ABSTRACT
Knowledge of the breeding biology of grassland birds is important given the accelerated rate of transformation of their habitats, which has led to noticeable population declines of many species. Although several species in South America are of conservation concern due to habitat alteration, information on their nesting biology is generally sparse. During three seasons we studied a breeding population of a poorly studied ground-nesting bird, the Grassland Sparrow (Ammodramus humeralis), in grasslands used for cattle grazing in central-eastern Argentina. We described its breeding parameters, estimated nestling growth curves, and analyzed daily nest survival rates (DSR) as a function of grassland characteristics, including grass density, grass height, and distance to forest edges. We found 34 nests placed among low and sparse vegetation and made exclusively of grass. The modal clutch size was three eggs. Incubation and nestling periods lasted 11 and 10.5 days, respectively. Nestlings had a fast-growing tarsus, which could be advantageous to escape from predators early. Only seven nests (20.6%) were successful and predation was the principal cause of nest failure (78% of the failures). DSR was 0.91, resulting in a cumulative survival of 11% for the 23.5 day nesting period. We found no effects of habitat features on DSR, which may be a consequence of the high predation rate and a very diverse predator community in the area. Studies at a broader scale could help to elucidate which habitats favor the reproduction of these species. We emphasize the importance of knowing basic ecological aspects of native grassland birds to develop management plans, especially given the lack of protected grassland areas in the Pampas Grassland ecoregion.
Introduction
The ecology and conservation of grassland birds have received increasing attention during the last decades due to noticeable population declines of many species across the globe [Citation1]. However, the breeding biology of some grassland ground-nesting species remains mostly unknown, in part due to the difficulty of finding a large number of nests in some grassland habitats [Citation2,Citation3]. This is unfortunate because knowledge on the breeding biology of grassland birds is important for understanding their life histories, habitat requirements, and population dynamics [Citation4,Citation5]. Given the rate at which natural grasslands have been altered over the last decades, broadening the information available on the breeding ecology of these species is crucial for their conservation and can help designing better management strategies for their habitats [Citation5,Citation6].
In southern South American grasslands, conversion to cropland and grazing have expanded and intensified rapidly during the last 20 years [Citation6,Citation7], with only a minor proportion remaining unused for cattle-grazing or agriculture [Citation8]. Birds breeding in modified grasslands can be affected by factors such as habitat fragmentation [Citation9] as well as the alteration of plant species composition [Citation10] and grassland structure induced by land management [Citation11,Citation12]. As a consequence of these alterations, several bird species in southern South American grasslands have become of conservation concern [Citation13], although the available information on their biology is generally sparse [Citation6] and few studies have evaluated the effects of changing habitat features on their nesting success [Citation9].
The Grassland Sparrow (Ammodramus humeralis) is a ground-nesting passerine endemic to South America which inhabits grasslands at low altitude from Colombia and Venezuela to central Argentina, being a year-round resident across its distribution range [Citation14]. It is currently listed as Least Concern under the IUCN Red List criteria, although its habitat extension is being reduced [Citation15]. Current knowledge about its breeding and nesting biology is focused on tropical South American grasslands and consists of observations of only a few nests during one breeding season [Citation14,Citation16]. For the southern portion of its range, there are only some occasional nest records from northern Argentina [Citation17].
To better understand the biology of birds in these continuously altered habitats, we studied a breeding population of the Grassland Sparrow during three breeding seasons in the Pampas Grasslands of central-eastern Argentina. We estimated the species’ descriptive breeding parameters and nestling growth curves. We also evaluated if daily nest survival rates (DSR) were affected by habitat features that could influence nest survival according to previous knowledge on other grassland birds, including proximity to trees and forest edges [Citation18–20], nest concealment, and grass height [Citation21]. We expected nest survival to be lower in nests close to trees and edges, with lower nest concealment and located among shorter grass.
