ABSTRACT
The high Andean Polylepis woodlands are home to a unique biota, including an outstanding avifauna. They are one of the most threatened mountain ecosystems worldwide; accordingly, they have been the object of the first restoration initiatives in South America. Here, we evaluated the effectiveness of 20-year Polylepis australis woodland restoration efforts to recover the woodland bird communities. We recorded bird diversity and abundance in an ongoing restoration site (with a high proportion of woodland) and in a control site, where no active restoration efforts were made (with a high proportion of grasslands and bare soil), 15 and 20 years after the start of the restoration project. We compared the avifauna of these sites with that of reference mature woodlands, using published records of the study region. At the ongoing restoration site, bird diversity increased over time, as well as abundances of species associated with P. australis, whereas those parameters remained stable at the control site. Our results are the first evidence that active restoration of P. australis entails passive restoration of the avifauna occurring in these unique upland forests.
Introduction
Woodlands of the genus Polylepis occur across broad altitudinal, latitudinal and precipitation ranges. They are present along the Andes and nearby mountain ranges, from Venezuela to central Argentina, being one of the forest communities with the greatest latitudinal extension in the world [Citation1]. These woodlands also form the highest tree lines in the world [Citation2]. They are characterized by their high level of endemism [Citation3,Citation4], and are currently regarded as one of the most threatened ecosystems in the Tropical Andes [Citation5].
These particular upland woodlands retain soils and nutrients [Citation6,Citation7], capture large amounts of atmospheric carbon, regulate run-off, and improve water capture in regions with many foggy days [Citation8]. Birds are essential for woodland ecosystem functioning (e.g. seed dispersal, pest control, pollination); thus, when the abundance of different plant species is reduced, birds are strongly affected due to decreases in food availability and nesting substrates. For this reason, the number of bird species and their abundances are approximately twofold in Polylepis forests compared with the surrounding degraded areas [Citation9]. Hence, the conservation and restoration of these highland forests have a critically important dual role: protecting a rich biodiversity while providing valuable environmental and socioeconomic services [Citation10,Citation11].
The main factors controlling pre‐human Polylepis woodland dynamics were precipitation and landscape heterogeneity [Citation12]. A review by Renison et al. [Citation13] found that, at present, Polylepis woodland distribution is strongly associated with sites of low anthropogenic impact, indicating that human-ignited fires and browsing by domestic livestock would be the main causes for the decline of these forests. This finding partly explains that these woodlands occur mainly in topographic areas characterized by very steep and inaccessible escarpments [Citation14]. In particular, Polylepis australis (hereafter Polylepis) woodlands in the mountains of central Argentina have been drastically reduced due to overgrazing by introduced livestock for over 400 years [Citation15]; grazing caused an alarming soil erosion process that resulted in 20% loss of soil surface above 1500 m asl [Citation16]. These forests currently cover only 12% of the landscape [Citation17], and the remnant patches are severely degraded, showing a significant loss of structural complexity [Citation18].
Polylepis woodlands hold a unique biota, particularly of habitat specialist birds [Citation19]. Most of these birds have undergone a continuous population decline, which is likely linked to the loss of habitat and fragmentation [Citation20]; for this reason, avian communities are one of the conservation priorities [Citation21]. Of the bird species recorded throughout the distribution of the genus Polylepis, two are categorized as critically endangered, eight are endangered, eight are vulnerable, and nine are near threatened (http://www.iucnredlist.org/). In addition, 70% of these species have restricted distribution ranges, whereas 52% have a high degree of association with Polylepis woodlands [Citation22]. At the landscape level, birds associated with Polylepis are present mainly in remnant woodland patches of different sizes and shapes, often of complex topography, surrounded by a grassland or shrubland matrix [Citation9,Citation23]. At the patch level, resource availability, the characteristics of the edge vegetation, growth mode, and degree of disturbance have a marked influence on Polylepis bird assemblages [Citation24–26].
Due to the alarming conservation status of Polylepis woodlands, one of the first restoration projects was implemented in 1997 in Córdoba province, Argentina. After more than 20 years of restoration activities, soil loss was largely reduced and more than 30% of the forest cover was restored [Citation27]. However, even though plantation of trees in degraded areas favors forest cover, it does not necessarily imply the recovery of the associated wildlife [Citation28]; indeed, restoring habitat for wildlife depends on multiple factors and spatial scales [Citation29]. For this reason, this study evaluated, for the first time, if the Polylepis restoration project also contributed to the recovery of the native avifauna associated with these woodlands. We hypothesized that the active restoration of a Polylepis forest would increase bird diversity, since vegetation patches with a complex architecture provide more resources and opportunities for microhabitat segregation. In this study, we provide field data of the avifauna in an ongoing restoration site (hereafter, restoration site) and in a control site, and compare them with avifauna data of mature Polylepis woodlands located in protected or inaccessible areas in the study region, using published records [Citation9,Citation30,Citation31].
