ABSTRACT
Charybdis hellerii is an invasive swimming crab widely disseminated in the western Atlantic. This species became a threat to colonized ecosystems, competing with local species for resources. The extension of distribution and increasing population size of C. hellerii has been associated with the scarcity of indigenous predators and cases of predation report octopuses as the main native predators. In this study, we present the first evidence that native fish can consume C. hellerii. 53 individuals of the goldspotted snake eel, Myrichthys ocellatus, were collected at seagrass and macroalgal beds in Pernambuco State, Brazil, and had their stomach contents analyzed. Three juvenile C. hellerii were found along with native prey. Myrichthys ocellatus feeds mainly on small crabs, thus C. hellerii individuals were consumed before reaching sexual maturity. Oppositely to octopuses and other crab predators, M. ocellatus is of little fishing interest and is commonly found in macroalgae beds, seagrass meadows and sandy areas near reefs. Our results suggest that a higher number of carcinophagous taxa may prey on this invasive crab and we emphasize that the conservation of these species is paramount for controlling C. hellerii populations in invaded areas.
Invasive alien species (IAS) are one of the worst threats to marine biodiversity worldwide [Citation1,Citation2]. Non-indigenous species have been increasingly reported to colonize the western Atlantic, at times with great success in establishing populations [Citation3–5]. The introduction of IAS has become both biological and economical threats to local ecosystems; the colonization by the lionfish Pterois volitans (Linnaeus, 1758) has been acknowledged as the most deleterious invasion to Caribbean systems [Citation6,Citation7]. While lionfish has been recently recorded on the Brazilian coast [Citation8,Citation9], the IAS with most detrimental effects in Brazil are benthic: Isognomon bicolor (C.B. Adams, 1845) (a Caribbean bivalve), Tubastraea coccinea Lesson, 1830, Tubastraea tagusensis Wells, 1982, Chromonephthea braziliensis van Ofwegen, 2005 (Indo-Pacific corals) and Charybdis hellerii (A. Milne-Edwards, 1867) (an Indo-Pacific swimming crab) [Citation10–12].
Charybdis hellerii has colonized the Atlantic Ocean through the Mediterranean Sea with the opening of the Suez Canal, reaching the western Atlantic by the end of the 80`s [Citation13]. This species spread along the Caribbean in the following years, probably transported by ballast water and local currents [Citation14,Citation15]. The rapid growth, continuous reproduction, along with the generalized habitat and diet requirements of C. hellerii, allowed a successful establishment and spread of this IAS, which is now found in virtually the entire Brazilian coast, from Pará to Santa Catarina states [Citation13,Citation16].
Local impacts caused by C. hellerii are still under-reported; however, available literature indicates potential economic and biological effects [Citation10]. In Twin Cays, Belize, this crab is possibly displacing other decapods previously found in higher abundances at the area (e.g. spider crabs (Mithrax spp.), swimming crabs (Callinectes spp.) and spiny lobsters (Panulirus spp.)) [Citation17]. In some Brazilian states (e.g. São Paulo and Santa Catarina), C. hellerii has outnumbered local crab populations, competing for resources with native species [Citation4,Citation16]. The use of this invasive crab in local fisheries is minimal; for example, in Venezuela (Falcón State), C. hellerii represented 5% of the captures in crab artisanal fisheries in 2003–2004 [Citation18]. Moreover, C. hellerii was also identified as a potential host for the white spot disease virus, which causes high mortality in shrimp [Citation10].
In spite of the complexity of processes that underlie biological invasions, IAS success can be linked to reduced population control by predators (i.e. the enemy release hypothesis) [Citation19–21]. In fact, the instances of predation on C. hellerii by indigenous species are very limited and highlight octopuses as the most common consumers [Citation22,Citation23]. Here, we present the first report indicating that fish may act as predators of this invasive species. Predation on C. hellerii by native fish was revealed by the analysis of stomach contents of the goldspotted snake eel, Myrichthys ocellatus (Lesueur, 1825). This species is found from Bermuda and South Florida, in the Caribbean, to Santa Catarina, in Brazil [Citation24,Citation25].