Methods
Study site
The study was conducted on a private farm in Punta Piedras, Buenos Aires province, Argentina (35°20’S; 57°12ʹW). It is located within the Flooding Pampas, a subregion of the Pampas Grasslands mostly used for cattle grazing due to its precipitation regime and soil properties, which make it inconvenient for large-scale agriculture [Citation22]. The climate of the region is temperate-humid, with a mean annual rainfall of 900 mm concentrated from winter to the end of spring (April to December) in most years [Citation22,Citation23]. The site is composed of natural grasslands and remnants of native forests. Most of its grassland cover is used for extensive cattle-grazing, and is composed mainly of native grasses, such as Nassella spp., Paspalidium spp., Leersia hexandra and Baccharis spp., and some exotic species [Citation24,Citation25]. Potential terrestrial nest predators include White-eared Opossum (Didelphis albiventris), Pampas Fox (Lycalopex gymnocercus), Lesser Grison (Galictis cuja), Big-hairy Armadillo (Chaetophractus villosus), small rodents, Black-and-white Tegu (Salvator merianae), and snakes (Philodryas spp.); potential avian nest predators include Guira Cuckoo (Guira guira), Chimango Caracara (Milvago chimango), Southern Crested Caracara (Caracara plancus) and Long-winged Harriers (Circus buffoni) [Citation26,Citation27].
Field procedure
We collected data on Grassland Sparrow (hereafter “sparrow”) nests during three breeding seasons (October–February 2017–2018, 2018–2019, and 2019–2020) as part of a survey of breeding grassland birds. We found nests by flushing incubating females either by dragging a 20 m long rope between two people or by systematic walking with sweeping sticks [see Citation4]. We established temporary plots of 100 × 100 m and used portable poles as a reference to systematically walk over all the area while avoiding walking over the already covered path. We carried on 100 m long transects of variable until the 100 m wide of the plots were covered with the rope or sticks. The search was repeated in the same plots once a week and the total area covered each season was approximately 250 ha. In addition, some nests were found by observing the adults’ behavior while building nests or feeding nestlings [Citation28]. Once a nest was found, we georeferenced it with a GPS and placed a small flag (a 5 cm red tape attached to a 50 cm long wire) 4 m north of it to facilitate relocation during monitoring. This type of marking is unlikely to be used as a cue by predators occurring at our study site [Citation29]
We monitored nests every 2–3 days until the nestlings successfully fledged or the nest failed. We considered a nest successful when at least one fledgling left the nest. We considered a nest predated when the eggs or nestlings disappeared between two consecutive visits and no parental activity was detected in the surroundings. We considered a nest abandoned if eggs were found cold in successive visits after incubation had started or if nestlings were found dead in the nest with no signs of predation.
When clutches were complete (i.e. no new eggs were found between visits), we measured eggs for length and breadth to the nearest 0.1 mm using vernier calipers, and we weighed them to the nearest 0.1 g using Pesola spring balances. We estimated egg volume following Hoyt [Citation30]. We measured nestlings’ wing chord and tarsus length to the nearest 0.1 mm using calipers and weighed them to the nearest 0.1, 0.2, or 0.25 g using Pesola spring balances of 10, 20, or 50 g capacity, depending on nestling size. Eggs and nestlings were marked with non-toxic waterproof ink for individual identification over successive visits. To minimize the risk of premature fledging, nestling features were obtained until they reached an age of 8–9 days, and in subsequent visits, nests were checked from a distance of 1–2 m. This study was conducted with research permits from the regional nature conservation authority (OPDS #17,717, Direccion de Áreas Naturales Protegidas, Buenos Aires province, Argentina).
