ABSTRACT
The Osteocephalus buckleyi species group is widely distributed in primary and secondary forests of the Amazon Basin and Guiana Region. Based on integrative analysis, including morphological and genetic data, we estimate the phylogenetic relationships and species boundaries among populations of the Osteocephalus buckleyi group from the Ecuadorian Amazon, focusing on the O. verruciger-O. cannatellai species complex. Our results uncovered the existence of one confirmed candidate species from Sangay National Park and one unconfirmed candidate species. Here, we describe the new species which is morphologically and ecologically distinct from other Osteocephalus species. The new species is unusual because it shows quite distinct morphology, but low genetic distances compared to its closest relatives.
Zoobank.org registration LSID: urn:zoobank.org:pub:01F86A33-2D07-4D9E-A8A2-FF35622E3DB9
Introduction
The genus Osteocephalus Steindachner [Citation1] is widely distributed in South America, from Venezuela, Guianas, and the Amazon basin to central Bolivia, and from the eastern Andean slopes of Bolivia to Colombia [Citation2]. It is distributed from 0 to 2000 m.a.s.l., in primary and secondary forests [Citation3]. Osteocephalus comprises a total of 27 recognized species [Citation4] classified into five species groups [Citation5]: O. alboguttatus, O. buckleyi, O. leprieurii, O. planiceps, and O. taurinus.
The Osteocephalus buckleyi species group includes 13 species: O. buckleyi [Citation6], O. cabrerai [Citation7], O. camufatus Jungfer et al. [Citation8], O. cannatellai Ron et al. [Citation9], O. carri [Citation10], O. duellmani Jungfer [Citation11], O. festae [Citation12], O. germani Ron et al. [Citation9], O. helenae [Citation13], O. mimeticus [Citation14], O. mutabor Jungfer and Hödl [Citation15], O. verruciger [Citation16], and O. vilmae Ron et al. [Citation9]. Eight of these species occur in Ecuador [Citation17]. One putative morphological synapomorphy for the O. buckleyi group is its reproduction, which occurs along streams, where eggs are deposited [Citation5].
The Osteocephalus buckleyi species group was the first formally defined within Osteocephalus. It was proposed by Cochran and Goin [Citation10]; 1 year later, Duellman and Trueb [Citation3] published the first review of the genus. The most recent reviews of the group carried out by Ron et al. [Citation9] and Jungfer et al. [Citation5] revealed that it still has unresolved taxonomic issues due, to among others, the presence of undescribed species because of its similar morphology and the presence of cryptic species. In this study, we evaluated the species limits within O. verruciger and O. cannatellai species complex, and describe a new species discovered during an expedition to Sangay National Park, in the Amazonian slopes of the Andes of Ecuador. We also present a new phylogeny for Osteocephalus, focusing on the O. buckleyi species group. The species description is based on an integrative analysis of genetic and morphological data.
Methods
Morphological analyses
We examined alcohol-preserved specimens of the Osteocephalus buckleyi group (Appendix A) deposited at the herpetological collection of Museo de Zoología at Pontificia Universidad Católica del Ecuador (QCAZ). We included the type material of O. cannatellai (QCAZ 49572) and one syntype of Hyla verrucigera (ZMB 16589) from the Museum für Naturkunde, Leibniz Institute for Evolutionary and Biodiversity Research in Berlin, Germany.
The format for species diagnosis and description follows Duellman and Trueb [Citation3]. Notation for hand and foot webbing is based on Savage and Heyer [Citation18]. Sex and reproductive status were determined by gonad inspection. Morphometric measurements were taken with a digital caliper (to the nearest 0.01 mm). Adult specimens were measured for the following variables [Citation19]: snout-vent length (SVL); head length (HL); head width (HW); tympanum diameter (TD); femur length (FEL); tibia length (TL); foot length (FL); and eye diameter (ED) (). Color data in life were based on digital photos. We analyzed the SVL for adult males and females to determine size differences between species with a Student’s t-test using the program JMP® 9.01 [Citation20].
Table 1. Morphological measurements of Osteocephalus sangay sp. n
Table 2. Snout-vent length (SVL) measurements of clades A–F. All measurements in mm
DNA extraction, amplification, and sequencing
Genomic DNA was extracted from muscle and liver tissue preserved in 95% ethanol using standard phenol-chloroform extraction protocol [Citation21]. Polymerase chain reaction (PCR) was used to amplify partial mitochondrial genes of 12S rRNA, 16S rRNA, NADH dehydrogenase I (ND1), and cytochrome c oxidase I (CO1). PCR amplifications were performed under standard protocols and primers used are presented in Appendix B. Products were purified and sequenced by the Macrogen Sequencing Team (Macrogen Inc., Seoul, Korea).
Phylogenetic analyses
Sequences of five species (O. cannatellai, O. mutabor, O. sangay sp. n., O. verruciger, and O. vilmae) were edited, assembled and aligned using the software Geneious 7.1.7 (GeneMatters Corp [Citation22]), with default settings for MAFFT multiple Alignment [Citation23], for all mitochondrial genes. To determine phylogenetic relationships within the Osteocephalus buckleyi species group, we added sequences of five mitochondrial genes 12S, 16S, ND1, CO1 and Cytochrome-b (Cytb) from GenBank (available at: http://www.ncbi.nlm.nih.gov/genbank/) published by Faivovich et al. [Citation24], Ron et al. [Citation25], Ron et al. [Citation9], Jungfer et al. [Citation5], Moen and Wiens [Citation26], Moravec et al. [Citation27], Salducci et al. [Citation28], and Wiens et al. [Citation29]. As outgroup, we included sequences of O. yasuni, O. leprieurii, and O. fuscifacies based on phylogenies of Jungfer et al. [Citation5] and Ron et al. [Citation9]. All newly generated sequences and their GenBank accession numbers are listed in . The sequence matrix was imported to Mesquite 3.04 [Citation30] where manual adjustments were made to correct alignment errors.
