ABSTRACT
Polylepis forests are one of the most threatened high Andean ecosystems, with 15 species and eight subspecies being categorized as critically endangered, vulnerable or near threatened by IUCN. However, their conservation status is poorly evaluated and could be outdated. As a case study, we evaluated Polylepis flavipila, a species endemic to the Peruvian central Andes, that is categorized as Vulnerable in Peru and is not mentioned in the Global Threatened Species Red List. We used two methods to categorize P. flavipila: (1) a species-level assessment using criteria proposed by IUCN and (2) a population-level assessment of four forests using the more specific criteria proposed by Navarro and collaborators. We recorded 350 relicts of P. flavipila forests as identified from herbariums and other sources. Forest cover was reduced 53% over 45 years as evaluated using satellite images from 1975 and 2020 and we estimated a total area of 458 and 216 km2, respectively. Thus, according to the IUCN criteria, P. flavipila should be classified as Endangered. At the population level, the application of the criteria of Navarro and collaborators results in different threat categories: one of the studied forests is classified as Critically Endangered, two forests as Vulnerable and one as Least Concern. We stress the need for updated categorizations for the 45 described Polylepis tree and shrub species based on the following facts: the only species we tested should change category, the IUCN categorizations were performed 16 to 22 years ago, and there have been many changes in the taxonomy of the genus. The assessment using IUCN criteria should also be complemented with more detailed evaluations at the population level since important differences were detected at a smaller scale, which could help target conservation and restoration resources more efficiently.
Introduction
Species of the genus Polylepis (Rosaceae) dominate the high mountain forest and woodland canopies along the Andes and associated mountains, and are considered one of the most threatened Andean ecosystems [1,2]. These forests and woodlands (hereafter, forests) provide diverse ecosystem and economic services in the Andean region, and have a high level of endemism and biological wealth that must be preserved and recovered [Citation1–3]. Globally, 15 species and eight subspecies of the Polylepis genus are categorized as endangered on the IUCN Red List, 20 of which were categorized in 1998 and three in 2004, with two species being categorized as Near Threatened (NT), 20 as Vulnerable (VU) and one as Critically Endangered (CR). There are few studies that have assessed species in detail (see [Citation4,Citation5]), and with the recent taxonomic revision of the genus, a threat categorization has been proposed for the 45 newly proposed Polylepis species [Citation6]; however, this has not been done on the basis of a detailed study for each species.
This fact is reflected in the IUCN Red List and in the Regional Red List of some countries, and there is evidence of either a lack of information on population trends of individual species or an apparent underestimation of conservation status (categorized as low risk although it has a high extinction risk, e.g. P. microphylla) or overestimation of conservation status (categorized as high risk when the species does not deserve it, e.g. P. racemosa) [Citation7,Citation8]. Inaccurate information could lead to inappropriate conservation and restoration interventions and a bad management of investment (money and human resources). Furthermore, there have been many changes in the genus taxonomy, with records of higher endemism levels than initially estimated [Citation9–11]; therefore, an update of the threat categories for all species seems necessary.
The IUCN Red List of Threatened Species provides categorization of the extinction risk of wild species, which helps focus research, action and conservation legislation policies [Citation12,Citation13]. However, applying the criteria established by the IUCN may be difficult since detailed information is required for each species [Citation14]. The conservation priority of a species not only takes into account the extinction risk [Citation15,Citation16] but also other factors, such as ecology, phylogenetics, and historical and cultural uses [Citation17]. However, this information is limited to very few species [Citation14,Citation18]. In the case of plants, the distribution data obtained for the Red List assessment usually depend on herbarium records [Citation19,Citation20]. Another problem in assessing the conservation status of species lies in the spatial-temporal scale [Citation21–23]. The categorization of species is based on their distribution range, among other factors; in the case of plant species, there are other factors that must be taken into account, such as: limitations of dispersion and characteristics of their spatial distribution, for example, Polylepis is known to have seed dispersion limitation which makes it difficult to regenerate and has a highly fragmented distribution [Citation14,Citation24,Citation25]. Therefore, it is advisable to develop complementary methods that contribute to the process of species categorization at different scales [Citation6,Citation26].
For Polylepis forests in Bolivia, Navarro et al. [Citation27] presented a method for categorizing species populations at a smaller scale than the IUCN approach. The authors used biogeographical, bioclimatic, ecological and floristic criteria, and included a characterization of the avifauna and an estimate of the general conservation status; this approach provides a clearer idea of the conservation status at the population level and complements the information from the IUCN. The Navarro method for categorizing Polylepis populations has not been used outside Bolivia; however, enough information is available to perform this categorization in several forests of Central Peru.