Nest and vegetation measurements
Immediately after the nest attempt ended, we measured the nest for internal and external diameter, depth, and height from the ground to the entrance. We measured the height of the clump of vegetation used as cover and took measurements of visual obstruction using a modified version of a Robel pole [Citation31]. We placed a pole divided into 10 cm sections and recorded the first visible section from the ground to the top, looking from a height of 1 m and a distance of 4 m in the four cardinal directions (NSEW). Each observation provided a score from 1 (lowest obstruction) to 10 (highest obstruction) which were averaged to obtain the final VOI (Visual Obstruction Index) for each nest. Upper concealment was measured by placing an 8 cm diameter disc divided into eight black-and-white sections in the nest and recording the number of sections visible from 1 m overhead. The score was calculated as 8 minus the number of visible sections, with a higher score indicating better concealment [Citation21]. We also obtained the geolocation of the nearest perch to each nest (any bush, tree, or fence pole ≥1 m in height was considered a perch) and mapped wooded edges in the area using SPOT6 satellite images (1.5 m spatial resolution), provided by the CONAE (Comisión Nacional de Actividades Espaciales). We obtained distances to perches and to the closest forest edge with QGIS software, version 3.10.2 [Citation32]. Wooded areas ≥500 m2 were considered as forests for this study (see also Suppl. Material S1).
Data analysis
Each nest was assigned a clutch-initiation date corresponding to the laying of the first egg. Clutch-initiation dates were determined directly for nests found under construction or during egg-laying and through backdating from hatching dates for nests found during incubation. For nests found during the nestling stage, hatching dates were estimated from nestling measurements. For nests found during incubation that did not survive until hatching (N = 18), we assumed that they were found halfway through incubation [see details in Citation33]. The incubation period was defined as the number of days elapsed from laying to hatching of the last egg. The nestling period was defined as the number of days elapsed since hatching of the last egg until fledging [Citation33]. We calculated hatching success as number of eggs hatched/number of eggs that survived through the incubation period, and nest productivity as the number of fledglings/clutch size. Values are reported as means ± SD (standard deviation).
Using non-linear mixed models [nlme package, version 3.1–148; Citation34] we estimated nestling growth curves as a function of nestling age, fitted to a Richards equation [Citation35]. We only included nestlings with three or more measurements (N = 15 nestlings from six nests) and used nestling and nest identity as random effect factors to estimate curve parameters, due to the lack of independence among repeated measures [see Citation36 for details]. Nestling age was estimated with a precision of 0.5 days. We followed Byers et al. [Citation14] and Fecchio et al. [Citation37] for a reference of adult size. We performed all statistical analyses using software R (version 3.6.3) [Citation38]. We report all results as means ± SE.
We estimated the daily nest survival rate (DSR) for the species using generalized linear models with a logistic-exposure link function [Citation39]. This model considers each visit interval’s fate as the response variable (coded as 0 = failed during the interval and 1 = survived the interval). We first created a null model for which DSR is constant across nests and then we estimated the cumulative nest survival probability for the species by raising the DSR to a power equal to the length (in days) of a complete breeding cycle for an average size clutch [~23.5 days from egg-laying to fledging; Citation16; this study]. We assessed the effects of habitat characteristics by building a model set which included explanatory variables that a priori may influence nest survival of grassland birds, including clump height, VOI, upper obstruction, distance to perches, and distance to forest edges [Citation18–20]. We also controlled for effects of time-specific variables that could influence nest DSR [Citation40,Citation41], which included year (a three-level factor, one corresponding to each season), linear effects of time within a season (standardized as day 1 = October 1), and linear effects of nest age (age 0 = day the first egg of each nest was laid). Additive models were built using combinations of a temporal factor with any of the habitat features, allowing a maximum of three parameters in a single model to prevent overparameterization due to sample size. We checked for overdispersion using the Pearson X2 test on the global model [Citation42]. Models were ranked using Akaike’s information Criterion corrected for small sample sizes (AICc), where the model with the lowest AICc value is the best-approximating model of the candidate model set and the differences in AICc value between each model and the best model (ΔAICc) allow for quick comparisons [Citation42].
Results
We found 34 nests over the entire duration of the study (11 in 2017–2018, 12 in 2018–2019, and 11 in 2019–2020). The earliest clutch was initiated on October 26 and the latest on January 31. The earliest and latest fledging dates were December 1 and January 25, respectively. The last active nest was predated during incubation on February 15. The peak of clutch initiation for all seasons was in November (). Two nests were found during construction, five during egg-laying, 26 during incubation, and one during the nestling stage. Nests were located under clumps of grass or in depressions on the ground and were built exclusively with grasses. They had a cup-like general shape, with one side higher than the other, acting as a roof and forming a tunnel-like structure with an oblique entrance (). Descriptive nest and surrounding vegetation measurements are shown in .
