ABSTRACT
Island endemic reptiles face many threats that potentially have a negative effect on their conservation status, such as habitat loss, interactions with introduced predators and competitors, and stochastic environmental events. Since confirmation of the continued existence of the Barbados Leaf Toed gecko (Phyllodactylus pulcher) on the island of Barbados, West Indies, efforts have been made to increase ecological knowledge of the gecko in order to inform conservation direction and safeguard this critically endangered endemic species. The present study is the first to determine the microhabitat use of P. pulcher and that of the non-native gecko Hemidactylus mabouia on Barbados, and makes comparison to patterns of displacement of native geckos following Hemidactylus spp. invasions seen elsewhere. Factor Analyses of gecko locations and habitat data collected during night-time surveys indicated a strong non-random selection by P. pulcher for structurally diverse, rock habitat with an abundance of natural crevices. Where H. mabouia is present in natural habitat along the Barbados coast, the species also selected for exposed rocky habitat, albeit to a significantly lesser degree than did P. pulcher. Measures of body condition suggest the non-native gecko is less able to exploit such habitats and that its ability to thrive on Barbados is due to utilisation of a broader niche as befitting a habitat “generalist”, rather than competitive displacement of the native gecko from cliff side habitat. Our findings highlight the importance for protection of the remaining natural cliff side habitat and promotion of biosecurity on Barbados as being key to the survival of P. pulcher.
Introduction
Predation from introduced mammalian predators, such as the Asian mongoose (Herpestes javanicus), rats (Rattus spp.), and cats (Felis catus) is undoubtedly the major cause of population declines of many endemic island reptile species [Citation1]. However, there is also growing evidence that competitive interactions between non-native and native reptile species can significantly affect abundance, distribution, and community structure [Citation2–6]. Yet, unlike predation, there are few documented cases where competition has been implicated as the causal mechanism threatening the extinction of reptile species in particular [but see Citation6,Citation7].
Interspecific competition occurs when ecologically similar species overlap in their utilisation of food, space, or time niches [Citation8]. Interactions caused by this overlap are generally categorised as either direct interference, with one species aggressively displacing the other from a shared resource, or indirect, such as exploitative competition, where the ability of a competitor to acquire a key resource prevents or reduces the other species from obtaining the same resource [Citation9]. The outcomes of conflict arising from such overlap are often asymmetrical, most demonstrably in relation to body size [Citation8,Citation9], which in time can lead to niche segregation, character displacement, exclusion of inferior competitors from a particular niche, and potentially, extinction [Citation8–12].
Lizards have long been an important model species for research on the occurrence of competition in nature and investigations into its role in shaping ecological communities [Citation10,Citation13–15]. Furthermore, the incidence of introductions of non-native lizard species to island ecosystems has provided opportunities for researchers to observe the mechanisms of competition underlying successful colonization events [Citation2,Citation7,Citation16,Citation17].
House geckos in the genus Hemidactylus are amongst the most successful colonising invasive species and are reported to be negatively impacting native gecko species on a global scale [Citation18–20], particularly in urban habitats [Citation21,Citation22]. On the islands of Curaçao and Bonaire in the southern Caribbean, the relatively rapid displacement of the Dutch Leaf-Toed gecko (Phyllodactylus martini) from otherwise occupied buildings, to structures nearer the forest, has been observed following the introduction of H. mabouia to the islands in the late 1980’s [Citation23,Citation24]. Hemidactylus mabouia is well established in the wider Caribbean [Citation25], and earliest reports of the species being present on Barbados, where it is ubiquitous on and in buildings (RW, pers obs), date back to the 1700’s [Citation26]. In light of large-scale patterns of negative impacts on native geckos, the long-established presence of H. mabouia on Barbados may well have contributed to the displacement, and thus documented scarcity of, the ecologically and morphologically similar Barbados Leaf-Toed gecko (Phyllodactylus pulcher) from suitable habitat on the mainland [Citation27].
Endemic to the island of Barbados, P. pulcher is unique amongst the region’s reptile fauna as being the only species of the genus described from the Lesser Antilles [Citation28]. Rediscovered in 2011, only recently has detailed information on the natural history and limited range of the Barbados Leaf-Toed gecko been published [Citation29]. Owing to its scarcity and restricted range the species now has an IUCN classification of Critically Endangered [Citation30].
Experimental trials testing the diurnal refuge preference of both P. pulcher and H. mabouia, have shown considerable overlap in refuge preference, and a tendency for both species to actively select for refugia conditioned with scent from the potential competitor, suggesting there may be considerable niche overlap and contest for preferred refugia in the wild [Citation31]. Furthermore, niche separation has been observed in surveys of anthropogenic features in coastal cliff habitats, where in contrast to patterns of displacement of native species by H. mabouia seen elsewhere, P. pulcher appears more abundant than the non-native on coastal cliff edge walls [Citation31].
Established theory on competition posits that competition is expected to decrease over time as species coevolve or disappear from areas of sympatry by local extirpation [Citation4,Citation14]. Any competitive interaction between H. mabouia and P. pulcher may therefore have been mediated by time and be reflected in current variations in species distribution, abundance, individual fitness, and microhabitat use [Citation8–10,Citation14].