Methods
Study area description
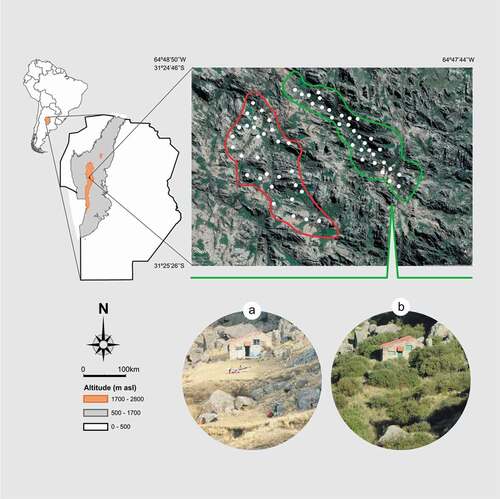
The study was conducted in the mountains of central Argentina, known locally as “Sierras Grandes de Córdoba”; it is characterized by a heterogeneous landscape of hills and plateaus with gentle valleys and deep ravines [Citation14]. Mean temperature is 5°C in winter and 11.4°C in summer, and mean annual precipitation is 900 mm, concentrated in the warmest months, between October and April [Citation11]. The site under restoration, located in Los Gigantes massif, encompasses a 22-ha area characterized by a heterogeneous combination of open valleys, rocky outcrops, deep ravines, ridges, and slopes at altitudes ranging from 2,200 to 2,300 m asl. Restoration activities consisted of excluding livestock using wire fences and planting about 35,000 native tree saplings mainly of Polylepis (86%), with Maytenus boaria (9%) and Escallonia cordobensis (5%) from 1997 to 2007 [Citation27]. Ongoing activities include planting of native grasses and forbs in gullies showing active soil erosion and the systematic control of the existing non-native shrubs and trees (https://www.ecosistemasarg.org.ar/proyectos).
Site physiognomy
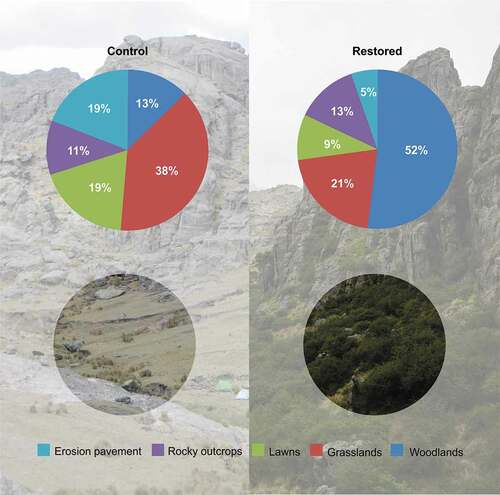
Two sampling areas were selected (): the site where the first project for the ecological restoration of Polylepis woodlands was implemented and a control site (30 ha) of similar topographic characteristics and elevation, adjacent to the restoration site, where no restoration activities had been conducted (). The landscape of both selected sites (restoration and control) was very similar in 1997 according to the vegetation map reported in Cingolani et al. [Citation32]. We classified the landscape into five physiognomic types, following Flores et al. [Citation15]: Woodlands, Grasslands, Short-grass terrains or Lawns, Rocky outcrops, and Erosion pavement. We used 2017 satellite images obtained from Google Earth version 7.3 for the study period (https://www.google.com/earth/index.html, accessed 6 April 2019). We visually differentiated the physiognomic units based on color and texture, and on previous experience and field surveys in the study area. The proportion of each physiognomic unit was calculated by dividing the area covered by each type into the total area, using Qgis version 3.0.