Table 1. Nest features, nest-site features, and egg measures of Grassland Sparrow (Ammodramus humeralis) in grazed grasslands in central-eastern Argentina. Values are reported as mean ± SD (standard deviation) and range
Figure 1. Distribution of Grassland Sparrow nests initiated every month during three breeding seasons (2017–2020) in a natural grassland in Buenos Aires province, Argentina
Figure 2. Profile scheme of Grassland Sparrow nest (A), empty nest (B), clutch with four eggs (C) and 6-day old nestling (D). Figures A, B and D taken by MAC. Picture C taken by E. Grim

Mean clutch size was 3.1 ± 0.6 eggs (range = 2–4 eggs, N = 30), and modal clutch size was 3 eggs. The eggs were immaculate matte white and elliptical ovate in shape (). Egg measures are summarized in . The incubation period lasted 11.0 ± 0.7 days (range = 10.0–12.0 days, N = 7), and nestlings stayed in the nest for 10.5 ± 1.2 days (range = 9.0–12.0 days, N = 7). Hatching success was 0.8 ± 0.2 (range = 0.5–1.0, N = 16). None of the nests suffered clutch reduction during egg or nestling stages. Nest productivity was 0.8 ± 0.1 (range = 0.7–1.0, N = 7 successful nests).
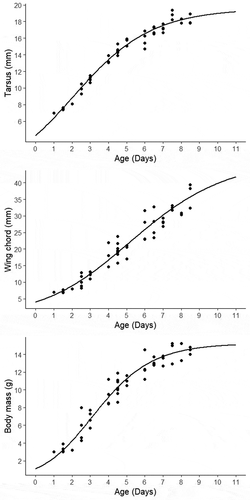
We present nestling growth curve parameters estimated from measurements of tarsus, wing chord, and body mass (), which were used to predict values for each measure at different ages (). According to predicted curves, sparrow nestlings reached 50% of adult size at an age of 2.8 days for the tarsus, 6.8 days for wing chord, and 3.6 days for their body mass. At fledging, the tarsus, wing chord, and body mass have an estimated of 95%, 86%, and 95% adult size, respectively.
Table 2. Richard’s growth curve parameters for tarsus, wing chord, and body mass of Grassland Sparrow nestlings. Only nestlings with three or more measures were used (N = 15 nestlings from six nests). A = upper asymptote, ti = time (in days) when maximum growth was reached, K = maximum relative growth rate; d = shape parameter, and RD = residuals’ standard deviation for the predicted curve. Other values are reported as mean ± SD
Figure 3. Growth curves of Grassland Sparrow nestlings for tarsus, wing chord and body mass. Curves were fitted to a Richard’s equation using non-linear mixed models. Black dots represent the real measures obtained from nestlings (N = 15 nestlings from six nests)
Apparent nest success was 20.6% (N = 7 nests). The main cause of nest failure was predation (77.8% of the failures, N = 21 nests), while 2 nests (7.4% of the failures) were abandoned due to flooding and 4 nests (14.8% of the failures) were abandoned for unknown reasons (three of them during incubation and one after hatching). The average DSR was 0.91, which resulted in a cumulative survival probability of 10.9% for an average breeding cycle of ~23.5 days. The global model showed no evidence of overdispersion (C^ = 1.01, Pearson's X2 P > 0.05). The null model of constant DSR was the most supported model (i.e. lowest AICc value, ) within the set, showing little evidence of habitat or temporal effects on nest DSR.