The aims of the present study were (a) to provide an assessment of the primary patterns of microhabitat use by P. pulcher throughout its range and (b) to quantify and compare the spatial patterns of microhabitat use and individual fitness between P. pulcher and a potential competitor, H. mabouia.
Methods
Study site
This study was undertaken in Barbados, the most easterly of the Caribbean islands (13°N, 59°W). With a total area of 431 km2, the coral limestone island rises to a maximum elevation of only 340 m, creating largely xeric conditions [Citation32]. Natural vegetation on Barbados has become reduced to around 2% of its historical extent prior to European settlement (ca. 800 ha remain), with indigenous flora largely restricted to small patches of woodland, gullies and coastal fringes [Citation33].
Survey method
Fieldwork was conducted in coastal habitat found within 100 m of the waterline in the six island parishes on the south, east, and northern coastline of Barbados. This survey area ensured coverage of habitat seen to be used by P. pulcher during targeted surveys of 2012 and 2013 [Citation29]. The west and southwest coasts were not surveyed due to the habitat within 100 m of the waterline being either intertidal or highly urbanized (). Urban environments account for 74% of the land use within 100 m of this leeward shore, as opposed to 15% on the east and south east coasts [Citation34].
Figure 1. Barbados, its parish boundaries, and extent of the 2014 gecko study area (100 m from waterline shown in black and red). The red area indicates portion of survey area that was also the focus of a separate analysis comparing gecko habitat use on the North coast to elsewhere. Distribution of the number of points surveyed within the study area for each parish is given in parenthesis
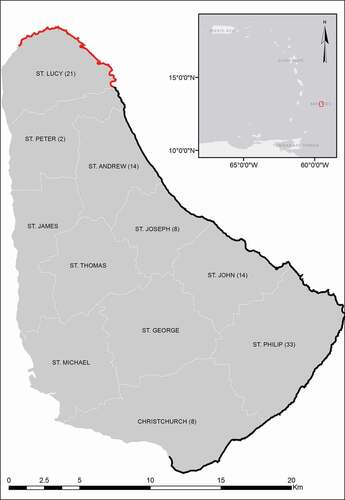
We used ArcMap ver.10.1 (Environmental Systems Research Institute) to create potential survey points at 25 m spacing in a grid formation throughout the study area. 2249 points were generated by this grid, from which one hundred points were then randomly selected (in ArcMap) to form the basis of the habitat use survey. This sample area represented 5% of the potential study area generated. To ensure equal survey effort throughout the study area, we stratified the distribution of the 100 points to be surveyed (during the random selection) by the total number of potential survey points falling within each of the six parishes that comprised the survey area ().
All 100 points were surveyed over the course of a four-week period, beginning 4 April 2014. Points were navigated to within ± 1 metre accuracy using hand held GPS (Garmin etrex 10™), and upon approach an assessment was made in consideration of safety and accessibility to carry out the survey at that location. In incidences where predetermined points were deemed unsafe or inaccessible, an adjacent (accessible/safe) point on the grid was surveyed instead.
Point surveys entailed the night-time visual search for both gecko species within a 12.5 m radius around each point. We judged this search area to be of a practical size to carry out a comprehensive search for geckos during 30 min of search effort (3 × 10 surveyor mins), based on previous experience surveying for the species on Barbados [Citation29]. We undertook searches between the hours of 18:00 and 23:30, to coincide with the period of peak activity for both gecko species [Citation29,Citation35]. The search team consisted of three experienced surveyors who searched for a period of 10 minutes using head torches (white light). Surveyors searched immediate habitat by scanning trees, the ground, rock faces, and likely gecko refuges such as crevices, loose rocks, logs, and debris piles. We took care to spread the search effort within the search area, and to not degrade habitat quality by undue disturbance of habitat features and careless interference of potential refugia [Citation36].
We recorded locations (at point of first sighting) of all geckos seen during the 10 min search, and opportunistically en-route to and from survey points, on hand held GPS. In addition, we temporarily marked locations with biodegradable flagging tape. All observed geckos were identified to species by pattern (P. pulcher has a distinctive lateral stripe running from the snout through the centre of the eye, and a bolder, more symmetrical dorsal pattern than H. mabouia), body shape, and posture. When possible, we caught geckos (by hand) for morphological analysis, placing them in individual breathable cloth bags prior to processing. Following gecko searches, we recorded data on microhabitat variables within a 1 m radius of the first observed location of all gecko encounters, as outlined in . To establish whether gecko habitat use was non-random, we collected the same microhabitat data from random control points that simulated gecko locations [after Citation37]. Since habitat structure (e.g. vertical surfaces, raised perches) is important to many geckos, we generated these simulated locations by releasing a half inflated, untied balloon from the point of every real gecko sighting, taking the point of first impact of the deflated balloon as the location for the control point. This ensured we accounted for structural features in the collection of habitat data. If we didn’t find any geckos during the initial search, we took habitat data from two random points generated from the survey point itself (established when optimal GPS proximity to 0 metres was achieved) using the method above. This method therefore resulted in a minimum of two locations where we recorded habitat data around each survey point. Additional habitat data were recorded from point of first sighting of geckos we observed opportunistically, and geckos seen during a separate mark-recapture study undertaken at St Phillip (also in April 2014) [Citation29].