The Andean forests of Peru cover 0.17% of the national territory, with the genus Polylepis being one of the most representative taxonomic groups of this type of plant formation [Citation28]. Livestock and agriculture expansion in the Andes in recent centuries, in addition to burning of pastures, logging, and firewood extraction, is the main cause of the degradation of these ecosystems, increasing soil erosion, loss of biodiversity [Citation2,Citation29,Citation30], and vulnerability to climate change [Citation31].
Polylepis flavipila is one of the 14 species of the genus endemic to Peru [Citation4,Citation9–11,Citation32]. It is distributed throughout the western and central ranges of the Peruvian Andes, in Lima and Huancavelica departments between 3800 and 4700 m a.s.l. and is characterized by its growth in arid and cold habitats [Citation33]. These forests harbor a great diversity of species in different taxonomic groups [Citation34,Citation35]. Currently, this species is partially protected within the Nor-Yauyos Cochas Landscape Reserve (national protected area), although these particular populations are affected by hemiparasite infestations and human-induced modification of spatial distribution and diametrical structure [Citation36,Citation37]. Until 2006, P. flavipila was considered a subspecies of P. incana [Citation32]; therefore, there are few herbarium records with accurate identification, and based on this, the species was categorized as Vulnerable (VU) in the Peruvian Endemic Species Red Book [Citation20] and it is not on the Global Threatened Species Red List [Citation5,Citation8]. There is still confusion about the taxonomic identification of P. flavipila due to its similarity to other species [Citation4]; in addition, few studies have been carried out in Central Peru, largely because the presence of these forests is unknown as evidenced in the Pan Andean map [Citation33–36,Citation38].
Here, we set the following objectives: 1) to determine the general conservation status according to the IUCN criteria, 2) to determine the local-scale conservation status of four P. flavipila forests according to the criteria of Navarro et al. [Citation27], and 3) to discuss the assessment of conservation status at different scales and its implications in updating the categorizations of the remaining Polylepis species, conservation and restoration work.
Materials and methods
Study area
Our study area is located in the Andes of central Peru (see ), and covers approximately 210 km from north to south and 160 km from east to west. This area is characterized by an average annual rainfall of 523 ± 105 mm, with a wet season from December to March and a dry season from April to November. The average annual temperature is 4 ± 0.7°C and the topography is very rugged. Puna grasslands predominate, with the presence of lagoons, snowy peaks, bofedales (high altitude peatlands) and patches of forests with canopies dominated by Polylepis, Gynoxys, Escallonia trees and shrubs.
Figure 1. Polylepis flavipila forest locations as recorded in our database and the four forest sites selected for the small-scale detailed categorization proposed by Navarro et al. [Citation25]. Forest 1: Shaitura – Shutco; forest 2: Shajsee – Shajsee; forest 3: San Luis; forest 4: Ccarhuancho
![Figure 1. Polylepis flavipila forest locations as recorded in our database and the four forest sites selected for the small-scale detailed categorization proposed by Navarro et al. [Citation25]. Forest 1: Shaitura – Shutco; forest 2: Shajsee – Shajsee; forest 3: San Luis; forest 4: Ccarhuancho](/cms/asset/e1869b93-79f8-4d6a-b30a-ff7eae747cb7/tneo_a_1920295_f0001_oc.jpg)
Data sampling
We built a data base of P. flavipila locations throughout its distribution range, based on information from the San Marcos herbarium, USM (Universidad Nacional Mayor de San Marcos, Peru), the Augusto Weberbauer herbarium, MOL (Universidad Nacional Agraria La Molina, Peru), the Tropicos online herbarium [Citation39], the geographical distribution data from the Global Biodiversity Information Facility [Citation40], and scientific publications and books with records of the presence of the species [Citation20,Citation32–36,Citation41]. For the western Peruvian Cordillera, the database was complemented with observations of satellite images taking into account elevation (>4000 m a.s.l.), shape and fragmented distribution with subsequent field trips to verify the presence of the locations. All collected samples were identified using the taxonomic key of Kessler and Schmidt-Lebuhn [Citation32] and were corroborated by Tatiana Boza of the Zurich Herbarium (personal communication). Subsequently, we mapped each location, excluding those that we regarded as incorrectly georeferenced; the boundaries of each forest were delimited with the Google Earth program, and the current total area was determined with using QGIS software [Citation42].
Species evaluation according to IUCN criteria
IUCN Red List guidelines [Citation43] include five criteria for helping prioritize conservation efforts at the regional and/or global level, when any of the five criteria are met, the species of interest is assigned a category of threat [Citation15] (see ).
