidae) from southeastern Brazil, with bioacoustic data on seven species of the genus. Zootaxa. 2012;3321:37–55.
- Carvalho TR, Giaretta AA. Taxonomic circumscription of Adenomera martinezi (Bokermann, 1956) (Anura: Leptodactylidae: Leptodactylinae) with the recognition of a new cryptic taxon through a bioacoustic approach. Zootaxa. 2013;3701(2):207–237.
- Caminer MA, Ron SR. Systematics of treefrogs of the Hypsiboas calcaratus and Hypsiboas fasciatus species complex (Anura, Hylidae) with the description of four new species. Zookeys. 2014;370:1–68.
- Cocroft RB, Ryan MJ. Patterns of advertisement call evolution in toads and chorus frogs. Anim Behav. 1995;49(2):283–303.
- Forti LR, Costa WP, Martins LB, et al. Advertisement call and genetic structure conservatism: good news for an endangered neotropical frog. Peer J. 2016;4:e2014.
- Obrist MK, Pava G, Sueur J, et al. Bioacoustics approaches in biodiversity inventories. Abc Taxa. 2010;8:68–99.
- Sugai LSM, Llusia D. Bioacoustic time capsules: using acoustic monitoring to document biodiversity. Ecol Indic. 2019;99:149–152.
- Frost DR. Amphibian species of the World: an online reference. Version 6.0. New York (NY): American Museum of Natural History; [ cited 2019 Feb 2]. Available from: http://research.amnh.org/herpetology/amphibia/index.html
- Faivovich J. A cladistic analysis of Scinax (Anura: Hylidae). Cladistics. 2002;18(4):367–393.
- Faivovich J, Haddad CFB, Garcia PCA, et al. Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bull Am Mus Nat Hist. 2005;(294):1–240.
- Duellman WE, Marion AB, Hedges SB. Phylogenetics, classification, and biogeography of the treefrogs (Amphibia: Anura: Arboranae). Zootaxa. 2016;4104(1):1–109.
- Ferrão M, Colatreli O, de Fraga R, et al. High species richness of Scinax treefrogs (Hylidae) in a threatened amazonian landscape revealed by an integrative approach. PloS One. 2016;11(11):e0165679.
- Fouquet A, Vences M, Salducci M-D, et al. Revealing cryptic diversity using molecular phylogenetics and phylogeography in frogs of the Scinax ruber and Rhinella margaritifera species groups. Mol Phylogenet Evol. 2007;43(2):567–582.
- Conte CE, Araujo-Vieira K, Crivellari LB, et al. A new species of Scinax Wagler (Anura: Hylidae) from Paraná, southern Brazil. Zootaxa. 2016;4193(2):245–265.
- Ferrão M, Moravec J, de Fraga R, et al. A new species of Scinax from the Purus-Madeira interfluve, Brazilian Amazonia (Anura, Hylidae). ZooKeys. 2017;(706):137–162.
- Ferrão M, de Fraga R, Moravec J, et al. A new species of Amazonian snouted treefrog (Hylidae: scinax) with description of a novel species-habitat association for an aquatic breeding frog. PeerJ. 2018;6:e4321.
- Ferrão M, Moravec J, Kaefer IL, et al. New species of Scinax (Anura: Hylidae) with red-striped eyes from Brazilian Amazonia. J Herpetol. 2018;52(4):472–488.
- Brusquetti F, Jansen M, Barrio-Amorós C, et al. Taxonomic review of Scinax fuscomarginatus (Lutz, 1925) and related species (Anura; Hylidae). ZoolJ Linn Soc. 2014;171(4):783–821.
- Duellman WE, Wiens JJ. Hylid frogs of the genus Scinax Wagler, 1830, in Amazonian Ecuador and Peru. Occas Pap Mus Nat Hist Univ Kans. 1993;(153):1–57.
- Magrini L, Carvalho-e-Silva SP, Bedá AF, et al. Calls of five species of the Scinax ruber (Anura: Hylidae) clade from Brazil with comments on their taxonomy. Zootaxa. 2011;3066:37–51.
- Carvalho TR, Martins LB, Giaretta AA. The complex vocalization of Scinax cardosoi (Anura: Hylidae), with comments on advertisement calls in the S. ruber clade. Phyllomedusa. 2015;14(2):127–137.
