?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We describe two new species of terrestrial-breeding frogs from the Cordillera de Colán, in northeastern Peru. We used Parsimony and Maximum Likelihood approaches to infer a molecular phylogeny on a dataset composed of 75 terminals, including three terminals representing the new species, and 4202 bp of concatenated mtDNA and nuDNA fragments. Our phylogenetic analyses support the placement of the two new species in Lynchius and Oreobates, respectively. The new species of Lynchius occurs in two localities from 1,977 to 2,006 m a.s.l., and is characterized by having a dorsum covered by conical tubercles and a brown dorsal coloration lacking a pattern of blotches on the hidden surfaces of flanks and hindlimbs. The new species of Oreobates is only known from one location at 2608 m a.s.l. and is characterized by the absence of axillary and inguinal glands, and the presence of white or cream blotches on the dark brown hidden surfaces of the body.
Introduction
The Huancabamba Depression, located in southern Ecuador and northern Peru approximately between 4°S–7°S, is a major structural and physiographic break in the Andes, and includes Abra de Porculla (2145 m a.s.l.), which is the lowest pass between Colombia and southern Chile [Citation1]. The Huancabamba Depression is formed by a complex system of relatively low ridges, basins, and valleys [Citation2] associated with a structural deflection of the Andean faults that corresponds to two major tectonic segments of the Andes [Citation3]. The complex topography of this region is associated with drainages that separate distinct north-south mountain ranges. The Río Marañón, which changes direction from north to east at Huancabamba, forms the northern boundary of the Cordillera Central. Mountains south of the Marañón are drained by rivers that flow both northward into the Marañón (Río Chiriaco and Río Utcubamba), and southward into the Río Huallaga (Río Mayo), isolating mountaintop highland areas such as the Cordillera de Colán [Citation2].
The Cordillera de Colán is a mountain ridge of moderate elevation (<3700 m a.s.l.), and its herpetofauna is poorly known. An ornithological expedition by Louisiana State University in 1978 conducted the major herpetological survey in the Cordillera de Colán and resulted in the discovery of several new species of amphibians that were later described by W. E. Duellman and colleagues (e.g. Gastrotheca abdita [Citation4], Hyloxalus spilotogaster [Citation5], Pristimantis avicuporum [Citation2], P. atrabracus [Citation2], P. serendipitus [Citation2], P. cuneirostris [Citation2], P. metabates [Citation2], and Telmatobius colanensis [Citation6]). In addition, other species independently discovered in the Cordillera de Colán were “Colostethus” poecilonotus [Citation7] and Hyloscirtus diabolus [Citation8]. However, the herpetofauna of these mountains is far from being well surveyed, and the true dimension of its amphibian biodiversity and endemicity remains unknown.
The New World terrestrial-breeding frogs are part of the superfamily Brachycephaloidea, which includes 1186 species [Citation9], representing about 33% of all New World frog species and nearly 17% of named species worldwide [Citation10]. Nevertheless, species diversity in this group constantly grows with the discovery of new species when remote regions are surveyed, museum specimens are examined, or cryptic species complexes are phylogenetically analyzed (e.g. Elmer and Cannatella [Citation11]; Ortega-Andrade and Venegas [Citation12]; Motta et al. [Citation13]; Rodríguez and Catenazzi [Citation14]; Páez and Ron [Citation15]; Santa-Cruz et al. [Citation16]).
Brachycephalid frogs show a high degree of evolutionary convergence, especially throughout the Andes [Citation17,Citation18]. Similar lifestyles related to high-Andean environments, such as grasslands and cloud forests, resulted in the convergent evolution of similar body shapes, posing a challenge for classifications based solely on morphology [Citation17]. Species that occupy Andean highlands and share the “phrynopoid” morphology (i.e. characterized by small round bodies, short extremities, and rounded, non-expanded tips of fingers and toes) were considered part of Phrynopus, until the analyses of molecular data rejected the monophyly of the genus, revealing a scenario where high-elevation lineages have independent origins [Citation17–19]. Most of the Andean Brachycephalid clades are now represented by the genera Bryophryne, Lynchius, Microkayla, Niceforonia, Noblella, Oreobates, Phrynopus, Psychrophrynella, and Qosqophryne [Citation9], most of them restricted to the Andes from southern Ecuador to Bolivia [Citation10,Citation13,Citation19–21].
We report the discovery of two new species of terrestrial-breeding frogs from Cordillera de Colán, a poorly sampled area in northern Peru. The new species resemble species of the genera Lynchius, Oreobates, and Phrynopus in their external morphology, and we use phylogenetic analyses of nuclear and mitochondrial genes to assess their phylogenetic relationships, especially regarding Andean Brachycephalid genera. We also combine morphological, molecular, and distribution data to support the recognition of the two species that we name and describe herein.