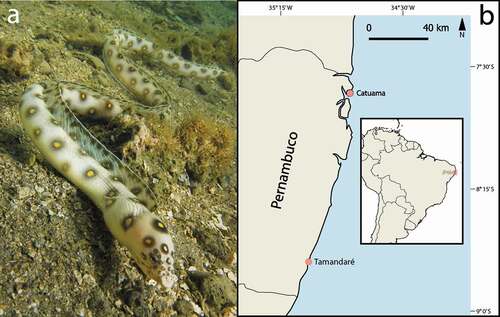
Samples were collected on the beaches of Catuama (7°37ʹ40.7 “S; 34°48ʹ19.9” W) and Tamandaré (08º45ʹ35ʹ’S; 35º06ʹ17ʹ’W), located on the Northern and Southern coasts, respectively, of the State of Pernambuco, Brazil (). The Catuama beach is influenced by the Itamaracá estuarine complex and comprises the largest seagrass meadows in the region where individuals of M. ocellatus were captured. Tamandaré, on the other hand, is part of the most extensive marine protected area in the Brazilian coast, the Costa dos Corais Marine Protected Area. The Tamandaré reef complex includes one of the largest fringing reef formations in the South Atlantic. In this site, M. ocellatus was captured at the macroalgal beds adjacent to shallow reefs (also see study location in [Citation26]). The goldspotted eel is very common in the study area [Citation27,Citation28], but there are no records of C. hellerii presence on both sites sampled prior to this study.
Figure 1. The goldspotted eel Myrichthys ocellatus, a novel predator of Charybdis hellerii (a). Location of collection sites, Catuama and Tamandaré beaches in Pernambuco, northeastern Brazil (b)
Individuals were captured during free dives performed between August and December 2019, on low tides at a maximum depth of 2 m, using hand nets (~40 cm diameter, 1.5 cm mesh). A total of 53 individuals were collected, 25 being captured in Catuama and 28 in Tamandaré, with sizes ranging from 33.5 to 66 cm in total length, with an average of 46.6 cm. After collection, specimens were fixed in a 4% formaldehyde solution. In the laboratory, stomach contents were removed on a petri dish and identified under stereo-microscope (40x magnification). Each individual prey was measured with a digital caliper (0.1 mm accuracy) and preserved in 70% ethyl alcohol.
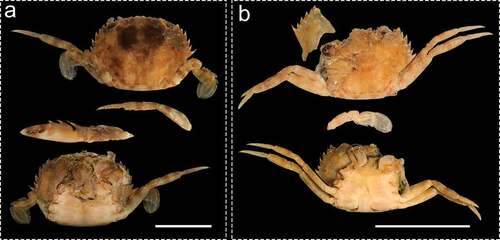
Three individuals of Charybdis hellerii were found in the stomach contents of Myrichthys ocellatus (). Specimens found were sexually immature; two males and a female measuring 11, 12 and 19 mm in carapace width (CW), respectively. The female was consumed by an individual of M. ocellatus with 47 cm of total length (TL) and one of the males was found as a prey of a fish with 39 cm TL, both collected in Catuama beach. The male with 11 mm CW was found within stomach contents of a 52 cm TL goldspotted eel sampled in Tamandaré.
Figure 2. Dorsal and ventral views of the Charybdis hellerii juvenile specimens found in Myrichthys ocellatus stomach contents at Catuama and Tamandaré beaches in Pernambuco, northeastern Brazil. a) female b) male. Scale bars denote 1 cm
Myrichthys ocellatus preys mainly on mobile invertebrates, especially crabs of the families Portunidae, Xanthidae and Majidae [Citation27–29]. During our stomach contents analysis, we found that the bulk of M. ocellatus diet is composed by these native crab species (e.g., Callinectes spp., Pitho sp., Epialtus sp.), also registering the ingestion of mollusks, isopods and shrimps to a lesser extent.