Table 3. New DNA sequences used in the phylogenetic analysis
As there was a possibility that each of our sampled genes or codon positions in protein-coding genes were shaped by different evolutionary processes, sequences were partitioned according to gene and codon position. We used software PartitionFinder v.2.1.1 [Citation31] to simultaneously determine the best evolution model for each partition and the best partition strategy for the complete matrix.
Phylogenetic relationships were inferred under maximum likelihood and Bayesian criteria, using GARLI 2.0 [Citation32] and MrBayes 3.2.2 [Citation33], respectively. Maximum likelihood analyses were carried out using default values, except for the setting maximum program memory usage (availablememory = 10,000), and the number of generations without topology improvement required for termination (genthreshfortopoterm = 500,000). We ran a total of 20 independent searches, 10 of them starting from randomly generated trees (streefname = random) and 10 from stepwise addition trees (streefname = stepwise). We evaluated the exhaustiveness of the global search by comparing the final maximum likelihood value among the 20 replicate searches. We considered that the replicate searches were effective in finding the best tree when more than 90% of them had maximum likelihood values within two units of the best global search. Support was evaluated using 100 bootstrap pseudoreplicates under the same search parameters used to find the best tree.
Bayesian inference analyses consisted of four parallel runs using the Monte Carlo Markov Chain (MCMC) algorithm for 6 × 106 generations and sampling every 1,000 generations. Each run had four chains (3 hot and one cold), with a temperature of 0.1. Convergence into a stationary distribution and effective sample sizes (ESS) for all parameters were analyzed using Tracer software version 1.6 [Citation34]. We discarded as burn-in 10% of the sampled generations and combined the four runs to summarize the posterior probabilities of nodes in a maximum clade credibility tree. Pairwise genetic uncorrected p-distances for the 16S rRNA gene were calculated using MEGA 7 [Citation35] software. This gene has been commonly used to compare divergence between amphibian species [Citation36 Citation37,]. We obtained p-distances in the 16S rRNA gene between all species, except for clade G from Colombia, as sequences were not available.
Results
Phylogenetic relationships
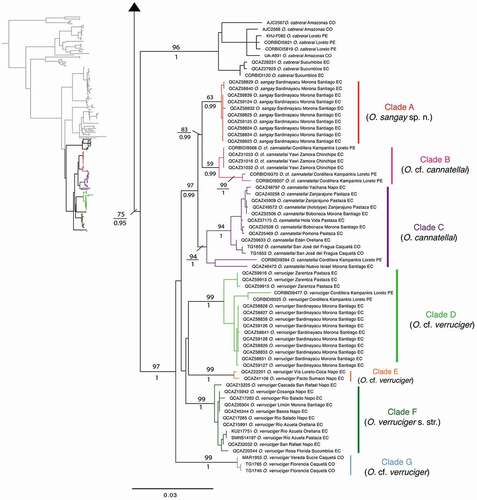
The complete DNA sequence matrix contained five mitochondrial genes and up to 4,856 bp for 180 individuals. The software PartitionFinder choose four partitions as the best strategy (best model in parentheses): 12S, 16S, ND1 1st and 3rd position, and CO1 3rd position (GTR + I + G); ND1 2nd position and Cytb 1st position (HKY + I + G); COI 1st position and Cytb 2nd and 3rd position (K80 + I + G); and CO1 2nd position (F81 + I). Topologies of maximum likelihood and Bayesian inference were very similar except for weakly supported nodes (posterior probabilities, pp <0.96 and bootstrap <64). Phylogenetic relationships among species of the Osteocephalus buckleyi group were consistent with those reported by Ron et al. [Citation9] and Jungfer et al. [Citation5].
We found a strongly supported clade for specimens identified as O. verruciger, O. cannatellai, and the population from Sangay National Park (). These specimens are distributed among seven subclades, all well supported (pp >0.99; bootstrap >94) except for clade B (pp = 0.99; bootstrap = 59). Clades A and B form a clade sister to clade C. Clade A includes specimens from Sardinayacu, Sangay National Park, Province of Morona Santiago; its sister clade is composed by O. cannatellai from southeastern Ecuador (province of Zamora Chinchipe) and northeastern Peru (Cordillera Kampankis); clade C comprises O. cannatellai from eastern Ecuador (provinces of Morona Santiago, Napo, Orellana, Pastaza, and Sucumbíos), southeast Colombia (department of Caquetá), and northeastern Peru (Cordillera Kampankis) (). The remaining clades (D–G) include individuals traditionally ascribed to O. verruciger. Phylogenetic relationships among them are weakly supported (pp <0.5; bootstrap <50).
Figure 1 Maximum likelihood phylogram depicting relationships within the Osteocephalus buckleyi species group. Phylogram derived from analysis of 4,856 bp of mitochondrial DNA (gene fragments 12S, 16S, ND1, CO1 and Cytb). Museum catalog numbers and localities are shown for each sample. Bootstrap values and Bayesian posterior probabilities are shown above and below the branches, respectively; missing values indicate values below 50 (bootstrap) or 0.5 (posterior probability). Outgroup species (O. fuscifacies, O. leprieurii and O. yasuni) are not included. BR: Brazil; CO: Colombia; EC: Ecuador; GU: Guyana; PE: Peru; VE: Venezuela
Figure 2. Distribution of the Osteocephalus verruciger-O. cannatellai complex (clades A–G). Localities are based on sequenced specimens from Ecuador, Peru and Colombia deposited at Museo de Zoología of Pontificia Universidad Católica del Ecuador QCAZ, Centro de Ornitología y Biodiversidad CORBIDI, and Natural History Museum University of Kansas. Black points refer to locations visited by Rich Haensch [Citation39], collector of O. verruciger syntypes
![Figure 2. Distribution of the Osteocephalus verruciger-O. cannatellai complex (clades A–G). Localities are based on sequenced specimens from Ecuador, Peru and Colombia deposited at Museo de Zoología of Pontificia Universidad Católica del Ecuador QCAZ, Centro de Ornitología y Biodiversidad CORBIDI, and Natural History Museum University of Kansas. Black points refer to locations visited by Rich Haensch [Citation39], collector of O. verruciger syntypes](/cms/asset/af998059-dab5-49ed-aeab-d7350fbdd9ed/tneo_a_1729306_f0002_oc.jpg)
Sequence divergence (uncorrected p-distance for gene 16S) within the O. verruciger-O. cannatellai complex (clades A to G) is low and ranges from 0.7% to 1.5% among clades and 0% to 0.1% within clades (). The genetic distance between Sangay (clade A) and its closest relative (clade B, O. cannatellai) is only 0.7%. However, the Sangay population shows a quite distinct morphology compared to Osteocephalus cannatellai. Therefore, we conclude that the Sangay population represents a new species that we describe in the following section. The recognition of the new species renders O. cannatellai paraphyletic because some populations of O. cannatellai (clade B) are more closely related to the new species than to other populations of O. cannatellai (clade C; ). We discuss the status of both clades of O. cannatellai in the taxonomic review section.