Table 1. IUCN classification criteria [Citation43]. Abbreviations: CR: Critical Endangered, EN: Endangered, VU: Vulnerable
Our study species is subject to several ongoing human pressures [Citation36,Citation37], but no published literature is available to determine past population changes. For this reason, the current forest area estimate was compared with the area estimated 45 years ago. We chose a time difference of 45 years because the population dynamics of this forest type is slow compared to that of other forests [Citation44,Citation45]. To estimate forest area in the past, we used LandSat 2 satellite images from 1975 downloaded from Earth Explorer [Citation46].
The Vegetation Index (NDVI) was calculated, and polygons of current forest areas were superimposed on the previously calculated NDVI layer, both layers having a resolution of c. 1 km2 (c. 1945 pixels2), projected in the WGS 84 Coordinate Reference System/UTM 18 N zone. The past areas were determined based on the pixel size, obtained from the information extracted from the 1975 NDVI, the area was calculated according to the pixel size and it was considered that those of greater range around the forest polygon indicate greater presence of tree species than the surrounding grasslands and bare soils [Citation47]; this was performed using the QGIS program [Citation42].
For criterion B, which refers to the species’ geographical distribution area, the ConR package [Citation48] was used to estimate the extent of occurrence (B1) and the area of occupancy (B2), based on occurrence records collected and assembled in the field. The area of occupancy was calculated with a resolution of 2 km2 since the average of each patch area does not exceed 2 km2. The extent of occurrence was determined with the minimum convex polygon around all georeferenced data of the occurrence sites.
Criterion C, which refers to population size and decline; criterion D, which refers to cases where populations are extremely small; and criterion E, which refers to quantitative analyses of population viability, were not calculated due to the lack of information on the number of mature individuals per population and their dynamics.
Population evaluations according to the criteria of Navarro and collaborators [Citation27]
We selected four populations of P. flavipila for which there were flora, fauna and population structure evaluations available (see [Citation33–37]). Two forests (Shaitura-Chutco and Shajsee-Shajsee) are located in Lima department, within the Nor-Yauyos Cochas Landscape Reserve, and the other two (San Luis and Ccarhuancho) are in Huancavelica department, outside a Protected Natural area (). Hereafter, they will be referred to as forests 1, 2, 3 and 4, respectively. In these four populations, the seven criteria suggested by Navarro et al. [Citation27] were applied ().
Table 2. Classification criteria according to Navarro et al.[Citation27], for the evaluation of conservation status (CS) and conservation priority (CP) of Polylepis forests
Figure 2. Satellite view of the four forests under detailed study in 2020. (a) forest 1: Shaitura – Shutco (lat. −12.3411; long. −75.7763), (b) forest 2: Shajsee – Shajsee (lat. −12.3725; long. −75.7528), (c) forest 3: San Luis (lat. −12.6189; long. −75.1620) (d) forest 4: Ccarhuancho (lat. −13.3016; long. −74.9307)
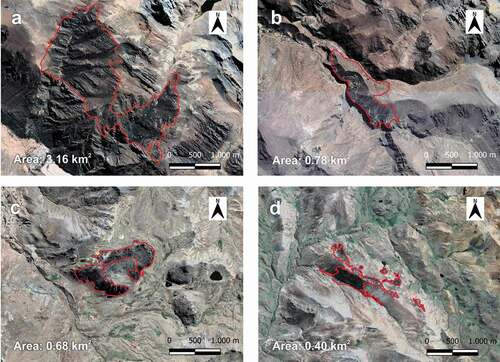
Criterion 1 refers to forest loss over 10 years; as IUCN criterion A2, this criterion was calculated using the vegetation index (NDVI) determined from LandSat 2 satellite images from 1975 [Citation46]. Criterion 2 addresses forest fragmentation evaluating the degree of forest fragmentation and quality of the matrix regarding the presence of human activities that do not allow the dispersion of forest organisms. For this evaluation, we mapped forests in the field using GPS and complemented with information from satellite images extracted from Earth Explorer [Citation46], determining each relict area and the distance between them. The presence of human activities was also evaluated, considering a qualitative classification based on logging, burning, presence of livestock, agricultural areas and roads within the forest; 1 for lightly disturbed, 2 for moderately disturbed and 3 for highly disturbed.