Table 1. Summary of habitat variables recorded during random surveys, methods of recording, and equipment used
Habitat use data analysis
We explored microhabitat use data by factor analysis to reduce the habitat variables into a smaller number of components that may describe underlying patterns. Data were initially transformed prior to analysis using Log10 + 1 for continuous variables and arcsine-square root for percentages. We considered Factors with eigen values ≥1 as significant and used them in further analysis. Within factors, we considered variables with loadings greater than 0.4 as important [Citation37,Citation38]. We then applied analysis of variance (ANOVA) to determine whether factor scores varied between gecko microhabitats and microhabitat data from random points (simulated locations), followed by Post-hoc analysis using Fisher’s LSD to identify where significant variations lay between groups. We ran two separate factor analyses to ask specific questions about the gecko habitat use data set. The first run was primarily concerned with the entire data derived from the random point survey to determine if geckos were using microhabitat non-randomly, and if variation in habitat use existed between the two species. In the second analysis, we investigated whether P. pulcher is potentially being excluded from suitable habitat in the north of the island. We based this investigation on observations of a contrast between the frequency of encounters of P. pulcher and H. mabouia on the north and south coast, despite similar appearances in macrohabitat at both locations [Citation39]. In this latter analysis, we compared habitat use data collected from P. pulcher locations throughout the entire study area to data from random points and H. mabouia locations along the coast at North Point, St Lucy (). In order to compare the availability and character of naturally formed refuges between these locations in greater detail, we included observed frequencies of various sized crevices (see ), under-rock refuges, and holes/burrows as habitat variables in this second analysis. Due to sand substrate being recorded at only one point, and an absence of anthropogenic features at these survey locations, associated habitat variables were omitted from the analysis.
Morphometric data analysis
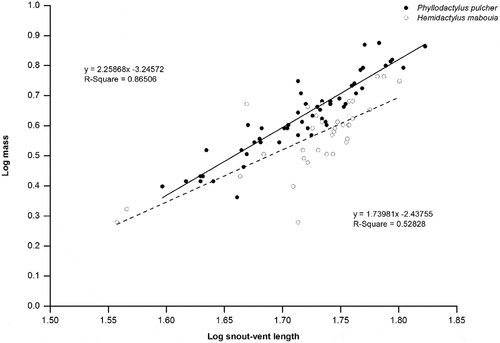
We collected morphological data from geckos encountered during the survey as part of a wider study [Citation39], and used the data to generate body condition scores for all captured animals. Geckos were caught by hand and weighed to 0.5 g using a 60 g Pesola spring balance. Snout-to-vent length (SVL) was measured to 0.5 mm using digital Vernier callipers. All geckos were returned to the point of capture following sampling. We tested for variation in body size (SVL) between species using students t-test (with data having met assumptions for parametric tests), and made additional comparison (z test) to data reported from other H. mabouia populations in the literature [Citation35,Citation40,Citation41]. We tested for variance (ANOVA) in body condition between P. pulcher and H. mabouia. using an index of body condition created from the residuals of linear regression on log transformed SVL and mass data of individuals [Citation42]
Results
Habitat data were collected from a total of 85 P. pulcher locations, 69 H. mabouia locations, and 184 simulated control locations This included data obtained from the point surveys, locations of geckos seen opportunistically and en route to/from survey points, and data obtained during mark-recapture sessions (see methods) ().
Table 2. Summary of number of locations at which habitat use data was collected, attributable to species, control points, and survey method. Opportunistic sightings refer to geckos encountered en route to and from random survey points
Factor analysis 1: microhabitat selection
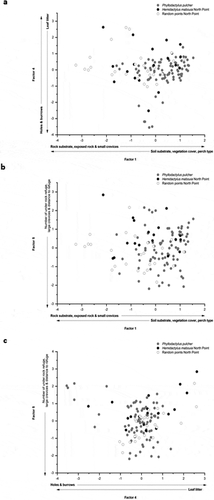
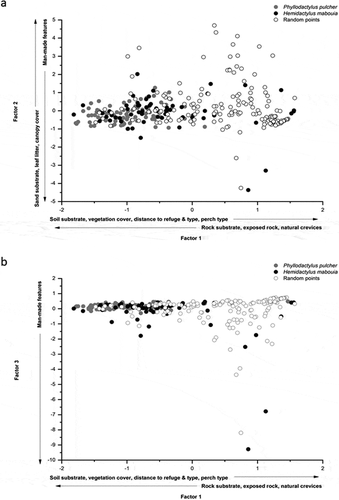
Factor analysis of 18 habitat variables recorded at gecko locations (n = 154) and simulated locations (n = 184) produced five significant factors, cumulatively accounting for 66% of the variance within the data (). Factor 1 was defined by positive loading values for Percentage of substrate that was soil, Percentage of vegetation ground cover, Perches on ground vegetation, and Increasing distance to the nearest refuge. There were negative loading values for Percentage of rock substrate, Percentage of exposed bare rock, Number of naturally formed crevices, and Vertical orientation on perches. The Factor 1 axis therefore represents the gradient from sites of grassland habitat (highly loading factor scores), where the only available perch is ground vegetation and refuges are sparse (such habitat often provided no refuge within the survey radius); to structurally diverse habitat of exposed rock with naturally formed crevices (negative factor scores; )). The second factor was defined by positive values for Percentage of sand substrate, Percentage leaf litter cover, Canopy cover, and the Number of refuges other than crevices. These positive scores denote littoral woodland habitat and dune habitat of tall vegetation, where holes and burrows are abundant in the soft substrate. Negative values were for Percentage of artificial substrate and Percentage of ground covered by anthropogenic materials – indicative of habitat features such as walls of residential properties, synthetic debris, or construction materials. These anthropogenic features were also the only variables of significance in Factor 3, again with negative loadings, but including Frequency of artificially formed crevices ()). Factor 4 had positive loading variables attributable to woodland habitat, such as Amount of leaf litter, Percentage of canopy cover and Substrate temperature. Negative loadings were with Percentage of sand substrate and amount of exposed bare soil/sand, both characteristics associated with dunal habitat. The fifth factor defined by positive loadings for Percentage of substrate that was bare soil/sand, Type of nearest refuge (in this case holes/burrows), and Substrate temperature ().