Table 4. Pairwise genetic distances (uncorrected p) of 16S DNA sequences among Osteocephalus verruciger-cannatellai complex
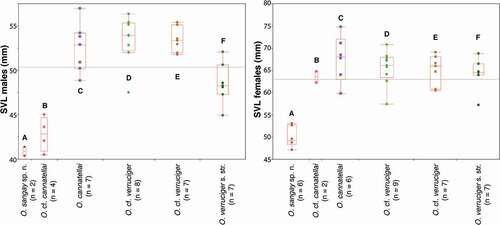
We found significant size differences (adult males and females) between most clades (). Males of clade A (O. sangay sp. n.) are smaller than those of clades C – F (all P values for t tests < 0.001), males of clade B are smaller than those of clade C (t = −6.74, df = 29, P < 0.001); clade F has smaller males than clade D (t = −4.05, df = 29, P < 0.001) and E (t = −3.97, df = 29, P < 0.001); and clade C has the largest males among clades A–F. Females of clade A are smaller than those of clades B–F (all P values for t tests < 0.001), females of the other clades (B–F) are similar in size ().
Figure 3. Boxplots for snout-vent length (SVL) from females and males of the Osteocephalus verruciger-cannatellai complex. The line in the middle of the box represents the median, and the lower and upper ends of the box are 25% and 75% quartiles, respectively. Letters correspond to those of clades on
Taxonomic review
To assign binomens to clades A–G, we examined the holotype of Osteocephalus cannatellai (QCAZ 49572), one syntype of O. verruciger (ZMB 16589) () and published descriptions of types of O. festae, O. mimeticus, O. mutabor, and O. verruciger. We document those assignments in the following section.
Figure 4. Dorsal, lateral and ventral views of the syntype of Hyla verrucigera (ZMB 16589). Photographs by Frank Tillack

Taxonomic status of Osteocephalus cannatellai
Based on morphology and phylogenetic position of the holotype of O. cannatellai, we assign clade C as O. cannatellai sensu stricto. We suggest that clade B is considered O. cf. cannatellai until a thorough review of both clades is carried out. Differences in size and color suggest that clade B represents a species distinct from O. cannatellai.
Taxonomic status of Osteocephalus verruciger
The description of Hyla verrucigera is based in two syntypes (ZMB 16589 and ZMH A00946). One syntype (ZMH A00946) is an adult specimen and is lost [Citation38]. The type locality is “Ecuador” and the description by Werner [Citation16] is short (Appendix C). It was collected between 1899 and 1900 by Rich Haensch, who traveled through seven provinces in Ecuador () [Citation39,Citation40]: Bolívar (Balsapamba), Guayas (Palmar), Napo (Ahuano, Archidona, Baeza, Papallacta, and Pucurcu), Orellana (Coca), Pastaza (Canelos, Sarayacu), Pichincha (Santa Inés) and Tungurahua (Baños de Agua Santa). This syntype () is a young male with faded coloration, so it cannot be unambiguously assigned to a specific clade within O. verruciger. However, the itinerary of the collector indicates that the type material should fall within clades D, E, or F (). Trueb and Duellman [Citation38] examined syntype ZMB 16589 and considered it “nearly identical” to a juvenile from Cordillera del Dué (Sucumbíos province; KU 123186), which should be part of clade F. The description of the syntype by Trueb and Duellman [Citation38] suggests that it has lost color since they examined it in the 1960s. We tentatively follow Trueb and Duellman [Citation38] in considering clade F as O. verruciger sensu stricto, but note that it could also belong to clades D or E.
Werner [Citation16] description of Hyla verrucigera (Appendix C) indicates that the lost syntype is an adult female with heavily tuberculated dorsal areas. This is inconsistent with the morphology of adult females of O. verruciger because heavy tuberculation is only present on adult males. It is likely that the sex of the syntype reported by Werner was incorrect. No other hylid from the foothill forests in the Amazon basin of Ecuador fits Werner [Citation16] description of a heavily tuberculated dorsum. Therefore, we consider highly unlikely that the syntype belongs to a different species of hylid.
Systematic accounts
Osteocephalus sangay sp. n., LSID: urn:lsid:zoobank.org:act:6C9A96AB-76E8-45FC-84EF-D82988F7DD02
Common name
Proposed standard English name: Sangay casqued treefrog. Proposed standard Spanish name: Rana de casco del Sangay.
Holotype
() QCAZ 58832 (field no. SC-PUCE 48058), adult female from Ecuador, Morona Santiago Province, Sangay National Park, Sardinayacu, Refuge 2 (2.0903°S, 78.1897°W), 1551 m above sea level, collected by Daniel Rivadeneira, Francy Mora, Juan Carlos Sánchez, and Andrea Correa on 18 January 2015.