Criterion 3 addresses forest degradation. We evaluated whether the forest canopy was closed or open, presence of characteristic plant species and dasometric data taken in the field. We considered the Total Height (TH), Diameter at Canopy Height (DCH), Crown Diameter (CD) and distance between each tree. The information and results of the study by Camel et al. [Citation37], who evaluated the structure of 8 Polylepis forests in the central region of Peru, were also incorporated, and remote sensing analysis of satellite images. For the analysis of flora, we used the plant lists reported in Trinidad and Cano [Citation34] and Ames-Martínez et al. [Citation33]. Criterion 4 deals with avifauna suitability in each forest; the presence of threatened, specialist and/or endemic birds in Polylepis forests is evaluated. For this criterion, the study of bird diversity in Polylepis forests in the Central Peru, carried out by Quispe-Melgar et al. [Citation35] was used, who evaluated the alpha, beta and gamma diversity of birds associated with Polylepis forests in 12 different locations, also considering the four evaluation forests of the present study.
Criterion 5 estimates the current or projected level of threats. This was done by assessing the proximity of forests to main and secondary access roads, as well as the distance to human populations, using Google Earth software to estimate these values; for this, a qualitative scale was considered, being 0 for no distances (0 km), 10 long distances (50.1 km to more), 20 close distances (15.1–50 km), 30 medium close distances (5.1–15 km) and 40 for very close distances (0–5 km) (see ). Criterion 6 estimates the forest geographical exclusivity level, by assessing whether the species under study is dominant and endemic to the country. Criterion 7 refers to the adequacy levels and cultural landscape balance, and evaluates whether the forest is well integrated spatially and whether the nearest human communities make good use of its resources. This criterion was evaluated through inquiry to local people and direct observations.
Once the score assignment was completed, according to the weighting (), each forest was assigned to the category corresponding to the conservation status and priority (see ). The nomenclature applied to the conservation value scale is from the IUCN.
Table 3. Score Assignment [Citation27]. Abbreviations: CS, Conservation status; CP, Conservation priority
Results
IUCN criteria
We recorded 350 relicts of P. flavipila, all of them included in Huancavelica (251 relicts) and Lima (99 relicts) departments. In total, they cover an area of 216.17 km2 between 3800 and 4700 m a.s.l. in the western Andean cordillera (). The largest forests are found in the department of Lima, representing 70.45% of total area. Nor-Yauyos Cochas Landscape Reserve contains some of the largest forests with a total of 21.51 km2 (9.94% of the total species distribution area). Most of the forests are located in the department of Huancavelica; however, the total area of the species in this department is 63.87 km2, representing 29.55% of the total distribution area; these values indicate a high degree of fragmentation in this department. We estimated that 45 years ago, P. flavipila forests covered 457.98 km2, including the two departments; hence, the area was reduced by 52.8%. This value exceeds the threshold of 50% of criterion A2; therefore, it is categorized as Endangered (EN A2abcd).
The extension of occurrence (EOO) is 10,335 km2, being lower than the threshold of 20,000 km2 of criterion B1, which would lead to a category of Vulnerable (VU B1b(i,ii,iii)). At a resolution of 1.5 km2, the area of occupancy (AOO) is equivalent to 303.75 km2, and at a resolution of 2 km2, the area is 496 km2. In both cases, the value is lower than the threshold of 500 km2 of category B2; therefore, it is categorized as Endangered (EN B2b(i,ii,iii)). Thus, based on IUCN criteria A2 and B2, it is concluded that P. flavipila is Endangered (EN A2abcd; B2b(i,ii,iii)) (see ).
Table 4. P. flavipila classification according to IUCN criteria. Abbreviations: CR, Critical Endangered; EN, Endangered; VU, Vulnerable
Criteria of Navarro and collaborators [Citation27]:
In relation to criterion 1, average forest loss for the four selected study sites was 24.75% between 1975 and 2020, ranging from 21.3% to 31.1% for forest 1 and 4, respectively. In the analysis of criterion 2, forests 1 and 2 show large continuous areas with small clearings, and low fragmentation values, whereas forests 3 and 4 are fragmented in two to four patches per forest, thus presenting intermediate fragmentation values () and 2(d)) ().
Table 5. Criterion quantification applied to four P. flavipila forests according to Navarro et al. [Citation27]
In relation to criterion 3, forests 1 and 2 have greater area and more compact structure than forests 3 and 4. Forest 2, however, presents an altered structure as a consequence of hemiparasite attack, which is reflected in the loss of horizontal structure, with forest 1 being the better conserved; in forests 1 and 2, plant species characteristic of this ecosystems type are present. Forests 3 and 4 are more degraded, being forest 3 the most disturbed, here trees are of lower height, density of individuals is lower, and plant species composition differs from that of Lima forests, where diversity is greater in forests 1 and 2 ().