Materials and methods
Ethics and research
This study was carried out in accordance with the guidelines for the use of live amphibians and reptiles in field research [Citation22], compiled by the American Society of Ichthyologists and Herpetologists (ASIH) and the Society for the Study of Amphibians and Reptiles (SSAR). Specimens collected for this study are covered by the following research permits (given by the Ministerio de Agricultura and Servicio Nacional de Áreas Naturales Protegidas por el Estado) that include a permanent scientific collection of live specimens: 004–2019–SERNANP–JEF and 067–2019–MINAGRI–SERFOR–DGGSPFFS.
Morphology
The format for the descriptions follows Motta et al. [Citation13] for Lynchius, and Padial et al. [Citation23] for Oreobates. The terminology and definition of diagnostic characters follows Duellman and Lehr [Citation24]. Specimens were preserved in 10% formalin and stored in 70% ethanol. We deposited all specimens in the herpetological collection of Centro de Ornítología y Biodiversidad (CORBIDI), Lima, Perú. Sex was determined by the presence of vocal slits and by direct gonadal inspection. Measurements were taken with digital calipers and rounded to the nearest 0.1 mm. We measured SVL (snout–vent length), TL (tibia length), FL (foot length, distance from proximal margin of inner metatarsal tubercle to tip of Toe IV), HL (head length, obliquely from angle of jaw to tip of snout), HW (head width, at level of angle of jaw), ED (eye diameter, distance between the anterior and posterior borders of the visible eye), IOD (interorbital distance, distance between the medial edge of the orbits), EW (upper eyelid width, length of the visible eye along the outer edge of eyelid), IND (internarial distance, distance between the inner edges of nares), EN (eye–nostril distance, distance between the anterior corner of orbit and the posterior margin of nares). Fingers and toes are numbered preaxially to postaxially from I to IV and I to V, respectively. Comparative lengths of Toes III and V were determined when both were adpressed against Toe IV; lengths of Fingers I and II were compared when adpressed against each other. We obtained data for the comparison of L. megacephalus from Sánchez-Nivicela et al. [Citation25]. Specimens examined are listed in Appendix 1. Museum acronyms are those cited by Frost [Citation9], except that MHNC refers to Museo de Historia Natural de Cusco, Universidad Nacional de San Antonio Abad del Cusco, Cusco, Peru (MHNCP in Frost [Citation9]).
DNA extraction, amplification, and sequencing
We sequenced a fragment of the mitochondrial 16S rRNA gene. We extracted DNA from liver samples with a commercial extraction kit (IBI Scientific, Peosta, USA). We followed the standard protocols [Citation18] for extraction, amplification, and sequencing of DNA, using the same primers and amplification protocols of von May, Lehr and Rabosky [Citation26]. We used a Proflex thermal cycler (Applied Biosystems), purified PCR products with Exosap-IT (ThermoFisher), and shipped purified samples to MCLAB (South San Francisco, CA, USA) for sequencing.
Phylogenetic analyses
To infer the phylogenetic relationship of the new species, we supplemented our sequences with sequences available on GenBank for species of Lynchius (n = 7), Oreobates (n = 24), and Phrynopus (n = 21). We also included species of Bahius (n = 1), Barycholos (n = 2), Bryophryne (n = 1), Euparkerella (n = 1), Holoaden (n = 1), Microkayla (n = 3), Noblella (n = 3), Niceforonia (n = 6), and Psychrophrynella (n = 1). We rooted all our analyses with the distant species Haddadus binotatus. Our final sample includes 72 species, in addition to three terminals representing the two new species. We chose the mitochondrial 12S rRNA and partial sequence of 16S rRNA genes, as well as nuclear genes recombination-activating gene 1 (RAG1) and tyrosinase precursor (tyr) to perform our analyses. These gene fragments were available to most of our terminals and have been successfully used in several phylogenetic studies of the Brachycephaloidea [Citation10,Citation18]. Specimen voucher numbers for newly produced sequences and accession numbers for all sequences used in this study are listed in Table A1 (Appendix 2). The analyzed matrices, including partitions, are available in Zenodo (http://doi.org/10.5281/zenodo.4711865).
We used parsimony and maximum likelihood optimization to generate phylogenetic hypotheses. For parsimony analyses, we partitioned sequences of the genes 12S, 16S, RAG1, and Tyr into fragments of equal length separated by conserved regions with no gaps and few or no nucleotide substitutions (following Motta et al. [Citation13]). The conserved regions were identified after aligning the sequences in MAFFT v.7 (Katoh and Standley [Citation27]; under the G-INS-i strategy) and we used Geneious 11.1.2 to visualize and edit the sequences. This strategy generated putatively homologous fragments where length variation among DNA sequences was only due to insertions and/or deletions of nucleotides, a requisite for tree-alignment in POY [Citation28]. After the removal of gaps implied by MAFFT from sequence fragments, tree-alignment of unaligned sequences was performed under parsimony with equal weights for all classes of transformations using direct optimization [Citation28,Citation29] and iterative pass optimization [Citation30] algorithms in POY 5.1.1 [Citation31]. We calculated Goodman–Bremer values for each supported clade in TNT using the optimal tree-alignment matrix and the parameters specified in the bremer.run macro. We also calculated parsimony jackknife frequencies from 1000 pseudoreplicates searches using driven searches, gaps treated as fifth state, and removal probability of 0.36 (≈ e–1) [Citation32].