Figure 5. Adult preserved specimens of Osteocephalus sangay showing variation in dorsal and ventral coloration. From left to right, first and second rows: QCAZ 58832 (holotype, adult female), QCAZ 58824, 58839 (females); third and fourth rows: QCAZ 58825, QCAZ 59125 (females), QCAZ 58840, 59124 (males). All specimens are shown at the same scale. Photographs by Valeria Chasiluisa

Paratypes
ECUADOR: MORONA SANTIAGO PROVINCE: Sangay National Park, Sardinayacu: Laguna Chimerella (2.0885° S, 78.2069° W), 1650 m, QCAZ 58829 (subadult female), 58839 (adult female), 58840 (adult male), same date and collectors as the holotype; Refuge 2 (2.0903° S, 78.1897° W), 1551 m, QCAZ 58834 (juvenile male), same date and collectors as the holotype; Refuge 3 (2.0737°S, 78.2184°W), 1795 m, QCAZ 58823–58825 (adult females), collected by J. Pinto, D. Velalcázar, and D. Núñez on 18 January 2015; Refuge 3, path to Refuge 2 (2.0757° S, 78.2157° W), 1724 m, QCAZ 59124 (adult male), 59125 (adult female), collected by S. Ron, D. Paucar, P. Venegas, P. Baldeón, M. Caminer, and K. Nusirquia on 28 February 2015.
Diagnosis
The diagnosis and comparisons are based on six adult females and two adult males. Coloration corresponds to live individuals. Osteocephalus sangay sp. n. can be diagnosed by the following characters: (1) mean SVL 40.82 mm in males (range 40.32–41.31; n = 2), 49.65 mm in females (range 46.68–52.82; n = 6) (); (2) skin on dorsum bearing keratinized tubercles in males and scattered tubercles in females; (3) skin on flanks areolate; (4) hand webbing formula varying from I basal II12/3–3+III22/3–21/3IV to I basal II2–31/3III22/3–21/2IV; foot webbing formula varying from I12/3–2−II1+–2III1−–2−IV2−–1−V to I11/2–2+ II1+–21/3III11/3–2+ IV2-–1-V; (5) dorsum light to medium brown with irregular dark brown marks; (6) venter varying from cream to tan with dark brown dots; (7) cream suborbital mark present; irregular black labial stripe; (8) flanks cream with darker reticulations and brown blotches dorsally; (9) dermal roofing bones of the skull weakly exostosed; (10) bones green in life; (11) iris bronze with irregular black reticulations and olive green ring around the pupil; (12) paired lateral vocal sacs, behind jaw articulation; (13) larvae unknown.
Description of holotype
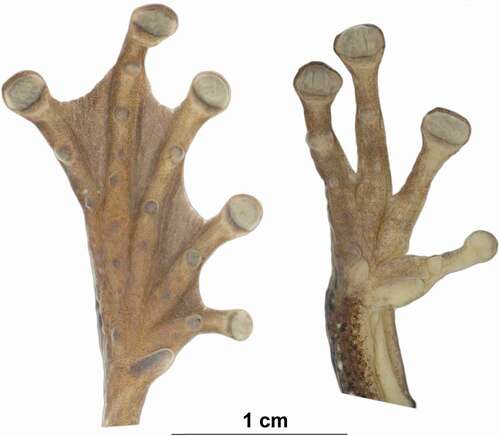
Adult female, SVL 52.39 mm, head length 17.95, head width 15.79, eye diameter 4.35, tympanum diameter 4.15, femur length 25.44, tibia length 27.23, foot length 22.02. Head narrower than body; slightly longer than wide; snout truncate in lateral and dorsal views; distance from nostril to eye longer than diameter of the eye; canthus rostralis rounded; loreal region concave; internarial region depressed; nostrils moderately protuberant; interorbital area slightly concave; eye large, protuberant; tympanum rounded, separated from eye by ca. one time its diameter; tympanic membrane evident; supratympanic fold thin, posterior end not reaching arm insertion. Thin arm; axillary membrane present; ulnar tubercles absent; relative length of fingers I < II < IV < III; fingers bearing large, oval discs; subarticular tubercles prominent, round to ovoid; single; supernumerary tubercles present; palmar tubercle small and round; prepollical tubercle large, elliptical; prepollex enlarged; fingers webbing formula I basal II2−–3+III3–21/2IV (). Toes bearing expanded discs, smaller than those of fingers; relative length of toes I < II < V < III < IV; outer metatarsal tubercle present, round; inner metatarsal tubercle present, ovoid, protuberant; supernumerary tubercles present; toe webbing formula I12/3–21/3II1+–2III1+–2−IV2−–−V. Skin on dorsum, head and dorsal surfaces of limbs with tubercles; skin on flanks areolate; skin on venter and ventral surfaces of thighs granular; ventral surfaces of shanks smooth. Cloacal opening directed posteriorly at upper level of thighs, round tubercles below vent. Tongue cordiform, widely attached to the mouth floor; dentigerous processes of vomers angular, behind ovoid choanae, each bearing 8 and 10 (left/right) vomerine teeth.
Color of holotype in life
Based on digital photographs ()). Dorsum light to medium brown with irregular dark brown marks; dorsal surfaces of forearms brown with few dark brown marks; dorsal surfaces of thighs, shanks, and feet light to medium brown with dark brown irregular flecks. Head with one big dark brown irregular blotch; sides of head brown with an oblique cream to tan bar below the orbit; tympanum dark brown; flanks cream to tan with dark brown reticulations and irregular dark brown blotches anteriorly; dorsal surfaces of thighs, shanks and forelimbs light to medium brown with dark brown flecks. Venter khaki to brown with dark brown marks; olive green ring around the pupil; bronze iris with black reticulations.