In relation to criterion 4, that is, avifauna, forests 1 and 2 have the highest number of species of interest for conservation (nine and eight, respectively), and the highest richness (39 and 38 species, respectively). In forests 1 and 2, the most representative species are Zaratornis stresemanni (White-cheeked Cotinga, VU and endemic), Conirostrum binghami (Giant Conebill, NT), Leptasthenura pileata (Rusty-crowned Tit-Spinetail, endemic) and Poospiza rubecula (Rufous-breasted Warbling Finch, EN and endemic). Forests 3 and 4 present fewer species of interest for conservation, with four and five, respectively, and reduced richness, with 32 and 36 species, respectively. In forests 3 and 4, the most representative species are Asthenes ottonis (Rusty-fronted Canastero, endemic), Conirostrum binghami (Giant Conebill, NT) and Oreonympha nobilis (Bearded Mountaineer, endemic). Thus, in criterion 4, the highest and lowest suitability corresponds to forests 1 and 3, respectively ().
In relation criterion 5, forests 1 and 4 are more distant to the main towns and roads and are less exposed to human impact than forests 2 and 3, which are closer to the main roads; therefore, for criterion 5, the level of threat is medium in the first two forests and high for the remaining two. In relation to criterion 6, the canopy of the four forests is dominated by P. flavipila, which is endemic to central Peru; therefore, the highest score is assigned to all forests. In relation to criterion 7, forests 1 and 2 are within a protected area and the communities closer to them have a better management of the forest resources than forests 3 and 4; therefore, the adequacy levels are high for forests 1 and 2, and medium for forests 3 and 4 ().
Overall, based on the criteria of Navarro and collaborators, these results indicate that forest 1 is classified as Least Concern (LC), forests 2 and 4 as Vulnerable State (VU) and forest 3 as Critically Endangered (CR), with two forests being of very high priority and two of high priority ().
Table 6. Score assigned to each forest using classification criteria of Navarro et al. [Citation27] for the evaluation of conservation status (CS) and conservation priority (CP) of Polylepis forests
Therefore, P. flavipila is Endangered (EN) according to the study based on the IUCN criteria, and among the four forests chosen for the analysis according to the criteria of Navarro and collaborators (2010), San Luis is Critically Endangered (CR), Shajsee-Shajsee and Ccarhuancho are Vulnerable (VU) and Shaitura – Chutco in a state of Least Concern (LC); where two forests are of very high priority and one of high priority and one of medium priority.
Discussions
Using the IUCN criteria, we conclude that Polylepis flavipila should be moved from Vulnerable to Endangered category. The reason for the change of category is due to the assessment made, taking into account the limited distribution of the species, which we update and detail in this paper; indeed, the species is restricted only to the departments of Lima and Huancavelica, occupying 0.002% of Peruvian territory, and much of its distribution coincides with relatively high human occupancy. The largest forests are found in Yauyos (70.45%), in Lima department; and in Nor-Yauyos Cochas Landscape Reserve (9.94%), where protection policies possibly contribute to a better management [Citation49,Citation50].
However, in the Huancavelica forests, which are forests outside of a Natural Protected Area; here, the number of relict forests is higher than in Lima, but they represent only 29.55% of the total species’ distribution area. This differences in covered area may be due to the history of degradation that affected Huancavelica department, one of the areas with greatest pressure on mercury mining by Spanish colonizers [Citation51]. It is also an area with very important livestock activity, imposing greater pressure of continuous burning of pastures and trees on forests [Citation52], and the greater aridity of the lands of the western Andes, which stresses the effects of fires [Citation53].
Using the criteria of Navarro et al. for categorization at a smaller scale results in a different categorization since forests in the Protected Natural Area (forests 1 and 2), are in the Least Concern (LC) and Vulnerable (VU) categories, respectively; whereas forests 3 and 4, which are not protected, are Critically Endangered (CR) and Vulnerable (VU), respectively. The differences in categorization between the forests situated in a protected area were due to the location of forest 1, a higher elevation and in very steep hill slopes, which facilitate its conservation [Citation37], whereas forest 2 is more accessible, is fragmented by a road, has a greater presence of livestock and is affected by infestation of the hemiparasite Tristerix chodatianus, accentuating the effects of human impact and making it more vulnerable to degradation [Citation36,Citation37,Citation54]. Forest 3 is more degraded than the others, due to high sheep densities, its proximity to a human community, and the use of trees for fences and firewood. These conditions would explain the low diversity of flora and fauna [Citation2,Citation27,Citation55]. Because forest 4 is far from a human community, it is less threatened than the other forests and has a greater presence of species characteristic of this type of ecosystem [Citation33,Citation35]. It is considered one of the forests of highest conservation priority in Huancavelica.