Table 3. Factor analysis loadings, eigenvalues, and percentage of variation explained by five significant factors resulting from factor analysis of habitat use variables collected from P. pulcher (n = 85) and H. mabouia (n = 69) sightings, and random points (n = 184) during surveys of the Barbados coast. Shaded columns indicate factors where significant variation in scores occurred between gecko species and random points. Factor loadings with absolute values ≥ 0.4 (indicated by an asterisk) are considered important [Field, 2000; in Pernetta et al. [Citation37]]
Figure 2. Relationship between factor scores of P. pulcher (grey circles; n = 87), H. mabouia (black circles; n = 69), and random habitat points (open circles; n = 184) for (a) Factors 1 and 2 and (b) Factors 1 and 3
ANOVA, applied to individual scores of the factor analysis to test for differences in habitat selection between both gecko species, and for comparison with random points, showed significant variation in mean factor scores between P. pulcher, H. mabouia, and random points, for Factors 1 (F2, 337 = 128.72, P < 0.0001), 2 (F2, 337 = 4.38, P = 0.01), and 3 (F2, 337 = 3.54, P = 0.03). There was no significant variation in mean factor scores between groups for Factors 4 (F2, 337 = 0.03, P= 0.975) and 5 (F2, 337 = 0.32, P = 0.728). Fisher’s LSD post-hoc test indicated where the significant variations lay between groups in regard to Factors 1, 2 and 3 ().
Table 4. Results of Fisher’s LSD post hoc test identifying variation in mean scores for factors 1, 2, and 3 between groups (P = P. pulcher, H = H. mabouia, R = random points). Asterisk denotes significant variation in mean scores between groups
There was significant variance in mean scores between both gecko species, and with random points, with regard to Factor 1. Although both gecko species scored negatively (P. pulcher, −0.9441 ± S.E. = 0.56; H. mabouia, −0.345 ± S.E. = 0.895), suggesting active selection by geckos for habitats with greater amounts of exposed rock and naturally formed crevices, the significant variation between species scores indicated that this preference was more strongly exhibited by P. pulcher. Random points differed significantly from both gecko species in regard to mean scores for Factor 2 (random points, 0.1455 ± S.E. = 1.1862; P. pulcher, −0.1945 ± S.E. = 0.4595; H. mabouia, −0.143 ± S.E. = 0.897), suggesting that woodland habitat and tall scrub habitat featured more significantly in the random samples than at gecko locations. The negative mean score of H. mabouia for Factor 3 (−0.269 ± S.E. = 1.490) was significantly different to the mean scores of both P. pulcher (0.1433 ± S.E. = 0.1664) and random points (0.0331 ± S.E. = 0.9881), indicating that more H. mabouia were seen in association with anthropogenic features during the surveys ()).
Factor analysis 2: Phyllodactylus pulcher microhabitat use vs. available microhabitat and Hemidactylus mabouia habitat use at North Point
Seven factors of significance were produced from analysis of the 17 variables; which cumulatively explained 73% of the variation in the data (). Results from an ANOVA applied to test variance in mean scores between gecko locations and random points for each of these factors indicated significant variation between groups for Factors 1, 4, and 5 (Factor 1, F2, 125 = 23.25, P < 0.0001; Factor 4, F2, 125 = 3.19, P = 0.04; Factor 5, F2, 125 = 4.96, P = 0.008). Factor 1 alone accounted for 24% of the variance in the data. There was no significant variation in mean factor scores between the three groups for Factor 2 (F2, 125 = 1.76, P = 0.176), Factor 3 (F2, 125 = 1.04, P = 0.356), Factor 6 (F2, 125 = 1.42, P = 0.247), or Factor 7 (F2, 125 = 2.74, P = 0.068).