Table 3. Ranking of models explaining daily nest survival of the Grassland Sparrow in central-eastern Argentina, including a null model of constant survival (S (.)). K = number of parameter estimates, Deviance = likelihood difference between each model and the saturated model (hypothetic model with perfect fit), AICc = Akaike’s Information Criterion corrected for small sample size (lower means more support), ∆AICc = difference in AICc units between a model and the best model, wi = model importance weight. We only present models within two AICc units from the best model
Discussion
We provide for the first time detailed data on the breeding biology and nest survival of the Grassland Sparrow in south temperate grasslands under cattle-grazing. Overall, our results indicate that the Grassland Sparrow has a short incubating period, fast nestling growth rates, and low nest survival in our study site.
The span of the breeding season for this species was previously only described for tropical populations at the northern limit of its range, where it lasts from April to August [Citation14]. The length of the breeding season we found was similar (~100 days) but with different starting dates (late October to middle February), as occurs in most breeding passerines in south temperate regions [see also Citation33,Citation43,Citation44]. Nest measurements, egg measurements, and clutch size (2–4 eggs) were similar to previous reports [Citation14,Citation17]. Although the sparrow is believed to inhabit tall and dense grasslands and to be intolerant to grazing for this reason [Citation14], we found all nests in relatively low (~50 cm height clumps) and sparse grass (~2 average VOI score), in plots used for extensive cattle-grazing. This indicates that short grasslands under low-intensity grazing should be considered as potential nesting areas for the species, although the nest density we found was relatively low compared to other species common in the same study site, such as Pipits (Anthus spp.) and the Grassland Yellow Finch (Sicalis luteola) (MAC, Unpubl. data), which could be more tolerant to the modifications caused by cattle management [Citation10].
The incubation and nestling period were short (11 and 10.5 days, respectively), as was found for some Ammodramus species (11.5 and 9.5 days) [Citation45,Citation46] and other ground-nesting passerines in North America [Citation47]. Incubation periods in these species are short compared to most tree-nesting subtropical passerines in South America (~14 days) [Citation48] and other species in the same study area (see, for example, 12 days [Citation33]; 15 days [Citation43]; 16 days [Citation44]). Given the high rate of nest failure at our study site, a short incubation period can be an advantage because it helps to reduce total exposure time, and may reflect an adaptation to a high exposure environment and high nest vulnerability [see also Citation49,Citation50]. Accordingly, sparrow nestlings’ growth was considerably fast, which may also be adaptive to a high nest predation risk [Citation51,Citation52]. The maximum growth rate for the tarsus occurred at 2 days from hatching, which is sooner than that of body mass and wing chord (3 and 5 days, respectively). Given that sparrows fledge by walking [Citation16, pers. comm.], a fast-developing tarsus may allow nestlings to rapidly acquire mobility outside of the nest, enhancing their chances of escaping from predators.
The estimated nesting success of the sparrow was low (~11%). Studies on other Ammodramus species in North America showed higher success in plots that were excluded from cattle-grazing at the time [~35%, Citation44,Citation46]. Since our study site is under continuous cattle-grazing, which can reduce nest success in several indirect or direct ways [Citation12,Citation53], part of the difference could be related to the different management practices [Citation54]. In addition, nesting success has been found to be generally lower in Neotropical birds [~15%, Citation55] than in Nearctic ones [up to 50%, Citation56]. This trend is usually attributed to predation rates, which have also been reported to be greater in the Neotropical region [Citation41,Citation57]. The low success rate we found could be a consequence of a wide diversity of nest predators in our study area [see also Citation33]. The high predation rate and wide predator diversity may also explain the low relevance of nest-site variables for DSR since they limit the availability of significantly safe sites for placing nests [Citation55,Citation58].
This study contributes to the knowledge of the nesting biology and success of a poorly studied grassland bird in a modified habitat. We found a very low nesting success in a grassland which has a long usage history as extensive rangeland. Although we were unable to find an effect of specific habitat features on nest survival, studies at a broader scale, including grasslands with different land-use regimes, could help to elucidate which habitats are favorable for the reproduction of this species [Citation59,Citation60]. As most grasslands in our study region are used for the cattle industry and are under a continuous process of modification [Citation23,Citation61, MAC, pers. obs.], we emphasize the importance of knowing basic ecological aspects of its fauna to develop management and conservation plans. Given the noticeable lack of protected areas in the Pampas Grassland ecoregion [Citation6] and that many grassland birds remain poorly studied, we encourage further research on how their breeding success is influenced by habitat features.