Conservation implications
We propose that the 45 Polylepis Andean tree and shrub species described to date must be re-assessed regarding their IUCN conservation status, and present-day categorizations must be interpreted with caution. We suggest this as the species assessment under study has an inadequate categorization, and the other species in the genus were categorized more than 16 to 22 years ago. To date, 12 new species have been added, which requires distribution maps for all species of the genus since they dominate tropical and subtropical high Andean forests and are considered keystone species.[Citation2,Citation8,Citation38].
A global species assessment has the advantage that it provides an overview of the conservation status for the sustainable management of each species; however, this study also shows that the assessment of extinction threat at the species level (in particular, for Polylepis species forests) differs from an assessment at the population level. In the former case, the evaluation of the species can hide information that overestimates or underestimates its actual conservation status, especially when the categorizations are based only on the IUCN B1 and B2 criteria. Hence, the result should be considered only as a first approximation to the actual condition of each population and the methods need to be complemented, at species and population levels, for a better understanding of the conservation status. On one hand, the global assessment provides a baseline of the conservation status of each species, allowing for strategic planning, decision-making and project development for the conservation of the species. On the other hand, the importance of the local assessment lies in the fact that it allows decision-making and sustainable management by each rural community associated to the forest, as well as the selection of priority forests for conservation and/or restoration. Each population has different ecological and human impact characteristics and histories [Citation56]; therefore, generalizing the conservation status could lead to making conservation or restoration efforts in areas of lower priority than necessary; in addition, identifying priority populations translates into greater effectiveness in the use of scarce resources, at the same time, implies that the intervention mode in each forest should be different. Thus, our study suggests that threat category may be influenced by the scale at which it is assessed.
We further suggest focusing active P. flavipila restoration efforts on the Huancavelica forests since they are highly fragmented; thus, they are more vulnerable to the effects of climate change or the pressure of continuous human activities. The current conditions of these forests may lead to a reduced forest area in the coming years, limiting its distribution to the north. Instead, the Lima forests deserve particular conservation efforts to support the ongoing actions of the Nor-Yauyos Cochas Landscape Reserve. Finally, it is necessary to continue studies at the population level, regardless of the conservation status at the species level, in order to improve decision-making for the conservation and restoration of these ecosystems.
Author contribution
FNAM and HRQM conceived and designed the research, gathered information and analyzed the data; FNAM, HRQM, and DR wrote, revised, and edited the manuscript.
Acknowledgments
We thank the “Servicio Nacional Forestal y de Fauna Silvestre” and the “Servicio Nacional de Áreas Naturales Protegidas por el Estado” for providing authorizations for research under R.D.G. No. 037-2017-SERFOR/DGGSPFFS and R.D. 009-2018-SERNANP-DGANP, respectively. Likewise, we are grateful to Wendy Carolay Navarro Romo, Ciro Ricardo Paredes Huamán (Negrito) and Jimy Ronie Llacuachaqui Rodríguez (Timys) for contributing to field work and making it so much fun.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tejedor-Garavito N, Álvarez E, Arango S, et al. Evaluación del estado de conservación de los bosques montanos en los Andes tropicales. Ecosistemas. 2012a;21(1–2):148–166.
- Fjeldså J, Kessler M. Conservación de la biodiversidad de los bosques de Polylepis de las tierras altas de Bolivia: una contribución al manejo sustentable en los Andes. Santa Cruz de la Sierra: DIVA technical report; 2004, 214 p.
- Fjeldså J. Polylepis forests - vestiges of a vanishing ecosystem in the Andes. Ecotrópica. 2002;8:111–123.
- Domic A, Palabral-Aguilera A, Gómez I, et al. Polylepis incarum (Rosaceae) una especie en Peligro Crítico en Bolivia: propuesta de reclasificación en base al área de ocupación y estructura poblacional. Ecología en Bolivia. 2017;52(2):116–131.
- Renison D, Cuyckens GAE, Pacheco S, et al. Distribución y estado de conservación de las poblaciones de árboles y arbustos del género Polylepis (Rosaceae) en las montañas de Argentina. Ecología Austral. 2013;23(1):27–36.
- Boza T. Taxonomic studies in Polylepis (Rosaceae) [PhD Thesis]. [Zurich]: University of Zurich; 2020.
- Tejedor Garavito N, Newton AC, Oldfield S. Regional red list assessment of tree species in upper montane forests of the Tropical Andes. Oryx. 2015;49(3):397–409.
- IUCN. The IUCN red list of theatrened species. Version 2020-1 [Internet]. 2020 [cited 2020 May 3]. Available from: https://www.iucnredlist.org.