Color of holotype in preservative
Dorsum brown with dark brown irregular blotches; a big dark brown blotch in the head between orbits extends to the flank; dorsal surfaces of forearms and thighs brown with dark brown and light brown flecks; dorsal surfaces of shanks and feet light brown with small brown flecks; flanks light cream with irregular blotches; venter light cream with light brown spots, more abundant in the anterior half of the body; ventral surfaces of hindlimbs and forelimbs light to medium brown; sides of head light brown with a light cream bar below the orbit; iris gray with black irregular reticulations ().
Variation
In life, based on digital photographs (), adult male (QCAZ 58840) with dorsal olive-green coloration and keratinized tubercles, canthal region green with cream subocular mark, tympanum light green, flanks light green to cream with dark brown reticulation, venter tan. In adult females (QCAZ 58825 and 58839) dorsal coloration is the same as in preserved specimens, tympanum light brown, flanks light brown with dark brown reticulation and irregular dark brown blotches posteriorly. Canthal region brown with a cream tan (QCAZ 58839) to light yellow (QCAZ 58825) subocular mark, venter tan with brown reticulation in QCAZ 58839, and cream with a faint light brown reticulation in QCAZ 58825.
In preservative (), dorsal background coloration varies from light brown (QCAZ 59125) to dark brown (QCAZ 58825), with irregular dark brown blotches. Young and adult males bear keratinized tubercles (QCAZ 58840 and QCAZ 59124), which are scattered in females (QCAZ 58824). Ventral surfaces vary from light cream (QCAZ 58825 and QCAZ 59125) to tan with dark brown dots (QCAZ 58839).
Comparisons with other species
Osteocephalus sangay is most similar to O. fuscifacies. Both species are similar in having brown to dark brown dorsal skin (females of O. sangay) and bronze iris with thin black reticulation in life. Osteocephalus sangay differs from O. fuscifacies in having: (1) dorsum with prominent and abundant tubercles in males, scattered tubercles in females (dorsum smooth in O. fuscifacies); (2) dorsal surfaces of limbs with irregular dark brown blotches (dark brown bars with light edges in O. fuscifacies); (3) a cream subocular mark (absent in O. fuscifacies); and (4) skin on flanks areolate (smooth in O. fuscifacies). Mitochondrial DNA sequences show that O. sangay and O. fuscifacies belong to different species groups.
Osteocephalus sangay is most closely related to O. cannatellai ( vs. c–f)) from which it differs in having: (1) brown dorsum in females, olive green in males (green dorsum with dark brown blotches in males and females of O. cannatellai); (2) dorsal tubercles in males and females (scattered tubercles in males, smooth skin in females in O. cannatellai); (3) inconspicuous tubercles in tarsus (prominent tubercles in O. cannatellai); (4) iris without a middle band (dark brown band in O. cannatellai); and (5) smaller size in females, SVL in females of O. sangay 49.04 mm (range 45.3–52.8; n = 7), SVL in O. cannatellai 66.5 mm (range 62.6–72.8; n = 33) [Citation9].
Figure 8. Dorsal and ventral view of species within the Osteocephalus verruciger-O. cannatellai complex. A–B Osteocephalus sangay sp. n., QCAZ 58825, (SVL = 48.40 mm), adult female; C–D O. cannatellai, QCAZ 67133, (SVL = 62.24 mm), adult female; E–F O. cf. cannatellai, CORDIBI 10534 (from Cordillera Kampankis, Peru), adult male; G–H O. cf. verruciger (clade D), QCAZ 58827, (SVL = 62.61 mm), adult female; I–J O. cf. verruciger (clade D), QCAZ 58833, (SVL = 47.50 mm), adult male. Photographs by G. Pazmiño, J. C. Sanchez and A. Catenazzi

Osteocephalus sangay is similar to O. verruciger ( vs. g–j)), both species share skin tubercles in males and females. Osteocephalus sangay differs from O. verruciger in having: (1) bronze iris with black reticulations (brown iris without reticulations in O. verruciger); (2) irregular blotches in dorsum and dorsal surfaces of forearms and thighs (absent in O. verruciger); (3) cream to tan venter with brown reticulations (dark brown mottled venter in O. verruciger); and (4) ventral surfaces of forearms and thighs light tan to brown (reddish brown in O. verruciger) [Citation38].
Osteocephalus sangay differs from O. taurinus in having a bronze iris with black irregular reticulation (golden iris with thick black lines radiating from the pupil in O. taurinus) and smaller body size in males and females (males 69.4–76.2 mm, n = 4; females 64.2–94.1 mm, n = 9 in O. taurinus) [Citation42]. Osteocephalus festae can be distinguished from O. sangay in having: (1) cream venter with chocolate blotches (venter without blotches in O. sangay); (2) cream thin labial stripe (irregular black labial stripe in O. sangay) [Citation25]; and (3) brown iris. Osteocephalus sangay differs from O. mutabor in lacking cream lines on heels and vent (present in O. mutabor) [Citation15]. Osteocephalus mimeticus differs from O. sangay in having black iris with some golden blotches and adult males with dorsal yellow brown blotches (absent in adult males of O. sangay) [Citation41].
Distribution and ecology
Osteocephalus sangay is known from eastern Ecuador, Morona Santiago province, Sangay National Park, at four nearby localities (maximum distance between localities: 3.7 km), and elevations between 1551 and 1795 m above sea level. Osteocephalus sangay occurs sympatrically with O. verruciger at the type locality (). This region corresponds to Eastern Montane Forest (based on Ron et al. [Citation17] classification). Specimens were found at night in primary terra firme forest, perching on vegetation up to 2 m above the ground.
Etymology
The specific epithet sangay refers to the area where the species was discovered: Sangay National Park, in Morona Santiago province. The park has more than 3220 lakes and three volcanoes: Sangay, Tungurahua and Altar. The word sangay originates from the native-American Shuar word “Samkay” that means to scare, in reference to violent explosions of this volcano. Sangay National Park is one of the most amphibian species-rich national parks in the world with a total of 100 species [Citation42].