Bird sampling
Bird sampling was conducted during the bird reproductive season (austral summer), in January 2012 and 2017, 15 and 20 years after the start of the forest restoration project, respectively. During each survey, diversity, abundance and composition of bird community were estimated using the point count method [Citation33]. In each study site, 30 count points were randomly located in accessible areas, and an intensive systematic sampling was performed to avoid double-counts between neighboring points [Citation34]. At each point, after waiting for 5 minutes, the same observer (an ornithologist specialized in bird identification in the study area) recorded all the bird species seen or heard within a 20-m radius for 10 min. Surveys started 30 min after sunrise and lasted 3 to 4 h; each point was sampled 10 times each year, at a minimum of one-day interval.
Data analyses
Bird communities were characterized in terms of species richness, bird abundance and Shannon diversity index [Citation35]. Differences in species diversity were calculated using a Test-t. To adjust for differences in species detectability, we compared species richness between sites and seasons using rarefaction curves. Rarefaction analysis calculates species richness after standardizing differences in abundance among samples by estimating the expected number of species of each sample if all samples were reduced to a standard size [Citation35]. All analyses were performed using the software PAST v 3.25 [Citation36]. We also compared the species recorded in this study with avifauna data of mature Polylepis woodlands located in protected or inaccessible areas in the study region, using published records [Citation9,Citation30,Citation31].
To identify patterns of bird community variation across the control and restoration sites, we used a Detrended correspondence analysis (DCA) [Citation37]. Moreover, to understand the importance of Polylepis restoration on avifauna, two additional DCAs were carried out focusing on the abundance of Anairetes parulus and Leptasthenura fuliginiceps, two characteristic species of these forests in the study area [Citation31].
Finally, to evaluate the species associated with a particular forest site (i.e. control and restoration sites), we performed an Indicator Species Analysis (ISA), following the method of Dufrêne and Legendre [Citation38]. This approach selects the characteristic species of each site based on the abundance and fidelity of occurrence of each species in a particular habitat type (group). We calculated Aij, i.e. the mean of abundance of species i in the group j, compared with all the study groups, and Bij, i.e. the relative frequency of occurrence of species i in the group j. Then, the indicator value (IV) was obtained as follows: IV = Aijх Bijх 100. This IV value was statistically tested using the Monte Carlo method [Citation39]. A perfect characteristic species of a site will be that showing an IV of a significance level <0.05, i.e. one that is always present and is exclusive to the restoration site or to the control site. We also evaluated the percentage of these species that belonged to each of the trophic guilds in birds.
Results
Site physiognomy
In the restoration site, woodlands had the largest cover, whereas in the control site, the dominant covers were grasslands and lawns, with a high percentage of bare soil ().
Bird sampling
A total of 30 bird species were recorded; specifically in the restoration site, 17 species were recorded in 2012 and 25 species in 2017 (Supplementary information 1). Bird abundance also showed variation between study years at the Polylepis restoration site, with 1005 individuals recorded in 2012 and 869 individuals in 2017. Between 15 and 20 years after the start of the restoration project in 1997, species diversity increased (H2012: 2.39, H2017: 2.74, t = 9.12, P < 0.0001) in the restoration site with respect to the control site (H2012: 2.60 and H2017: 2.55), whereas the control site showed no differences between study years (t = 1.47, P = 0.14). Rarefaction analysis confirmed that our results were not an artifact of differences in the number of collected individuals (Supplementary information 2).
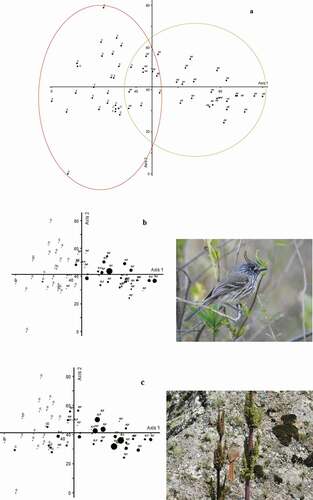
Differences in physiognomy between Polylepis forests restoration and control sites separated the bird species into two groups. The first and second axes of the DCA explained 41% of the total variability and clearly split the restoration site from the control site ()). A. parulus ()) and L. fuliginiceps ()) were recorded mainly in the restoration site.
Figure 3. (a) Detrended correspondence analysis (DCA) showing the ordination of bird species. The restoration and control sites are indicated with a green and red circle, respectively. (b) and (c) Anairetes parulus and Leptasthenura fuliginiceps abundances, respectively, in both sites; the point size indicates the abundance of the species at each survey points. RF: restoration site; C: control site
Representative species analysis
Four species (Monte Carlo test based on 1000 permutations; P < 0.05) resulted significant and closely associated with the restoration site; three of them (75%) were insectivores; in turn, six species were selected in the control sites, three of which (50%) were granivores ().