Aknowledgements
We thank ML. Shaw for allowing us to conduct this study in Estancia “Luis Chico”. We also thank C. Tiernan, A. Wolf, B. Vidrio, A. Valencia, T. Lansley, C. Dudley, A. Banges, M. Gilles, A. Hodges, L. Haag, S. Musgrave, A. Miller, B. Ewing, K. Depot and K. McPartlin for help during fieldwork. We appreciate the improvements in English writing made by K. Depot. We are also grateful to the CONAE for providing the satellite image. Fieldwork was supported by the Agencia Nacional de Promoción Científica y Tecnológica, Grant [PICT-2014-3347]. LNS is a CONICET Research Fellow.
Supplemental Material
Download MS Word (498.7 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental Material
Supplemental data for this article can be accessed here.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Jehle G, Yackel Adams AA, Savidge JA, et al. Nest survival estimation: a review of alternatives to the Mayfield estimator. Condor. 2004;106:472–484.
- Xiao H, Hu Y, Lang Z, et al. How much do we know about the breeding biology of bird species in the world? J Avian Biol. 2017;48:513–518.
- Vickery PD, Tubaro PL, Cardoso Da Silva JM, et al. Conservation of grassland birds in the Western Hemisphere. Stud Avian Biol. 1999;19:2–26.
- Winter M, Hawks SE, Shaffer JA, et al. Guidelines for finding nests of passerine birds in tallgrass prairie. Prairie Nat. 2003;35:197–211.
- Fondell TF, Ball IJ. Density and success of bird nests relative to grazing on western Montana grasslands. Biol Conserv. 2004;117:203–213.
- Azpiroz AB, Isacch JP, Dias RA, et al. Ecology and conservation of grassland birds in southeastern South America: a review. J Field Ornith. 2012;83:217–246.
- Modernel P, Rossing WAH, Corbeels M, et al. Land use change and ecosystem service provision in Pampas and Campos grasslands of southern South America. Environ Res Lett. 2016;11:113002.
- Gibson DJ. Grasses and grassland ecology. New York (NY): Oxford University Press; 2009.
- Pretelli MG, Isacch JP, Cardoni DA. Effects of fragmentation and landscape matrix on the nesting success of grassland birds in the Pampas grasslands of Argentina. Ibis. 2015;157:688–699.
- Isacch JP, Maceira NO, Bo MS, et al. Bird-habitat relationship in semi-arid natural grasslands and exotic pastures in the west pampas of Argentina. J Arid Environ. 2005;62:267–283.
- Zalba SM, Cozzani NC. The impact of feral horses on grassland bird communities in Argentina. Anim Conserv. 2004;7:35–44.
- Cardoni DA, Isacch JP, Iribarne O. Effects of cattle grazing and fire on the abundance, habitat selection, and nesting success of the Bay-capped Wren-Spinetail (Spartonoica maluroides) in coastal saltmarshes of the Pampas Region. Condor. 2012;114:803–811.
- DiGiacomo ASD, Krapovickas S Conserving the Grassland Important Bird Areas (IBAs) of Southern South America: Argentina, Uruguay, Paraguay, and Brazil. USDA Forest Service; 2005. Report No.: PSW-GTR 191. 2005
- Byers C, Olsson U, Curson J. Buntings and Sparrows. London: A&C Black; 2010.
- BirdLife International. IUCN Red list of threatened species: Ammodramus humeralis [Internet]. IUCN Red List of Threatened Species; 2020 [cited 2020 Jun 26]. Available from: https://www.iucnredlist.org/en
- Macondes-Machado LO. Biología y conducta reproductiva de Ammodramus humeralis. Hornero. 1988;13:71–73.
- De La Peña MR. Nidos y reproducción de las aves argentinas. Santa Fe: Ediciones Biológica; 2013.