This is the first record of a native fish using C. hellerii as prey, suggesting that a higher number of carcinophagous taxa may feed on this invasive crab as well. Other species have been suggested as potential predators of C. hellerii, such as larger native Portunidae crabs (e.g. Callinectes ornatus Ordway, 1863) and reef-associated groupers (e.g., Mycteroperca spp. and Epinephelus spp.) [Citation22,Citation28], but empirical evidence is still lacking.
Oppositely to the predation performed by octopuses or larger fish, which target larger prey [Citation23], M. ocellatus predates preferentially on small crabs, well below the size of first maturity for C. hellerii, which is approximately 37 mm CW for females [Citation29–32]. Bearing in mind that each female of C. hellerii can produce from 22,500 to 3,200,000 eggs per reproductive event [Citation14,Citation33], the predation of this exotic crab before breeding can represent an effective way of local control of its spread [Citation23].
Additionally, groupers, crabs and octopuses are of high fishing interest and their populations are in decline [Citation34–39], while snake eels are of little economic value and are not fished at all [Citation40]. One of our study sites, Catuama beach, is heavily targeted by fishing and show highly depleted fish communities when compared to other seagrass meadows in the region [Citation41]. Yet, M. ocellatus seems to have high abundance in the area, and may contribute in preventing the local establishment of C. hellerii. Indeed, seagrass-associated macrofauna surveys in this study site does not show indications of established populations; in a previous survey performed in 2017 by our study group, 4,500 invertebrates were sampled and not a single individual of C. hellerii was captured [Citation42].
The goldspotted eel is a crab predator found in various habitats, such as seagrass meadows, macroalgal beds, rocky shores and coral reefs [Citation26–28]. It is also a conspicuous member of the ichthyofauna along a large extent of the area colonized by C. hellerii in the western Atlantic [Citation43]. We hypothesize that M. ocellatus, along with other carcinophagous fish, may play an important role in the dynamics of C. hellerii invasion, to be evaluated in further studies. Our results emphasize the importance of the conservation of local carcinophagous species, given their potential role in the control of populations of this invasive crab.
Acknowledgments
We thank colleagues from the Laboratório de Pesquisa em Ictiologia e Ecologia de Recifes – LabPIER, Silva MC, Xavier TF and Silva CVC for their assistance in fieldwork.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Molnar JL, Gamboa RL, Revenga C, et al. Assessing the global threat of invasive species to marine biodiversity. Front Ecol Environ. 2008;6(9):485–492.
- McGeoch MA, Butchart SH, Spear D, et al. Global indicators of biological invasion: species numbers, biodiversity impact and policy responses. Divers Distrib. 2010;16(1):95–108. .
- De Assis JD, Alonso C, Christoffersen ML. First record of Ficopomatus uschakovi (Pillai, 1960) Serpulidae (Polychaeta: annelida) for the Western Atlantic. Rev Nordestina Biol. 2008;19(1):51–58.
- Sant’Anna BS, Watanabe TT, Turra A, et al. Relative abundance and population biology of the non-indigenous crab Charybdis hellerii (Crustacea: Brachyura: Portunidae) in a southwestern Atlantic estuary-bay complex. Aquat Invasions. 2012;7(3):347–356.
- Vieira LM, Mes J, Je W. Resurrection of the genus Licornia for Scrupocellaria jolloisii (Bryozoa) and related species, with documentation of L. jolloisii as a non-indigenous species in the western Atlantic. J Mar Biol Assoc UK. 2013;93(7):1911.
- Green SJ, Akins JL, Maljković A, et al. Invasive lionfish drive Atlantic coral reef fish declines. PloSOne. 2012;7(3):e32596.
- Albins MA, Hixon MA. Worst case scenario: potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environ Biol Fish. 2013;96(10-11:1151–1157.
- Ferreira CEL, Luiz OJ, Floeter SR, et al. First record of invasive lionfish (Pterois volitans) for the Brazilian coast. PloS One. 2015;10(4):e0123002.
- Bumbeer J, Da Rocha RM, Bornatowski H, et al. Predicting impacts of lionfish (Pterois volitans) invasion in a coastal ecosystem of southern Brazil. Biol Invasions. 2018;20(5):1257–1274.