Table 1. Bird species and trophic guilds significantly associated with the restoration and control sites
Discussion
We hypothesized that bird diversity at the Polylepis restoration site increased with increasing forest cover and complexity. Our results indicate that at least 20 years of active restoration of this woodland were necessary for the passive recovery of the native avifauna. Thus, this study shows the importance and effectiveness of restoration actions in sites like our study area, since they contribute to the persistence of viable populations of the different bird species associated with these mountain woodlands [Citation23,Citation40].
A review including 39 studies revealed that during secondary forest regeneration, the avifauna takes longer to recover than other taxa; thus, it can be predicted that bird species richness and composition will increase approximately 20 to 40 years after the start of forest restoration [Citation41]. In addition, bird species richness in High-Andean forest fragments was found to increase with increasing habitat quality and topography complexity [Citation42]. In our study, the abundance of birds associated with Polylepis, such as Anairetes parulus and Leptasthenura fuliginiceps [Citation43], markedly increased in the restoration site between 15 and 20 years after the start of the restoration project, suggesting that these birds bred there. In addition, 20 years after the start of the restoration activities, practically all the species recorded in well-preserved Polylepis woodland sites [Citation9,Citation30,Citation31], with the only exception to Catamenia analis and Knipolegus aterrimus, were observed.
Most of the bird species closely associated with the restoration site are insectivores and woodland habitat specialists. This result can be explained by the increased structural complexity of the Polylepis woodland over time after planting [Citation44], and the consequent availability of resources for the woodland avifauna, allowing the establishment of new species and the increased abundance of the species already present. Moreover, the occurrence of species with different life habits in the restoration site (such as Turdus chiguanco, Bubo magellanicus, Elaenia albiceps, Sappho sparganura and Psilopsiagon aymara) also suggests that the restoration process generated the ecological conditions for the development of new resources; in turn, these resources allow the development of other trophic guilds of birds (i.e. carnivores, omnivores, frugivores, nectarivores). Conversely, the bird species closely associated with the control site were mainly granivores associated with grasslands or rocky outcrops (such as Geospizopsis unicolor and Zonotrichia capensis), the predominant physiognomy of the upper belt mountains in central Argentina after the last century’s retraction of the Polylepis forests [Citation18].
Most of the bird species associated with Polylepis have small population sizes and restricted distribution ranges [Citation20]. Therefore, in the restoration site, the presence of species categorized as of conservation priority, such as Cinclodes olrogi [Citation22], reinforces the importance of projects like the present one to promote the recovery of specialist birds of this particular ecosystem [Citation45–47], whose bird richness depends on patch connectivity and conservation [Citation9]. In turn, a recent study found no evidence that Polylepis specialist bird species were sensitive to patch size, and indicated the high ecological value of small patches for their conservation [Citation48].
The evidence presented in this study shows that the active ecological restoration of Polylepis can be effective not only in regenerating these upland woodlands but also in the gradual recovery of its native avifauna. At least in this particular case, the presence and abundance of bird species associated with forest habitat were notably increased at the restoration site. Even though our study area was near the southern distribution of Polylepis genus, our results may encourage Polylepis forest restoration projects in other sites, in particular in the central area of their distribution, where most bird endemisms are found [Citation3,Citation24].
Due to the increasing number of Polylepis restoration projects (https://www.globalforestgeneration.org/Accin-Andina), we also suggest that in future evaluations, baseline studies should be conducted with the aim of determining not only the initial extent of Polylepis forest but also the initial bird biodiversity. In addition, species detectability and seasonality should be considered possible sources of bias [Citation49]. Further research in the study area is necessary to determine if other taxa present in reference Polylepis forests have also been recovered or, rather, if it will be necessary to apply active restoration measures until ecological restoration of the whole biota and ecosystem services of this montane forest is achieved. The results obtained after 20 years of the project implementation are very encouraging.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Acknowledgments
The authors thank Henrik von Wehrden for helpful guidance in the early stage of this study and all volunteers who made the Polylepis forest restoration possible. CONICET provided funding in the last stages of this study through a PIP grant to DR. Noelia Gaillardou helped us with the figures, and Jorgelina Brasca improved the English style.
Additional information
Funding
References
- Cuyckens GA, Christie DA, Domic AI, et al. Climate change and the distribution and conservation of the world’s highest elevation woodlands in the South American Altiplano. Global Planet Change. 2016;137:79–87.