Table 5. Factor analysis loadings, eigenvalues, and percentage of variation explained by seven significant factors resulting from factor analysis of habitat use variables collected from all P. pulcher (n = 84) in natural habitat, and H. mabouia locations (n = 18) and random points (n = 26) from the northern tip of the Barbados coast (North point). Shaded columns indicate factors where significant variation in scores occurred between gecko species and random points. Factor loadings with absolute values ≥ 0.4 (indicated by an asterisk) are considered important [Field, 2000; in Pernetta et al. [Citation37]]
Fisher’s LSD post hoc test showed mean scores differed significantly for all three groups with regard to Factor 1 (). Only P. pulcher locations had a positive mean score (mean (± S.E.) = 0.353 ± 0.691), which suggests the species was using habitat with higher Percentage of rock substrate, Exposed rock, and greater Frequency of small crevices than was observed at H. mabouia locations and at random points recorded at North Point. The negative mean scores for Factor 1 of H. mabouia and random points from North Point (H. mabouia, −0.309 ± S.E. = 1.00; random points, 0.925 ± S.E. = 1.198) suggest that these sites were of increased Soil substrate, Vegetation ground cover, and Perch types other than rock (vegetation, bare soil/sand). However, the significant difference between the two mean scores suggests H. mabouia were selecting habitat at a lesser extreme on this gradient than was observed at random points ()). The positive mean score of H. mabouia (0.350 ± S.E. = 1.280) differed significantly from the negative mean score of P. pulcher (−0.158 ± S.E. = 0.928) with regard to Factor 4 (). A positive mean score for this factor suggests H. mabouia at North Point were using habitat that had a higher Frequency of holes and burrows, whilst the negative score of P. pulcher locations indicated use of habitat with more Leaf litter ()). Hemidactylus mabouia from North Point also scored a positive mean for Factor 5 (0.641 ± S.E. = 0.916) which was negatively correlated with the Number of large crevices and under rock refuges, as well as the Distance to nearest refuge ()). This score was significantly different to the negative mean scores for both P. pulcher (−0.060 ± S.E. = 1.008) and random points (−0.251 ± S.E. = 0.872) ().
Table 6. Factor analysis 3: results of Fisher’s LSD post hoc test identifying variation in mean scores for factors 1, 4, and 5 between groups (P = P. pulcher, H = H. mabouia at North point, R = random points at North point). Asterisk denotes significant variation in mean scores between groups
Body size and condition
Mean adult body size (SVL) was similar (t-test, t84 = −1.30, P = 0.19) for both P. pulcher (53.5 ± S.E. = 0.6, n = 55) and H. mabouia (54.7 ± S.E. = 0.6, n = 34). However, mean adult SVL of H. mabouia was significantly shorter (Z test, z = −17.23, P= < 0.0001) than that recorded for the species on Anguilla, Lesser Antilles, (59.2 ± S.E. = 1.4, n= 17) by Howard et al. [Citation40], in Florida (z = −5.33, P= < 0.0001 (58.7 ± S.E. = 4.3, n = 20) by Meshaka [Citation35], and in Brazil (z = −152.63, P < 0.0001) (56.6 ± S.E. = 0.07; n = 176) by Anjos and Rocha [Citation41]. When comparing body mass vs length ratios, there were no significant differences between sex for P. pulcher (ANCOVA) (F1 = 1.67, P= 0.20) or H. mabouia (F1 = 1.06, P = 0.31). There was significant variation in body condition between the two gecko species in coastal habitat of Barbados, with P. pulcher individuals having greater body mass for a given SVL than H. mabouia (ANCOVA F1 = 38.86, P = < 0.0001) ().
Discussion
Through our analysis of the microhabitat variables recorded during the random point surveys, we were able to identify important habitat features conducive to the persistence of P. pulcher in its current range. Our results provide evidence for a strong non-random selection by P. pulcher for structurally diverse, rock habitat with an abundance of natural crevices. Phyllodactylus pulcher has also been found to occur at high densities in certain anthropogenic habitats; particularly unlit coastal walls made of coral stone and concrete block adjacent to cliff side habitat [Citation31]. The microhabitat selection demonstrated by P. pulcher in our results supports the theory that these high densities occur because some walls serve to augment microhabitat features of the connecting cliffs that are important to the species (i.e. increased number of small crevices) [Citation31]. Other Phyllodactylids are known to be habitat specialists, well-adapted to their natural environment [Citation43], and our results, in addition to aspects of P. pulcher morphology (i.e. enlarged terminal lamellae, slightly dorsoventrally compressed body and head, large eye diameter to head size, and laterally placed nostrils), strengthen the likelihood of P. pulcher being a specialist of cliff side habitat [Citation29]. Our results also lend support to the theory that availability of suitable habitat for P. pulcher is inextricably linked to the geological heterogeneity characterising the south, east and north coasts of Barbados, thus limiting the species wider distribution on the island [Citation29].