- Ferreira CEL, De Oliveira Ribeiro Junqueira A, Villac MC, et al. Marine bioinvasions in the Brazilian coast: brief report on history of events, vectors, ecology, impacts and management of non-indigenous species. In: Rilov G, Crooks JAeditors. Biological invasions in marine ecosystems. Berlin, Heidelberg: Springer; 2009. p. 459–477.
- Miranda RJ, Cruz IC, Barros F. Effects of the alien coral Tubastraea tagusensis on native coral assemblages in a southwestern Atlantic coral reef. Mari Biol. 2016;163(3):1–12.
- Miranda RJ, Tagliafico A, Kelaher BP, et al. Impact of invasive corals Tubastrea spp on native coral recruitment. Mar Ecol Prog Ser. 2018;605:125–133.
- Dineen JF, Clark PF, Hines AH, et al. Life history, larval description, and natural history of Charybdis hellerii (Decapoda, Portunidae), an invasive crab in the Western Atlantic. J Crust Biol. 2001;21(3):774–805.
- Lemaitre R. Charybdis hellerii (Milne Edwards, 1867), a nonindigenous portunid crab (Crustacea: Decapoda: Brachyura) discovered in the Indian River lagoon system of Florida. Proc Biol Soc Wash. 1995;108:643–648.
- McMillen-Jackson A. First record of the Indo-Pacific swimming crab, Charybdis hellerii (A. Milne-Edwards, 1867) in the Gulf of Mexico. Crustaceana. 2008;81(7):889–896.
- Sant’Anna BS, Branco JO, Oliveira MM. et al. Diet and population biology of the invasive crab Charybdis helleri in southwestern Atlantic waters. Aquat Invasions. 2015;11(8):814–823.
- Felder DL, Dworschak PC, Robles R, et al. Obvious invaders and overlooked infauna: unexpected constituents of the decapod crustacean assemblage at twin cays, Belize. In: Lang MI, Macintyre G, Ruetzler K, editors. Proceedings of the Smithsonian Marine Science Symposium. Smithson: Institution Scholarly Press; 2009 p.181–188.
- Morán R, Atencio M. Charybdis hellerii (Crustacea: Decapoda: Portunidae), especie invasora en la Península de Paraguaná, estado Falcón, Venezuela. Multiciencias. 2006;6(2):202–209.
- Colautti RI, Ricciardi A, Grigorovich IA, et al. Is invasion success explained by the enemy release hypothesis? Ecol Lett. 2004;7(8):721–733.
- Santos MCF, Coelho PA. Crustáceos exóticos reproduzindo em águas costeiras do nordeste do Brasil [Exotic Crustaceans Breeding in Coastal Waters of Northeastern Brazil]. Boletim Técnico-Científico do CEPENE, Tamandaré. 2007;15:57–61.
- Loebmann D, Mai ACG, Lee JT. The invasion of five alien species in the delta do parnaiba environmental protection area, Northeastern Brazil. Rev Biol Trop. 2010;58(3):909–923.
- Sampaio CLS, Rosa IL. Predation of an alien species of crab (Charybdis hellerii Milne Edwards) by a native Octopus species on NE Brazilian reefs. Coral Reefs. 2006;25(1):58.
- Da Silva EJ, Bezerra LEA, Martins IX. The tropical Octopus insularis (Mollusca, Octopodidae): a natural enemy of the exotic invasive swimming crab Charybdis hellerii (Crustacea, Portunidae). Pan-American J Aquat Sci. 2018;13(1):79–83.
- McCosker JE, Böhlke EB, Böhlke JE. Family Ophichthidae. In: Böhlke EB, editor. Fishes of the Western North Atlantic. Sears Foundation for Marine Research: Yale University, New Haven. 1989. p. 254–412.
- McCosker JE, Rosenblatt RH. A revision of the snake eel genus Myrichthys (Anguilliformes: ophichthidae) with the description of a new eastern Pacific species. Proc Calif Acad Sci. 1993;48(8):153–169.
- Chaves LTC, Pereira PHC, Feitosa JLL. Coral reef fish association with macroalgal beds on a tropical reef system in North-eastern Brazil. Mar Freshw Res. 2013;64(12):1101–1111.