- Hoch G, Körner C. Growth, demography and carbon relations of Polylepis trees at the world’s highest treeline. Funct Ecol. 2005;19:941–951.
- Fjeldså J. The avifauna of the Polylepis woodlands of the Andean highlands: the efficiency of basing conservation priorities on patterns of endemism. Bird Conserv Int. 1993;3:37–55.
- Fjeldså J, Bowie R, Rahbek C. The role of mountain ranges in the diversity of birds. Annu Rev Ecol Evol Syst. 2012;43:249–265.
- Purcell J, Brelsford A. Reassessing the causes of decline of Polylepis, a tropical subalpine forest. Ecotropica. 2004;10:155–158.
- Torres RC, Renison D, Hensen I, et al. Polylepis australis’ regeneration niche in relation to seed dispersal, site characteristics and livestock density. For Ecol Manage. 2008;254:255–260.
- Renison D, Hensen I, Suárez R, et al. Soil conservation in Polylepis mountain forests of Central Argentina: is livestock reducing our natural capital? Austral Ecol. 2010;35:435–443.
- Poca M, Cingolani AM, Gurvich DE, et al. La degradación de los bosques de altura del centro de Argentina reduce su capacidad de almacenamiento de agua. Ecol Austral. 2018;28:235–248.
- Bellis L, Pidgeon A, Alcántara C, et al. Influences of succession and erosion on bird communities in a South American highland wooded landscape. For Ecol Manage. 2015;349:85–93.
- Gareca EE, Hermy M, Fjeldså J, et al. Polylepis woodland remnants as biodiversity islands in the Bolivian high Andes. Biodivers Conserv. 2010;19:3327–3346.
- Cingolani AM, Poca M, Giorgis MA, et al. Water provisioning services in a seasonally dry subtropical mountain: identifying priority landscapes for conservation. J Hydrol. 2015;525:178–187.
- Valencia BG, Bush MB, Coe AL, et al. Polylepis woodland dynamics during the last 20,000 years. J Biogeogr. 2018;45(5):1019–1030.
- Renison D, Morales L, Cuyckens G, et al. Ecología y conservación de los bosques y arbustales de Polylepis: ¿qué sabemos y qué ignoramos? Ecol Austral. 2018;28:163–174.
- Cingolani AM, Renison D, Tecco P, et al. Predicting cover types in a mountain range with long evolutionary grazing history: a GIS approach. J Biogeogr. 2008;35:538–551.
- Flores CE, Cingolani AM, von Müller A, et al. Habitat selection by reintroduced guanacos (Lama guanicoe) in a heterogeneous mountain rangeland of central Argentina. Rangeland J. 2012;34(4):439–445.
- Cingolani AM, Vaiereti MV, Giorgis MA, et al. Can livestock and fires convert the sub-tropical mountain rangelands of central Argentina into a rocky desert? Rangeland J. 2013;35(3):285–297.
- Cingolani AM, Renison D, Tecco P, et al. Cabido M Predicting cover types in a mountain range with long evolutionary grazing history: a GIS approach. J Biogeogr. 2008;35:538–551.
- Renison D, Hensen I, Suarez R. Landscape structural complexity of high-mountain Polylepis australis forests: a new aspect of restoration goals. Restor Ecol. 2011;19:390–398.
- Herzog SK, Soria R, Matthysen E. Seasonal variation in avian community composition in a high-Andean Polylepis (Rosaceae) forest fragment. Wilson J Ornithol. 2003;115:438–447.
- Sevillano-Ríos CS, Rodewald AD. Avian community structure and habitat use of Polylepis forests along an elevation gradient. Peer J. 2017;5:e3220.
- Cuyckens GA, Renison D. Ecología y conservación de los bosques montanos de Polylepis: una introducción al número especial. Ecol Austral. 2018;28:157–162.
- Sevillano-Ríos CS, Rodewald AD, Morales LV. Ecología y conservación de las aves asociadas con Polylepis: ¿qué sabemos de esta comunidad cada vez más vulnerable? Ecol Austral. 2018;28:216–228.
- Lloyd H, Marsden SJ. Between patch bird movements within a high Andean Polylepis woodland/matrix landscape: implications for habitat restoration. Restor Ecol. 2011;19:74–82.
- Lloyd H. Abundance and patterns of rarity of Polylepis birds in the Cordillera Vilcanota southern Peru: implications for habitat management strategies. Bird Conserv Int. 2008;18:164–180.