Overall, our results demonstrate that although there was significant difference in microhabitat use by P. pulcher and H. mabouia, habitat use was still more similar between species than compared to random control points. We detailed the occurrence of H. mabouia in natural habitat along the Barbados coast, with factor analysis revealing that this species also selected for exposed rocky habitat, albeit to a significantly lesser degree than did P. pulcher. In one area in particular, the northern cliffs of St Lucy, H. mabouia appeared to be the more abundant species in this habitat, with very few P. pulcher being found in this area (this study n = 1; n = 5 Williams (2013) unpublished data). The ability of invasive Hemidactylus spp. to displace and cause local extirpation of native gecko species has received much attention, most notably in respect to urban and altered environments [Citation3–5,Citation22,Citation44]. In the Pacific Islands, where displacement of native geckos from many urban areas has been complete, the same has not been so pronounced in natural, forested areas of the same islands [Citation5]. The colonisation of Hemidactylus spp. into natural habitats is however less well understood [Citation20,Citation21 but see Citation6]. On Isla de Utila, in the Honduran Bay Islands, introduced Hemidactylus frenatus are more commonly found than the native Phyllodactylus palmeus in natural forested areas of the east coast [Citation45]. However, there is no indication given as to how prevalent the native species was in this habitat prior to the invasion of H. frenatus. Similarly, with no records of the historical range of P. pulcher, it is not known if this species was ever abundant on the northern cliffs (or inland habitats) of Barbados. Characteristics of the rocky habitat used by P. pulcher throughout its range, namely high frequencies of small and large crevices, under-rock refuges and increased leaf litter, were not features of the habitat used by H. mabouia at North Point (St. Lucy), or of microhabitat sampled at random points in that vicinity. Comparison of current P. pulcher habitat use with the available habitat does therefore suggest that the northern cliffs may not be entirely suitable for P. pulcher, regardless of the presence of H. mabouia. However, some degree of caution is needed in this interpretation considering the length of time H. mabouia and non-native predators have been resident on Barbados. Hemidactylus mabouia has been present since as early as 1700, and introduction of cane toads and mongoose likely occurred in the 1800s [Citation26]. The currently observed range, and specific habitat use of P. pulcher, may therefore be a manifestation of a reduced fundamental niche in a long-term response to combined pressures of predation and competition from introduced species [Citation8,Citation10,Citation11,Citation46,Citation47].
The average size (SVL) of H. mabouia on Barbados was significantly less than that recorded for the species in Anguilla [Citation40], Florida [Citation35],, and south-eastern Brazil [Citation41]. This discrepancy in size could be largely due to the Anguilla and Florida data being derived exclusively from individuals collected from around lit buildings, a prey rich habitat that the species is well known to exploit [Citation35,Citation44]. Anjos & Rocha [Citation41] did however collect geckos from more natural habitat. Furthermore, we found H. mabouia individuals to have lower body condition than P. pulcher in the habitats surveyed on Barbados. Body condition is a direct indicator of individual fitness, representative of the animal’s ability to obtain and synthesise food resources [Citation48]. These results therefore suggest that H. mabouia may be less efficient in exploiting prey resources in natural coastal habitat on Barbados than P. pulcher. This disparity is not surprising considering the affinities of H. mabouia to an edificarian lifestyle and as a human commensal [Citation40], as opposed to the apparent specialist cliff side niche of P. pulcher. Specifically, differences in foraging tactics appropriate to each species’ preferred habitat could well be the underlying cause for variation in body condition observed during this study. Hemidactylus spp. are typically ambush predators of invertebrate prey who benefit from structurally simple habitats and aggregations of winged insects around artificial light sources [Citation4,Citation49–51]. Conversely, P. pulcher may have a more active approach to encountering dispersed insect prey amongst structurally diverse rock habitat (RW pers obs). In experimental trials, the competitive advantage of H. frenatus over native Lepidodactylus lugubris in exploitative contest for insect prey was shown to diminish in structurally complex environments, and when prey is dispersed rather than clumped around light sources [Citation4,Citation5]. The smaller body size of H. mabouia in natural habitats of Barbados compared to those found in urban areas elsewhere [Citation24,Citation35,Citation40] also supports the theory that the species is foraging less efficiently in natural habitats surveyed during this study. If this is correct, and the potential impacts of the invasive species on the native gecko are alleviated as a result, then the ability of H. mabouia to thrive on Barbados may be more a reflection of the successful exploitation of vacant niches (less rocky sites, buildings further from coastal edges) as befitting a habitat “generalist”, rather than competitive displacement of the native gecko.
The structurally diverse coastal cliffs with abundant natural crevices on the south east coast of Barbados are the preferred habitat for P. pulcher. Much of the habitat currently used by the gecko lies within the Coastal Landscape Protection Zone (OS3), where all new developments must be set back 30 m from the cliff edge (Physical Development Plan 2017 Draft). Although primarily to protect developments from erosion-prone cliff edges, salt spray and storms, the designation is also to ensure access for public use remains along these coastal cliffs. The obligatory set-back should reduce direct impacts of tourism infrastructure on P. pulcher habitat. However, new developments will increase the level of artificial light at night preferred by foraging H. mabouia, as well as bringing more predatory species like rats and cats into areas utilised by P. pulcher. Although P. pulcher is found on Barbados’ only offshore island, the islet is small (0.15 ha) and only a small part of it provides suitable gecko habitat (Williams, 2012 unpublished data). Options to conserve preferred P. pulcher habitat, if pressure on its natural range from coastal development increases, may therefore be limited to the provision of coastal wall structures that augment key P. pulcher resources [Citation31] and/or construction of a biosecure area on the mainland; an expensive and high-maintenance option.
Acknowledgments
We are grateful to the Ministry of Environment & Drainage, Government of Barbados, for permission to undertake this work. The authors are grateful for the advice and support of Jenny Daltry (Fauna & Flora International) and Matt Morton (Durrell Wildlife Conservation Trust). Special acknowledgement goes to Paul Doyle and staff of The Crane Resort, Jeanette & Edwin Shorey, The Oran family, and especially those who assisted in the field, Mikhael Dulal-Sealy and Stephan Lesmond. Finally, we thank Damon Corrie for making the rediscovery that led to this study and the opportunity to secure a future for the species in Barbados.