- Ternes MLF, Giglio VJ, Mendes TC, et al. Follower fish of the goldspotted eel Myrichthys ocellatus with a review on anguilliform fish as nuclear species. Helgol Mar Res. 2018;72(1):2.
- Luiz Jr. OJ, Carvalho-Filho A, FerreiraCEL, et al. The reef fish assemblage of the Laje de Santos Marine State Park, Southwestern Atlantic: annotated checklist with comments on abundance, distribution, trophic structure, symbiotic associations, and conservation. Zootaxa. 2008;1807(1):1–25.
- Araújo ME, Pereira PHC, Feitosa JLL, et al. Feeding behavior and follower fishes of Myrichthys ocellatus (Anguilliformes: ophichthidae) in the western Atlantic. Neotropical Ichthyol. 2009;7(3):503–507.
- Mantelatto FLM, Dias LL. Extension of the known distribution of Charybdis hellerii (A. Milne-Edwards, 1867) (Decapoda, Portunidae) along the western tropical South Atlantic. Crustaceana. 1999;72(6):617–620.
- Mantelatto FLM, Garcia RB. Biological aspects of the nonindigenous portunid crab Charybdis hellerii in the western tropical south Atlantic. Bull Mar Sci. 2001;68(3):469–477.
- Bolaños J, Baeza J, Hernández J, et al. Population dynamics and reproductive output of the non-indigenous crab Charybdis hellerii in the south-eastern Caribbean Sea. J Mar Biol Assoc UK. 2012;92(3):469–474. .
- Siddiqu G, Ahmed M. Fecundities of some marine brachyuran crabs from Karachi (Pakistan). Pakistan J Zool. 1992;24:43–45.
- Arreguín-Sánchez F, Solís-Ramírez MJ, González De La Rosa ME. Population dynamics and stock assessment for Octopus maya (Cephalopoda: octopodidae) fishery in the Campeche Bank, Gulf of Mexico. Rev Biol Trop. 2000;48(2–3):323–331.
- Chiappone M, Sluka R, Sealey KS. Groupers (Pisces:Serranidae) in fished and protected areas of the Florida Keys, Bahamas and northern Caribbean. Mar Ecol Prog Ser. 2004;7: 261–272.
- Morris AV, Roberts CM, Hawkins JP. The threatened status of groupers (Epinephelinae). Biodivers Conserv. 2000;9(7):919–942.
- Leite TS, Haimovici M, Lins JE. A Pesca de Polvos no Arquipélago de Fernando de Noronha, Brasil [Octopus Fishing in the Fernando de Noronha Archipelago, Brazil]. Bol Inst Pesca. 2008;34:271–280.
- Penn JW, Caputi N, De Lestang S, et al. Crustacean Fisheries. In: Cochran JK, Bokuniewicz HJ, Yager PLeditors. Encyclopedia of Ocean Sciences (Third Edition). Oxford: Academic Press; 2019. p. 324–337.
- Sadovy De Mitcheson Y, Craig MT, Bertoncini AA, et al. Fishing groupers towards extinction: a global assessment of threats and extinction risks in a billion dollar fishery. Fish and Fisheries. 2013;14(2):119–136.
- FishBase.org [Internet].World Wide Web. [cited 2020 Sep 16]. Available from: http://www.fishbase.org
- Vieira MLM, Lima CLA, Souza JRB, et al. Effects of beach seine fishing on the biodiversity of seagrass fish assemblages. Reg Stud Mar Sci. 2020;40:101527.
- Vieira MLM Diversidade funcional de comunidades associadas a pradarias de Halodule wrightii (Ascherson, 1868) na costa do Estado de Pernambuco [Functional diversity of communities associated with seagrass meadows of Halodule wrightii (Ascherson, 1868) off the coast of the State of Pernambuco] [master’s thesis]. Recife (PE): Universidade Federal de Pernambuco; 2019.
- Araújo ME, Mattos FMG, Melo FPL, et al. Diversity patterns of reef fish along the Brazilian tropical coast. Mar Environ Res. 2020;160:105038.