- Winter M, Johnson DH, Faaborg J. Evidence for edge effects on multiple levels in tallgrass prairie. Condor. 2000;102:256–266.
- Grant TA, Madden EM, Shaffer TL, et al. Nest survival of Clay-colored and Vesper Sparrows in relation to woodland edge in mixed-grass prairies. J Wildl Manage. 2006;70:691–701.
- Ellison KS, Ribic CA, Sample DW, et al. Impacts of tree rows on grassland birds and potential nest predators: a removal experiment. Saino N, editor. PLoS ONE. 2013;8:e59151.
- Davis SK. Nest-site selection patterns and the influence of vegetation on nest survival of mixed-grass prairie passerines. Condor. 2005;107:605–616.
- Perelman SB, RJC L, Oesterheld M. Cross-scale vegetation patterns of Flooding Pampa grasslands. J Ecol. 2001;89:562–577.
- Matteucci SD, editor. Ecorregiones y complejos ecosistémicos argentinos. 1st ed. Buenos Aires: Facultad de Arquitectura, Diseño y Urbanismo, GEPAMA Grupo de Ecología del Paisaje y Medio Ambiente, Universidad de Buenos Aires; 2012.
- Hummel AE, Rodríguez RA, Coconier EG, et al. El Parque Costero del Sur como área importante para la conservación de las aves. In: Athor J, editor. Parque Costero del Sur - Naturaleza, conservación y patrimonio cultural. Buenos Aires: Fundación Félix de Azara; 2009. p. 82–87.
- Roitman G, Preliasco P. Guía de reconocimiento de herbáceas de la Pampa Deprimida. 1st ed. Buenos Aires: Fundación Vida Silverstre Argentina; 2012.
- Svagelj WS, Mermoz ME, Fernández GJ. Effect of egg type on the estimation of nest predation in passerines. J Field Ornith. 2003;74:243–249.
- Menezes JCT, Marini MÂ. Predators of bird nests in the Neotropics: a review. J Field Ornith. 2017;88:99–114.
- Ralph CJ, Geupel GR, Pyle P, et al. Manual de métodos de campo para el monitoreo de aves terrestres. Albany (CA): U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station; 1996. Report No.: PSW-GTR-159.
- Jacobson MD, Tsakiris ET, Long AM, et al. No evidence for observer effects on Lark Sparrow nest survival: observer effects on nest survival. J Field Ornith. 2011;82:184–192.
- Hoyt DF. Practical methods of estimating volume and fresh weight of bird eggs. Auk. 1979;96:73–77.
- Robel RJ, Briggs JN, Dayton AD, et al. Relationships between visual obstruction measurements and weight of grassland vegetation. J Range Manage. 1970;23:295–297.
- QGIS Development Team. QGIS geographic information system [Internet]. Open Source Geospatial Foundation Project; 2020. Available from: http://qgis.osgeo.org/
- Segura LN, Mahler B, Berkunsky I, et al. Nesting biology of the Red-crested Cardinal (Paroaria Coronata) in south temperate forests of central Argentina. Wilson J Ornithol. 2015;127:249–258.
- Pinheiro J, Bates D, DebRoy S, et al. Packacge nlme: linear and nonlinear mixed effects models [Internet]; 2020 [cited 2020 Jun 22]. Available from: https://cran.r-project.org/web/packages/nlme/nlme.pdf
- Tjørve E, Tjørve KMC. A unified approach to the Richards-model family for use in growth analyses: why we need only two model forms. J Theor Biol. 2010;267:417–425.
- Vrána J, Remeš V, Matysioková B, et al. Choosing the right sigmoid growth function using the unified-models approach. Ibis. 2019;161:13–26.
- Fecchio A, Lima MR, Silveira P, et al. High prevalence of blood parasites in social birds from a neotropical savanna in Brazil. Emu. 2011;111:132–138.
- R Core Team. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2020.
- Schaffer TL. A unified approach to analyzing nest success. Auk. 2004;121:526–540.
- Grand JB, Fondell TF, Miller DA, et al. Nest survival in Dusky Canada Geese (Branta canadensis occidentalis): use of discrete-time models. Auk. 2006;123:198–210.