- Carvalho TR, Teixeira BFV, Duellman WE, et al. Scinax cruentommus (Anura: Hylidae) in the upper Rio Negro drainage, Amazonas state, Brazil, with the redescription of its advertisement call. Phyllomedusa. 2015;14(2):139–146.
- Carvalho TR, Azarak P, Bang D, et al. A reassessment of the vocalization and distribution of Scinax exiguus (Duellman, 1986) (Anura, Hylidae) in the Amazonian savanna of Roraima, northern Brazil, with the description of its aggressive call. Neotrop Biodivers. 2017;3(1):196–202.
- Bang DL, Giaretta AA. Redescription of the advertisement calls of Scinax tigrinus and Scinax maracaya (Anura: Hylidae) and an evaluation of their differential diagnosis. Rev Bras Biocienc. 2016;14(3):181–186.
- Bang DL, Carvalho TR, Andrade FS, et al. Vocalization of Scinax haddadorum (Anura: Hylidae), with further notes on the vocalization of the morphologically similar Scinax rupestris. Neotrop Biodivers. 2017;3(1):117–124.
- Pombal JP Jr, Bastos RP, Haddad CFB. Vocalizações de algumas espécies do gênero Scinax (Anura, Hylidae) do sudeste do Brasil e comentários taxonômicos. Naturalia. 1995;20:213–225.
- Bilate M, Lack E. The advertisement call of Scinax similis (Cochran, 1952) (Amphibia, Anura). S Am J Herpetol. 2011;6(1):54–58.
- Novaes G, Zina J. Advertisement call of Scinax camposseabrai (Bokermann, 1968) (Anura: Hylidae), with comments on the call of three species of the Scinax ruber clade. Zootaxa. 2016;4084(2):258–266.
- Bevier CR, Gomes FR, Navas CA. Variation in call structure and calling behavior in treefrogs of the genus Scinax. S Am J Herpetol. 2008;3(3):196–206.
- Pombal JP Jr, Haddad CFB, Kasahara S. A new species of Scinax (Anura: Hylidae) from southeastern Brazil, with comment on the genus. J Herpetol. 1995;29:1–6.
- Bang DL, Giaretta AA. Filling a gap in the distribution of Scinax rostratus (Peters, 1863) (Anura, Hylidae) in northern Brazil, with further data on its advertisement call. Check List. 2017;13(3):2150.
- Gerhardt HC. Acoustic spectral preferences in two cryptic species of grey treefrogs: implications for mate choice and sensory mechanisms. Anim Behav. 2005;70(1):39–48.
- Juncá FA, Napoli MF, Nunes I, et al. A new species of the Scinax ruber clade (Anura, Hylidae) from the Espinhaço range, northeastern Brazil. Herpetologica. 2015;71(4):299–309.
- Bioacoustics Research Program. Raven pro: interactive sound analysis software (Version 1.5) [ Computer software]. Ithaca (NY): The Cornell Lab of Ornithology; 2014. Available from: http://www.birds.cornell.edu/raven
- Sueur J, Aubin T, Simonis C. Seewave: a free modular tool for sound analysis and synthesis. Bioacoustics. 2008;18(2):213–226.
- R Development Core Team. R: a language and environment for statistical computing. Version 3.1.1. Vienna (AU). 2015 [cited 2019 Jun 13]. Available from: http://www.R-project.org
- Bradbury JW, Vehrencamp SL. Principles of animal communication. Sunderland (MA): Sinauer Associates; 1998.
- Watkins WA. The harmonic interval: fact or artifact in spectral analysis of pulse trains. Massachusetts: Woods Hole Oceanographic Institution; 1968.
- Goicoechea N, De la Riva I, Padial JM. Recovering phylogenetic signal from frog mating calls. Zool Scr. 2010;39(2):141–154.
- Robillard T, Höbel G, Gerhardt HC. Evolution of advertisement signals in north American hylid frogs: vocalizations as end‐products of calling behavior. Cladistics. 2006;22(6):533–545.
- Erdtmann L, Amezquita A. Differential evolution of advertisement call traits in dart‐poison frogs (Anura: Dendrobatidae). Ethology. 2009;115(9):801–811.
Table A1. List of analyzed sound files of Scinax montivagus from Rio de Contas, state of Bahia, northeastern Brazil.