- Lloyd H. Influence of within patch habitat quality on high Andean Polylepis bird abundance. Ibis. 2008;150:735–745.
- Matthysen E, Collet F, Cahill J. Mixed flock composition and foraging behavior of insectivorous birds in undisturbed and disturbed fragments of high-Andean Polylepis woodland. Ornitol Neotrop. 2008;19:403–416.
- Renison D, Herrero L, Torres RC, et al. El rol de los voluntarios en la restauración ecológica del centro argentino. In: Ceccon E, Pérez DR, editors. Más allá de la ecología de la restauración: perspectivas sociales en América Latina y el Caribe. México: Universidad Nacional Autónoma de México Press; 2017. p. 55–76.
- Bremer L, Farley K. Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers Conserv. 2010;19(14):3893–3915.
- George TL, Zack S. Spatial and temporal considerations in restoring habitat for wildlife. Restor Ecol. 2001;9(3):272–279.
- García C, Renison D, Cingolani AM, et al. Avifaunal changes as a consequence of large‐scale livestock exclusion in the mountains of Central Argentina. J Appl Ecol. 2008;45(1):351–360.
- Bellis L, Rivera L, Politi N, et al. Latitudinal patterns of bird richness, diversity and abundance in Polylepis australis mountain forest of Argentina. Bird Conserv Int. 2009;19:265–276.
- Cingolani AM, Renison D, Zak MR, et al. Mapping vegetation in a heterogeneous mountain rangeland using Landsat data: an alternative method to define and classify land-cover units. Remote Sens Environ. 2004;92(1):84–97.
- Hilden O, Koskimies P, Pakarinen R, et al. Point count of breeding land birds. In: Koskimies P, Väisänen RA, editors. Monitoring bird populations. Finland Zoological Museum: Finnish Museum of Natural History Press; 1991. p. 27–32.
- Ralph CJ, Sauer JR, Droege S. Monitoring bird populations by point counts. Albany, California: Pacific Southwest Research Station; 1998.
- Magurran AE. Measuring biological diversity. New Jersey, USA: Blackwells; 2004.
- Hammer Ø, Harper DA, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron. 2019;4(1):9.
- Hill MO, Gauch HG. Detrended correspondence analysis: an improved ordination technique. In: van der Maarel E, editor. Classification and ordination. Dordrecht: Springer; 1980. p. 47–58.
- Dufrêne M, Legendre P. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr. 1997;67(3):345–366.
- McCune B, Grace JB, Urban DL. Analysis of ecological communities: MJM software design. Gleneden Beach, Oregon, USA; 2002.
- Lloyd H, Marsden SJ. Bird community variation across Polylepis woodland fragments and matrix habitats: implications for biodiversity conservation within a high Andean landscape. Biodivers Conserv. 2008;17:2645–2660.
- Dunn RR. Recovery of faunal communities during tropical forest regeneration. Conserv Biol. 2004;18(2):302–309.
- Fastré C, Strubbe D, Balderrama JA, et al. Bird species richness in High-Andean forest fragments: habitat quality and topography matter. Belgian J Zool. 2020;150(1):95–133.
- Fjeldså J. Polylepis forest-vestiges of a vanishing Andean ecosystem. Ecotropica. 2002;8:111–123.
- Renison D, Chartier MP, Menghi M, et al. Spatial variation in tree demography associated to domestic herbivores and topography: insights from a seeding and planting experiment. For Ecol Manage. 2015;335:139–146.
- Cierjacks A, Salgado S, Wesche K, et al. Post-fire population dynamics of two tree species in high altitude Polylepis forests of Central Ecuador. Biotropica. 2008;40:176–182.
- Domic AI, Camilo GR, Capriles JM. Small-scale farming and grazing reduce regeneration of Polylepis tomentella (Rosaceae) in the Semiarid Andes of Bolivia. Biotropica. 2014;46:106–114.
- Morales LV, Sevillano-Ríos CS, Fick S, et al. Differential seedling regeneration patterns across forest-grassland ecotones in two tropical tree line species (Polylepis spp.). Austral Ecol. 2018;43(5):514–526.
- Sevillano-Ríos CS, Rodewald AD. Responses of Polylepis birds to patch and landscape attributes in the High Andes. Neotrop Biodivers. 2021;7(1):5–22.
- Kéry M, Schmidt B. Imperfect detection and its consequences for monitoring for conservation. Commun Ecol. 2008;9(2):207–216.