Discussion
Our genetic and morphological results document the existence of an undescribed species of Osteocephalus from Sangay National Park in Ecuador. The new phylogeny for Osteocephalus is consistent with phylogenies published by Ron et al. [Citation9] and Jungfer et al. [Citation5]. We found strong support for a clade that closely allies O. verruciger and O. cannatellai. Our results indicate that this clade is a species complex composed of seven clades. We were able to confirm the species status of one of them, Osteocephalus sangay. Clade B, sister to the new species, presents a geographic distribution, morphological and molecular differences that suggest it could be an undescribed species masked within “O. cannatellai”. Nevertheless, more data are needed to better assess variation within O. cannatellai and solve the taxonomic status of clade B.
Our study found paraphyly among populations of O. verruciger (also reported by Ron et al. [Citation25] but in contrast with Ron et al. [Citation9], and Jungfer et al. [Citation5]). Ron et al. [Citation25] hypothesized that paraphyly could be a result of incomplete lineage sorting, mitochondrial gene capture or the existence of undescribed species within O. verruciger. In our phylogeny, each of the clades of O. verruciger is well supported (), and genetic distances between clades vary from 0.7% to 1.1% (uncorrected p-distance for 16S). More morphological and genetic data from a larger number of populations is needed to solve the status of O. verruciger and describe putative cryptic species.
Within the O. verruciger-O. cannatellai complex we found low genetic divergence between species (0.7% to 1.5% uncorrected p-distance for gene 16S). Previous studies have shown that sister species of hylids are usually separated by higher distances (e.g., Vieites et al. [Citation37]; Caminer and Ron [Citation43]). However, our finding is not completely unprecedented. Coloma et al. [Citation44], for example, found sister species of the Hyloscirtus larinopygion group with distances as low as 1.3%. The occurrence of Osteocephalus sangay and O. verruciger (clade D) in sympatry indicates the existence of reproductive barriers. Sympatric populations showed marked morphological differences without intermediate morphs. Lack of intermediates suggests that hybridization is absent or infrequent between both species.
The elevation range of O. sangay (1551–1795 m.a.s.l) overlaps with that of O. verruciger (1400–2000 m.a.s.l) and differs from the elevation range of O. cannatellai (200–910 m.a.s.l.) and O. cf. cannatellai (310–1240 m.a.s.l), its closest relatives. Non-overlapping altitudinal ranges suggest that divergent selection along the altitudinal gradient may have played a role in speciation of these frogs, as previously hypothesized for Andean amphibians by Graham et al. [Citation45], Salerno et al. [Citation46] and Ron et al. [Citation9].
Author contributions
SRR and MAC conceived and designed the study. VDC wrote the first draft of the manuscript. MAC, AVJ and SRR reviewed and improved the manuscript. VDC, AVJ and MAC analyzed the data.
Acknowledgments
We are particularly thankful to Frank Tillack, who provided photographs of the syntype of Hyla verrucigera. Ana Belén Carrillo and Claudia Terán carried out laboratory work. For specimen collection, locality data and field assistance, we are indebted to Daniel Rivadeneira, Francy Mora, Juan Carlos Sánchez, Javier Pinto, David Velalcázar, Andrea Correa, Darwin Núñez, Diego Paucar, Pablo Venegas, Pamela Baldeón, and Kunam Nucirquia. Research and collecting permits were issued by the Ministerio de Ambiente del Ecuador (MAE-DNB-ARRGG-CM-2014-0002). We specially thank Diego Ortiz for comments on the manuscript and to the personal of Museo de Zoología QCAZ.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Steindachner F. Über zwei noch unbeschriebene Batrachier aus des Sammlung des KK zoologischn Museum zu Wien. Archivio per La Zoologia L’Anatomia E La Fisiologia. 1862;1:77–82.
- Frost DR. Amphibian species of the world: an online reference [Internet]. Version 6.0. New York: American Museum of Natural History; 2018. [cited 2018 Dec 20]. Available from: http://research.amnh.org/herpetology/amphibia/index.html
- Duellman WE, Trueb L. Synopsis of neotropical Hylid frogs, genus Osteocephalus. Occasional papers of the Museum of Natural History vol 1. The University of Kansas; 1971. p. 1–47.
- AmphibiaWeb. AmphibiaWeb: information on amphibian biology and conservation [Internet]. Berkeley, CA, USA: University of California; 2018. [cited 2020 Jan 26]. Available from: https://amphibiaweb.org
- Jungfer KH, Faivovich J, Padial JM, et al. Systematics of spiny-backed treefrogs (Hylidae: Osteocephalus): an Amazonian puzzle. Zool Scr. 2013;42(4):351–380.
- Goin CJ. Synopsis of the genera of hylid frogs. Ann Carnegie Mus. 1961;36:5–18.
- Duellman WE, Mendelson JR. Amphibians and reptiles from northern Departamento Loreto, Peru: taxonomy and biogeography. University of Kansas. Sci Bull. 1995;55:329–376.
- Jungfer KH, Verdade VK, Faivovich J, et al. A new species of spiny-backed treefrog (Osteocephalus) from central Amazonian Brazil (Amphibia: Anura: Hylidae). Zootaxa. 2016;4114(2):171–181.
- Ron SR, Venegas PJ, Toral E, et al. Systematics of the Osteocephalus buckleyi species complex (Anura, Hylidae) from Ecuador and Peru. ZooKeys. 2012;229:1–52.
- Cochran DM, Goin CJ. Frogs of Colombia. Bull US National Mus. 1970;288:1–655.
- Jungfer KH. A new tree frog of the genus Osteocephalus from high altitudes in the Cordillera del Cóndor, Ecuador (Amphibia: Anura: Hylidae). Herpetological J. 2011;21(4):247–253.
- Peracca MG, del V. Dr. Enrico Festa nell’Ecuador e regioni vicine. Bollettino Dei Musei Di Zoologia Ed Anatomia Comparata. 1904;465:1–41. Università di Torino.