Disclosure statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Funding
References
- Powell R, Henderson RW. Conservation status of lesser Antillean reptiles. Iguana. 2005;11:2–17.
- Losos J, Marks J, Schoener T. Habitat use and ecological interactions of an introduced and a native species of Anolis lizard on Grand Cayman, with a review of the outcomes of anole introductions. Oecologia. 1993;95:525–532.
- Case TJ, Bolger DT, Petren K. Invasions and competitive displacement among house geckos in the Tropical Pacific. Ecology. 1994;75:464–477.
- Petren K, Case TJ. An experimental demonstration of exploitation competition in an ongoing invasion. Ecology. 1996;77:118–132.
- Petren K, Case TJ. Habitat structure determines competition intensity and invasion success in gecko lizards. Proc Natl Acad Sci USA. 1998;95:11739–11744.
- Cole NC, Jones CG, Harris S. The need for enemy-free space: the impact of an invasive gecko on island endemics. Biol Conserv. 2005;125:467–474.
- Case TJ, Bolger DT. The role of introduced species in shaping the distribution and abundance of island reptiles. Evol Ecol. 1992;5:272–290.
- Case TJ, Gilpin ME. Interference competition and niche theory. Proc Natl Acad Sci USA. 1974;71:3073–3077.
- Schoener TW. Field experiments on interspecific competition. Am Nat. 1983;122:240–285.
- Schoener TW. Resource partitioning in ecological communities. Science. 1974;185:27–39.
- Losos JB. Ecological character displacement and the study of adaptation. Proc Natl Acad Sci USA. 2000;97:5693–5695.
- Stuart YE, Campbell TS, Hohenlohe PA, et al. Rapid evolution of a native species following invasion by a congener. Science. 2014;346:463–466.
- Pianka E. The structure of lizard communities. Annu Rev Ecol Evol Syst. 1973;4:53–74.
- Schoener TW. Presence and absence of habitat shift in some widespread lizard species. Ecol Monogr. 1975;45:233–258.
- Pianka E, Huey R. Comparative ecology, resource utilization and niche segregation among gekkonid lizard in the southern Kalahari. Copeia. 1978;4:691–701.
- Nevo E, Gorman G, Soule M, et al. Competitive exclusion between insular Lacerta species (Sauria: lacertilia). Oecologia. 1972;10:183–190.
- Campbell TS, Echternacht AC. Introduced species as moving targets: changes in body sizes of introduced lizards following experimental introductions and historical invasions. Biol Invasions. 2003;5:193–212.
- Bomford M, Kraus F, Barry SC, et al. Predicting establishment success for alien reptiles and amphibians: a role for climate matching. Biol Invasions. 2009;11:713–724.
- Rodder D, Sole M, Bohme W. Predicting the potential distributions of two alien invasive housegeckos (Gekkonidae: Hemidactylus frenatus, Hemidactylus mabouia). North-West J Zool. 2008;4:236–246.
- Rocha CFD, Anjos LA, Bergallo HG. Conquering Brazil: the invasion by the exotic gekkonid lizard Hemidactylus mabouia (Squamata) in Brazilian natural environments. Zoologia-Curitiba. 2011;28:747–754.
- Hoskin CJ. The invasion and potential impact of the Asian House Gecko (Hemidactylus frenatus) in Australia. Austral Ecol. 2011;36:240–251.
- Yang D, Gonzalez-Bernal E, Greenlees M. Interactions between native and invasive gecko lizards in tropical Australia. Austral Ecol. 2012;37:592–599.
- Powell R, Henderson RW, Farmer M, et al. Introduced amphibians and reptiles in the Greater Caribbean: patterns and conservation implications. In: Hailey A, Wilson BS, Horrocks JA, editors. Conservation of Caribbean Island herpetofaunas. Volume 1: conservation biology and the wider Caribbean. Brill, Leiden, (Netherlands): Brill Academic Publishers; 2011. p. 63–143.
- Hughes DF, Meshaka WE, van Buurt G. The superior colonizing Gecko Hemidactylus mabouia on Curaçao: conservation implications for the native gecko Phyllodactylus martini. J Herpetol. 2015;49:60–63.
- Henderson RW, Breuil M. Lesser Antilles. In: Powell R, Henderson RW, editors. Island lists of West Indian amphibians and reptiles. 2012. p. 85–166. Bull Am Mus Nat Hist (USA);51(2).
- Grant C. Herpetology of Barbados B.W.I. Herpetologica. 1959;2:97–101.
- Fields A, Horrocks JA. An annotated checklist of the herpetofauna of Barbados. J Barbados Mus Hist Soc. 2009;55:263–283.
- Dixon JR. The Leaf-Toed Geckos, genus Phyllodactylus, of Northeastern South America. Southwest Nat. 1962;7:211–226.
- Williams RJ, Horrocks JA, Pernetta AP. Natural history, distribution, and conservation status of the Barbados leaf-toed gecko, Phyllodactylus pulcher Gray, 1828 (Squamata, Gekkonidae). Herpetol Notes. 2015;8:197–204.