- Boza TE, Quispe-Melgar HR, Kessler M. Taxonomic reevaluation of the Polylepis sericea complex (Rosaceae), with the description of a new species. Systemat Bot. 2019;44(2):324–334.
- Mendoza W, Cano A. Diversidad del género Polylepis (Rosaceae, Sanguisorbeae) en los Andes peruanos. Rev Peru Biol. 2011;18(2):197–200.
- Segovia-Salcedo MC, Domic A, Boza T, et al. Situación taxonómica de las especies del género Polylepis. Implicancias para los estudios ecológicos, la conservación y la restauración de sus bosques. Ecol Austral. 2018;28(1 bis):188–201.
- Rodrigues A, Pilgrim J, Lamoreux J, et al. The value of the IUCN Red List for conservation. Trends Ecol Evol. 2006;21(2):71–76.
- Stuart SN, Wilson EO, McNeely JA, et al. The Barometer of Life. Science. 2010;328(5975):177–177.
- Mace GM. The role of taxonomy in species conservation. Godfray HCJ, Knapp S, editors. Phil Trans R Soc Lond B. 2004;359(1444):711–719.
- Unión Internacional para la Conservación de la Naturaleza y de los Recursos Naturales. Categorías y criterios de la Lista Roja de la UICN Version 3.1: aprobado en la 51° Reunión del Consejo de la UICN, Gland Suiza, 2000. Gland: UICN; 2012.
- Mace GM, Collar NJ, Gaston KJ, et al. Quantification of extinction risk: IUCN’s system for classifying threatened species. Conserv Biol. 2008;22(6):1424–1442.
- Miller RM, Rodríguez JP, Aniskowicz-Fowler T, et al. National threatened species listing based on IUCN criteria and regional guidelines: current status and future perspectives. Conserv Biol. 2007;21(3):684–696.
- Hortal J, De Bello F, Jaf D-F, et al. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu Rev Ecol Evol Syst. 2015;46(1):523–549.
- Brummitt N, Bachman S, Moat J. Applications of the IUCN Red List: towards a global barometer for plant diversity. Endanger Species Res. 2008;6: 127–135.
- León B, Pitman N, Roque J. El libro Rojo de las plantas endémicas del Perú. Rev per biol.2006;13(2):9–22.
- Pearson RG, Dawson TP. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol Biogeogr. 2003;12(5):361–371.
- Wiens JA. Spatial Scaling in Ecology. Funct Ecol. 1989;3(4):385.
- Willis KJ, Whittaker R. Species Diversity–Scale Matters. Science. 2002;295(5558):1245–1248.
- Morales LV, Sevillano-Rios CS, Fick S, et al. Differential seedling regeneration patterns across forest-grassland ecotones in two tropical treeline species (Polylepis spp.). Austral Ecol. 2018;43(5):514–526.
- Toivonen J, Gonzales-Inca C, Bader M, et al. Elevational shifts in the topographic position of Polylepis forest stands in the Andes of Southern Peru. Forests. 2017;9(1):7.
- Alaniz AJ, Galleguillos M, Perez-Quezada JF. Assessment of quality of input data used to classify ecosystems according to the IUCN Red List methodology: the case of the central Chile hotspot. Biol Conserv. 2016;204:378–385.
- Navarro G, Arrázola S, Balderrama J. et al. Diagnóstico del estado de conservación y caracterización de bosques de Polylepis y su avifauna. Rev boliv de ecologia y conservación ambiental. 2010;28(1):1–35.
- MINAM. Mapa nacional de cobertura vegetal - Memoria descriptiva. Dirección General de Evaluación, Valoración y Financiamiento del Patrimonio Natura. Peru. 2015. 105 p.
- Argibay DS, Renison D. Efecto del fuego y la ganadería en bosques de Polylepis australis (Rosaceae) a lo largo de un gradiente altitudinal en las montañas del centro de la Argentina. Bosque (Valdivia). 2018;39(1):145–150.
- Renison D, Hensen I, Suarez R, et al. Soil conservation in Polylepis mountain forests of Central Argentina: is livestock reducing our natural capital?. Austral Ecol. 2009;35(4):435–443.
- Herzog SK, Martínez R, Jørgensen PM, et al. Climate change and biodiversity in the tropical Andes [Internet]. Unpublished; 2011 [ cited 2020 Jun 14]. Available from: http://rgdoi.net/10.13140/2.1.3718.4969.
- Kessler M, Schmidt-Lebuhn A. Taxonomical and distributional notes on Polylepis (Rosaceae). Org Divers Evol. 2006;6(1):67–69p.
- Ames-Martínez FN, Quispe-Melgar HR, Zuñiga López DG, et al. Bosques de Polylepis: biodiversidad en la región central del Perú. Editorial Continental. Peru. 2019: 200.