- Segura LN, Reboreda JC. Nest survival rates of Red-crested Cardinals increase with nest age in south-temperate forests of Argentina. J Field Ornith. 2012;83:343–350.
- Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed. New York (NY): Springer; 2002.
- Gonzalez E, Jauregui A, Segura LN. Breeding biology of the Yellow-browed Tyrant (Satrapa icterophrys) in south temperate forests of central Argentina. Wilson J Ornithol. 2019;131:534.
- Jauregui A, Gonzalez E, Segura LN. Nesting biology of the Narrow-billed Woodcreeper (Lepidocolaptes angustirostris) in a southern temperate forest of central-east Argentina. Stud Neotrop Fauna Environ.2019;54:114–120.
- Davis SK, Sealy SG. Nesting biology of the Baird’s Sparrow in southwestern Manitoba. Willson Bull. 1998;110:262–270.
- Jones SL, Dieni JS, Gouse PJ. Reproductive biology of a grassland songbird community in northcentral Montana. Wilson J Ornithol. 2010;122:455–464.
- Pietz PJ, Granfors DA, Grant TA. Hatching and fledging times from grassland passerine nests. In: Ribic CA, Thompson III FR, editors. Video surveillance of nesting birds [Internet]. University of California Press; 2012. p. 47–60. Berkerley, CA. [cited 2020 Jul 1]. Available from:http://california.universitypressscholarship.com/view/10.1525/california/9780520273139.001.0001/upso-9780520273139-chapter-4
- Auer SK, Bassar RD, Fontaine JJ, et al. Breeding biology of passerines in a subtropical montane forest in northwestern Argentina. Condor. 2007;109:321–333.
- Bosque C, Bosque MT. Nest predation as a selective factor in the evolution of developmental rates in altricial birds. Am Natur. 1995;145:234–260.
- Martin TE, Auer SK, Bassar RD, et al. Geographic variation in avian incubation periods and parental influences on embryonic temperature. Evolution. 2007;61:2558–2569.
- Remeš V, Martin TE. Environmental influences on the evolution of growth and developmental rates in passerines. Evolution. 2002;56:2505–2518.
- Cheng Y-R, Martin TE. Nest predation risk and growth strategies of passerine species: grow fast or develop traits to escape risk? Am Natur. 2012;180:285–295.
- Nelson KS, Gray EM, Evans JR. Finding solutions for bird restoration and livestock management: comparing grazing exclusion levels. Ecol Appl. 2011;21:547–554.
- Bleho BI, Koper N, Machtans CS. Direct effects of cattle on grassland birds in Canada. Conserv Biol. 2014;28:724–734.
- Mezquida ET. Nest site selection and nesting success of five species of passerines in a South American open Prosopis woodland. J Ornith. 2004;145:16–22.
- Martin TE. Nest predation among vegetation layers and habitat types: revising the dogmas. Am Natur. 1993;141:897–913.
- Martin TE. Life history evolution in tropical and South temperate birds: what do we really know? J Avian Biol. 1996;27:263–272.
- Cockle KL, Bodrati A, Lammertink M, et al. Cavity characteristics, but not habitat, influence nest survival of cavity-nesting birds along a gradient of human impact in the subtropical Atlantic Forest. Biol Conserv. 2015;184:193–200.
- Stephens SE, Koons DN, Rotella JJ, et al. Effects of habitat fragmentation on avian nesting success: a review of the evidence at multiple spatial scales. Biol Conserv. 2004;115:101–110.
- Klug P, Wolfenbarger LL, McCarty JP. The nest predator community of grassland birds responds to agroecosystem habitat at multiple scales. Ecography. 2009;32:973–982.
- Isacch JP, Martínez MM. Estacionalidad y relaciones con la estructura del hábitat de la comunidad de aves de pastizales de paja colorada (Paspalum quadrifarium) manejados con fuego en la provincia de Buenos Aires, Argentina. Ornitol Neotrop. 2001;12:345–354.