- Daltry JC. Data from: Phyllodactylus pulcher. The IUCN red list of threatened species. 2016. [cited 2017 Mar 19]. DOI:https://doi.org/10.2305/IUCN.UK.2016-3.RLTS.T48443321A48443325.en.
- Williams RJ, Pernetta AP, Horrocks JA. Out‐competed by an invader? Interference and exploitative competition between Tropical House Gecko (Hemidactylus mabouia Moreau de Jonnès, 1818) and Barbados Leaf‐Toed Gecko (Phyllodactylus pulcher Gray, 1828) for diurnal refuges in anthropogenic coastal habitats. Integr Zool. 2016;11:229–238.
- Corke D. The status and conservation needs of the terrestrial herpetofauna of the Windward-Islands (West-Indies). Bio Conserv. 1992;62:47–58.
- Government of Barbados. A national biodiversity strategy and action plan for Barbados. The ministry of physical development and environment. Bridgetown: Government of Barbados; 2002 [cited 2013 Aug 13]. Available from: http://www.biodiv.org/doc/world/bb/bb-nbsap-01en.pdf
- Helmer EH, Kennaway TA, Pedreros DH, et al. Land cover and forest formation distributions for St. Kitts, Nevis, St. Eustatius, Grenada and Barbados from decision tree classification of cloud-cleared satellite imagery. Caribb J Sci. 2008;44(2):175–198.
- Meshaka WE. Colonization dynamics of two exotic geckos (Hemidactylus garnotii and H. mabouia) in Everglades National Park. J Herpetol. 2000;34:163–168.
- Pike DA, Croak BM, Webb JK, et al. Subtle - but easily reversible - anthropogenic disturbance seriously degrades habitat quality for rock-dwelling reptiles. Anim Conserv. 2010;13:411–418.
- Pernetta AP, Bell DJ, Jones CG. Macro- and microhabitat use of Telfair’s skink (Leiolopisma telfairii) on Round Island, Mauritius: implications for their translocation. Acta Oecol. 2005;28:313–323.
- Ross TN, Pernetta AP, Jones CG, et al. Sexual size dimorphism and microhabitat use of the orange-tail skink (Gongylomorphus spp.) on Flat Island, Mauritius: conservation implications. Amphibia-Reptilia. 2008;29:349–359.
- Williams RJ. Ecology of the endemic Barbados leaf-toed gecko (Phyllodactylus pulcher) and the competitive interaction with the non-native tropical house gecko (Hemidactylus mabouia): implications for conservation [MRes thesis]. Brighton, United Kingdom: University of Brighton; 2014.
- Howard KG, Parmerlee JS, Powell R. Natural history of the edificarian geckos Hemidactylus mabouia, Thecadactylus rapicauda, and Sphaerodactylus sputator on Anguilla. Caribb J Sci. 2001;37:285–288.
- Anjos LA, Rocha CFD. Reproductive ecology of the invader species gekkonid lizard Hemidactylus mabouia in an area of southeastern Brazil. Iheringia Série Zoologia. 2008;98(2):205–209.
- Jakob EM, Marshall SD, Uetz GW. Estimating fitness: A comparison of body condition indices. Oikos. 1996;77:61–67.
- Dixon JR, Huey RB. Systematics of the lizards of the gekkonid genus Phyllodactylus of mainland South America. LA County Mus Contrib Sci. 1970;192:1–78.
- Short KH, Petren K. Rapid species displacement during the invasion of Florida by the tropical house gecko Hemidactylus mabouia. Biol Invasions. 2012;14:1177–1186.
- McCranie JR, Hedges SB. A new species of Phyllodactylus (Reptilia, Squamata, Gekkonoidea, Phyllodactylidae) from Isla de Guanaja in the Honduran Bay Islands. Zootaxa. 2013;3694:51–58.
- Cole NC, Harris S. Environmentally-induced shifts in behavior intensify indirect competition by an invasive gecko in Mauritius. Biol Invasions. 2011;13:2063–2075.
- Lisicic D, Drakulic S, Herrel A, et al. Effect of competition on habitat utilization in two temperate climate gecko species. Ecol Res. 2012;27:551–560.
- Labocha MK, Schutz H, Hayes JP. Which body condition index is best? Oikos. 2014;123:111–119.
- Bonfiglio F, Balestrin RL, Capellari LH. Diet of Hemidactylus mabouia (Sauria, Gekkonidae) in urban area of southern Brazil. Biociencias. 2006;2:107–111.
- Rocha CFD, Anjos LA. Feeding ecology of a nocturnal invasive alien lizard species, Hemidactylus mabouia Moreau de Jonnes, 1818 (Gekkonidae), living in an outcrop rocky area in southeastern Brazil. Braz J Biol. 2007;67:485–491.
- Albuquerque NR, Costa-Urquiza AS, Soares MP, et al. Diet of two sit-and-wait lizards, Phyllopezus pollicaris (Spix, 1825) (Phyllodactylidae) and Hemidactylus mabouia (Moreau de Jonnes, 1818) (Gekkonidae) in a perianthropic area of Mato Grosso do Sul, western Brazil. Biota Neotrop. 2013;13:376–381.