- Trinidad H, Cano A. Composición florística de los bosques de Polylepis Yauyinazo y Chaqsii-Chaqsii, Reserva Paisajística Nor Yauyos-Cochas, Lima. Rev Peru Biol. 2016;23(3):271.
- Quispe-Melgar HR, Sevillano-Ríos CS, Navarro Romo WC, et al. The Central Andes of Peru: a key area for the conservation of Polylepis forest biodiversity. J Ornithol. 2020;161(1):217–228.
- Camel V, Arizapana-Almonacid M, Pyles M, et al. Using dendrochronology to trace the impact of the hemiparasite Tristerix chodatianus on Andean Polylepis trees. Plant Ecol. 2019;220(9):873–886.
- Camel VF, Quispe-Melgar HR, Ames-Martínez FN, et al. Forest structure of three endemic species of the genus Polylepis (Rosaceae) in Central Peru. Ecol Austral. 2019;29(3):285–295.
- Arnal H, Sampson A, Aucca C, et al. Mapa de bosques altiandinos de Polylepis prioritarios para la conservación [Internet]. Am Bird Conservancy; 2007. Available from: https://zenodo.org/record/4174838.
- Missouri Botanical Garden. Tropicos [Internet]. 2020 [ cited 2020 May 3]. Available from: http://www.tropicos.org.
- GBIF. Global Biodiversity Information Facility [Internet]. 2020 [ cited 2020 May 4]. Available from: https://www.gbif.org.
- Vásquez E, Ladd B, Borchard N. Carbon storage in a high-altitude Polylepis woodland in the Peruvian Andes. Alp Bot. 2014;124(1):71–75.
- QGIS Development Team. QGIS Geographic Information System. Open-source geospatial foundation project. [Internet]. 2020 [cited 2020 May 8]. Available from: http://qgis.osgeo.org.
- Unión Internacional para la Conservación de la Naturaleza y de los Recursos Naturales, Red List Programme. Directrices para el uso de los criterios de la lista roja de la UICN a nivel regional y nacional: versión 4.0. 2010. Gland. UICN. 2012.
- Fadrique B, Báez S, Á D, et al. Widespread but heterogeneous responses of Andean forests to climate change. Nature. 2018;564(7735):207–212.
- Purcell J, Brelsford A, Kessler M. The world’s highest forest: a better understanding of the properties of Andean queñua woodlands has major implications for their conservation. In: American Scientist. Sigma Xi. The Scientific Research Society. 2004. p. 454–461.
- U.S. Geological Survey. Earth Explorer [Internet]. 2020 [ cited 2020 May 8]. Available from: http://earthexplorer.usgs.gov/.
- Pettorelli N, Vik JO, Mysterud A, et al. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol Evol. 2005;20(9):503–510.
- Dauby G. ConR: Computation of parameters used in preliminary assessment of conservation status [internet]. 2020 [ cited 2020 May 19]. Available from: https://gdauby.github.io/ConR/.
- SERNANP. Plan maestro de la Reserva Paisajística Nor-Yauyos Cochas 2016-2020. Peru; 2016. 104p.
- Naughton-Treves L, Holland MB, Brandon K. The role of protected areas in conserving biodiversity and sustaining local livelihoods. Annu Rev Environ Resour. 2005;30(1):219–252.
- Contreras C. La ciudad del mercurio, Huancavelica 1570-1700. Instituto de Estudios Peruanos. Lima, Peru. 1982.126p.
- Fjeldså J, Kessler M. Conserving the biological diversity of Polylepis woodlands of the highland of Peru and Bolivia: a contribution to sustainable natural resource management in the Andes. Copenhagen. NORDECO. 1996.
- Reynel C, Pennington T, Särkinen T. Apuntes sobre la formación del relieve en ámbitos seleccionados de los Andes Peruanos. Cómo se formó la diversidad ecológica del Perú. Lima-Peru. 2013:175–189.
- Renison D, Hensen I, Cingolani AM. Anthropogenic soil degradation affects seed viability in Polylepis australis mountain forests of central Argentina. For Ecol Manage. 2004;196(2–3):327–333.
- Sylvester SP, Heitkamp F, Sylvester MDPV, et al. Relict high-Andean ecosystems challenge our concepts of naturalness and human impact. Sci Rep. 2017;7(1):3334.
- Kessler M, Toivonen JM, Sylvester SP, et al. Elevational patterns of Polylepis tree height (Rosaceae) in the high Andes of Peru: role of human impact and climatic conditions. Front Plant Sci. 2014;5:194.