- Ruthven AG. The amphibians of the university of Michigan-Walker expedition to British Guiana. Occasional Papers of the Museum of Zoology vol. 69. University of Michigan; 1919. p. 1–14.
- Melin D. Contribution to the knowledge of the Amphibia of South America. Vol. 1. Göteborgs Kungl: Vetenskaps-och Vitterhets-samhälles. Handlingar. Serien B, Matematiska och Naturvetenskapliga Skrifter; 1941. p. 1–71.
- Jungfer KH, Hödl W. A new species of Osteocephalus from Ecuador and a redescription of O. leprieurii (Duméril and Bibron, 1841)(Anura: Hylidae). Amphibia-Reptilia. 2002;23(1):21–46.
- Werner F. Ueber reptilien und batrachier aus Ecuador und Neu-Guinea. Verhandlungen des Zoologisch-Botanischen Vereins in Wien. 1901;50:593–614.
- Ron SR, Merino-Viteri A, Ortiz DA. Anfibios del Ecuador [Internet]. Version 2019.0. Quito: Museo de Zoología, Pontificia Universidad Católica del Ecuador; 2018. [cited 2018 Dec 15]. Available from: https://bioweb.bio/faunaweb/amphibiaweb
- Savage JM, Heyer WR. Digital webbing formulae for anurans: a refinement. Herpetological Rev. 1997;28(3):131.
- Duellman WE. The hylid frogs of middle America. Monograph of the Museum of Natural History, the University of Kansas. 1970;1:1–753.
- SAS Institute. User guide. SAS institute. Cary [Internet]. Version 9.01. 2010. [cited 2018 Dec 15] Available from: http://www.jmp.com
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold spring harbor laboratory press; 1989, Cold Spring Harbor, New York, USA.
- Kearse M, Moir R, Wilson A, et al. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649.
- Katoh K, Toh H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics. 2010;26(15):1899–1900.
- Faivovich J, Haddad CF, Garcia PC, et al. Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bull Am Mus Nat Hist. 2005;294:1–240.
- Ron SR, Toral E, Venegas PJ, et al. Taxonomic revision and phylogenetic position of Osteocephalus festae (Anura, Hylidae) with description of its larva. ZooKeys. 2010;70:67–92.
- Moen DS, Wiens JJ. Phylogenetic evidence for competitively driven divergence: body-size evolution in Caribbean treefrogs (Hylidae: Osteopilus). Evolution. 2009;63(1):195–214.
- Moravec J, Aparicio J, Guerrero-Reinhard M, et al. A new species of Osteocephalus (Anura: Hylidae) from Amazonian Bolivia: first evidence of tree frog breeding in fruit capsules of the Brazil nut tree. Zootaxa. 2009;2215(1):37–54.
- Salducci MD, Marty C, Fouquet A, et al. Phylogenetic relationships and biodiversity in hylids (Anura: Hylidae) from French Guiana. C R Biol. 2005;328(10–11):1009–1024.
- Wiens JJ, Fetzner JW Jr, Parkinson CL, et al. Hylid frog phylogeny and sampling strategies for speciose clades. Syst Biol. 2005;54(5):778–807.
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. Version 3.04. 2015. [cited 2018 Dec 15] Available from: http://mesquiteproject.org
- Lanfear R, Calcott B, Ho SY, et al. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol. 2012;29(6):1695–1701.
- Zwickl DJ. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion [dissertation]. University of Texas; 2006.
- Ronquist F, Teslenko M, Van Der Mark P, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61(3):539–542.
- Rambaut A, Drummond AJ. Tracer: MCMC trace analysis tool v 1.6. University of Oxford; 2013. [cited 2016 Jul 13] Available from: https://tree.bio.ed.ac.uk/software/tracer/.
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874.
- Fouquet A, Gilles A, Vences M, et al. Underestimation of species richness in Neotropical frogs revealed by mtDNA analyses. PLoS One. 2007;2(10):e1109.
- Vieites DR, Wollenberg KC, Andreone F, et al. Vast underestimation of Madagascar’s biodiversity evidenced by an integrative amphibian inventory. Proc Nat Acad Sci. 2009;106(20):8267–8272.
- Trueb L, Duellman WE. The systematic status and life history of Hyla verrucigera Werner. Copeia. 1970;1970:601–610.
- Haensch R. Kurzer Bericht über die entomolog. Ergebnisse meiner Ecuador-Reise Berliner Entomologische Zeitschrift. 1903;48(3):149–156.
- Ron SR. Anfibios de Parque Nacional Yasuní, Amazonía ecuatoriana [Internet]. Version 1.7. Quito: Museo de Zoología, Pontificia Universidad Católica del Ecuador; p. 2001–2011.
- Jungfer KH. The taxonomic status of some spiny-backed treefrogs, genus Osteocephalus (Amphibia: Anura: Hylidae). Zootaxa. 2010;2407:28–50.
- Ron SR, Merino-Viteri A, Ortiz DA. Anfibios del Ecuador [Internet]. Version 2019.0. Quito: Museo de Zoología, Pontificia Universidad Católica del Ecuador. 2019. [cited 2019 Jan 3]. Available from: https://bioweb.bio/faunaweb/amphibiaweb
- Caminer MA, Ron SR. Systematics of treefrogs of the Hypsiboas calcaratus and Hypsiboas fasciatus species complex (Anura, Hylidae) with the description of four new species. Zookeys. 2014;370:1–68.
- Coloma LA, Carvajal-Endara S, Duenas JF, et al. Molecular phylogenetics of stream treefrogs of the Hyloscirtus larinopygion group (Anura: Hylidae), and description of two new species from Ecuador. Zootaxa. 2012;3364(1):1–78.
- Graham CH, Ron SR, Santos JC, et al. Integrating phylogenetics and environmental niche models to explore speciation mechanisms in dendrobatid frogs. Evol. 2004;58(8):1781–1793.
- Salerno PE, Ron SR, Señaris JC, et al. Ancient tepui summits harbor young rather than old lineages of endemic frogs. Evol. 2012;66(10):3000–3013.
- Goebel AM, Donnelly JM, Atz ME. PCR primers and amplification methods for 12S ribosomal DNA, the control region, cytochrome oxidase I, and cytochromebin bufonids and other frogs, and an overview of PCR primers which have amplified DNA in amphibians successfully. Mol Phylogenet Evol. 1999;11(1):163–199.
- Hedges SB, Duellman WE, Heinicke MP. New World direct-developing frogs (Anura: Terrarana): molecular phylogeny, classification, biogeography, and conservation. Zootaxa. 2008;1737(1):1–182.
- Palumbi SR, Martin A, Romano S, et al. The simple fool’s guide to PCR. version 2.0 vol. 45. University of Hawaii, Honolulu. 1991. 1–24.
- Folmer O, Black M, Hoeh W, et al. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3(5):294–299.
Appendies
Appendix A. Examined specimens.
Osteocephalus cannatellai. ECUADOR: NAPO PROVINCE: Reserva Ecológica Yachana (0.84575°S, 77.22872°W), 317 m (QCAZ 48797); ORELLANA PROVINCE: San José de Payamino (0.48169°S, 77.29633°W), 285 m (QCAZ 56646); Comunidad Sinchichicta, Río Napo, Pad 16 (0.68041°S, 75.75683°W), 202 m (QCAZ 57929); PASTAZA PROVINCE: Villano B, Campo Villano, Bloque 10-Agip Oil (1.45338°S, 77.44171°W), 393 m (QCAZ 56636); Comunidad Kurintza, Campo Villano, Bloque 10-Agip Oil (1.50919°S, 77.51327°W), 269 m (QCAZ56371); Río Bobonaza (1.74611°S, 77.43720°W), 325 m (QCAZ 52767); ZAMORA CHINCHIPE PROVINCE: Centro Shuar Yawi (4.44148°S, 78.64934°W), 935 m (QCAZ 31047, 31051, 31053); Reserva Natural Maycu (4.20896°S, 78.63527°W), 882 m (QCAZ 67133).
Osteocephalus mutabor. ECUADOR: PASTAZA PROVINCE: Centro Ecológico Zanjarajuno, Río Pucayacu (1.37213°S, 77.85224°W), 943 m (QCAZ 36935); Parque Nacional Llanganates, Comunidad Zarentza (1.36263°S, 78.05820°W), 1350 m (QCAZ 59892, 59899, 59900); PERU: unknown locality, Germán Chávez field series (RO PM07P).
Osteocephalus verruciger. ECUADOR: MORONA SANTIAGO PROVINCE: Comunidad 9 de Octubre (2.22543°S, 78.26811°W), 1588 m (QCAZ 62213); Parque Nacional Sangay, Sardinayacu (2.07378°S, 78.21847°W), 1795 m (QCAZ 58808, 58826–28, 58831, 58833, 58838, 58841, 58847–48, 59126–59128); NAPO PROVINCE: Río Pucuno, sendero Pacto Sumaco-Volcán Sumaco (0.63391°S, 77.59228°W), 1602 m (QCAZ 41109–10, 41113–15, 41152, 41154); Pacto Sumaco, vertiente E del Volcán Sumaco (0.56969°S, 77.59412°W), 1570 m (QCAZ1560, 1562); Sumaco, 1800 m (QCAZ8964); WildSumaco (0.67610°S, 77.59932°W), 1487 m (QCAZ 57342, 62032); Bosque Protector “La Cascada” (0.14572°S, 77.49593°W), 1435 m (QCAZ 54619); PASTAZA PROVINCE: Parque Nacional Llanganates, Comunidad Zarentza (1.35644°S, 78.05807°W) 1367 m (QCAZ 59908, 59913, 59915–16, 59919, 59932, 59935–36); SUCUMBIOS PROVINCE: Hostería El Reventador (0.07374°S, 77.59327°W), 1881 m (QCAZ 52341, 66712–15, 66717–18).
Osteocephalus vilmae. ECUADOR: ORELLANA PROVINCE: Comunidad Boamano, Río Araña (1.25374°S, 76.37248°W), 211 m (QCAZ 54274); Parque Nacional Yasuní, km 80 on the road Pompeya Sur-Iro (0.8401°S, 76.30177°W), 233 m (QCAZ 51205); PASTAZA PROVINCE: Lorocachi (1.62525°S, 75.99035°W), 214 m (QCAZ 56026).
Appendix B. Primers used for DNA amplification.
Appendix C. Original description of Hyla verrucigera by Werner [Citation16].
Hyla verrucigera nov. spec.
Relative with H. buckleyi and H. leprieuri.
Tongue behind clearly free and notched, circular. Toothed palate between the back halves of the choanae, forming a figure in shape of\/. Depressed (flat) head, as long as wide, wider than body. Rounded snout, 1–11/2 times as long as diameter of the eye. Loreal region curved, concave. Snout edge clearly, almost straight. Tympanum very clearly, approximately half the width of the ocular diameter; on this a strong fold. Interorbital space twice as wide as upper eyelid.
Three external fingers of the hand with one-third palmate. Lacking pollex rudiment. Natatorial membranes of toes only reach in the fourth finger until the base of the penultimate, otherwise until the last phalanx. Discs of fingers slightly smaller than tympanum, on toes only half as wide. Posterior legs reach nostrils and one eye with the tibial joint.
Dorsal surfaces of head, trunk and back legs (except the thighs) strongly tuberculated. Anterior legs, dorsal and ventral thighs smooth, granulated only in the thorax and venter. Body sides are also smooth, clearly distinct from the tuberculated dorsum. Dark brown dorsum, monochromatic. Ventral part light brown, dirty white venter, black brown mottled.
Total length of 51 mm.
I have two copies, a larger one (female) and a juvenile.