Multiple sequence alignments for maximum-likelihood analyses were performed in MAFFT online v7 using the G-INS-i strategy, which is considered appropriate for alignments that consist of large numbers of sequences [Citation27,Citation33]. We applied the default transition:transversion cost ratio of 1:2, but changed the gap opening penalty to 1 time substitutions to avoid penalizing insertions and deletions more than we did in the parsimony analysis. The optimal maximum likelihood trees were estimated using 100 runs plus 1000 replicates of bootstrap in RAxML 8.2.10 [Citation34] implemented in CIPRES under the GTR+G model for the combined matrix. Uncorrected p-distances were estimated in Mega v4.0 [Citation34,Citation35] for the aligned 16S fragment.
Taxonomy
We relied on the observation of morphological features and color patterns, as well as the inferred phylogenetic relationships, to justify our taxonomic conclusions. We considered the combination of these data as species delimitation criteria following a general lineage or unified species concept [Citation36,Citation37].
Results
Phylogenetic relationships
Parsimony tree searches identified an optimal tree of 10,427 steps that was visited 5425 times during 1185 rounds of build + TBR, 23,656 of fusing, and 660 of ratchet. A round of swapping under iterative pass optimization recovered a single tree of 10,383 steps and a tree-alignment of 4202 characters. The optimal MAFFT similarity-alignment used in the maximum likelihood analysis comprises 3535 character columns and the maximum likelihood score of the optimal tree was −47,278.957778.
Parsimony and maximum likelihood analyses recovered the genera Lynchius, Oreobates, and Phrynopus as monophyletic, but the relationships among these genera differed between the two analyses (). In parsimony, Oreobates and Phrynopus formed the sister group of Lynchius, while in maximum likelihood, Phrynopus was sister to Lynchius and Oreobates. Results from both analyses supported the placement of the two new species in Lynchius and Oreobates, respectively.
Figure 1. Phylogenetic trees resulting from the analysis of data sets of 4202 aligned bp (POY) and 3535 aligned bp (MAFFT) and composed of the mitochondrial genes 12S and 16S, and fragments of the nuclear protein-coding genes RAG1 and Tyr. (a) Maximum parsimony optimal tree of 10,427 transformations. Numbers above branches indicate Goodman–Bremer values, and those under branches are jackknife proportions. (b) Maximum likelihood optimal tree (log likelihood −47,278.957778) and bootstrap node values
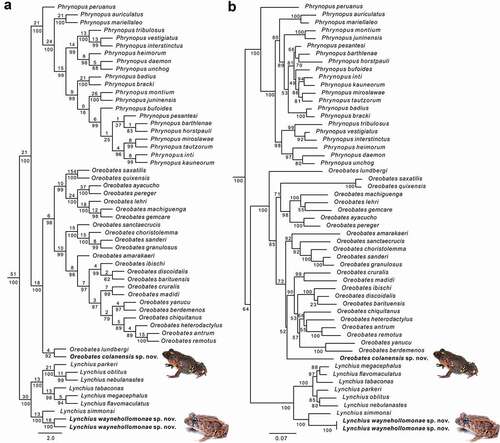
Within Lynchius, the relationships among species are similar between parsimony and maximum likelihood analyses. In both analyses, the new species of Lynchius is sister to L. simmonsi (uncorrected p-distance = 9.8% for 16S), forming a clade that is the sister group of the remaining species of Lynchius.
Within Oreobates, the two phylogenies differed mostly regarding the position of O. amarakaeri and the new species. In parsimony, the new species is sister to O. lundbergi (uncorrected p-distance = 14.6% for 16S), forming a clade that is the sister group of the remaining species of Oreobates (), while Oreobates amarakaeri is sister to a clade including O. antrum, O. barituensis, O. berdemenos, O. crepitans, O. cruralis, O. discoidalis, O. heterodactylus, O. ibischi, O. madidi, O. remotus, and O. yanucu. In maximum likelihood, the new species is sister to the clade, including O. antrum, O. barituensis, O. berdemenos, O. crepitans, O. cruralis, O. discoidalis, O. heterodactylus, O. ibischi, O. madidi, O. remotus, and O. yanucu, while O. amarakaeri is sister to a clade including O. choristolemma, O. granulosus, O. sanctaecrucis, and O. sanderi ().
The phylogenetic position and morphological distinctiveness of the newly collected specimens support the description of the two new species, which we name and diagnose below.
Lynchius waynehollomonae sp. nov.
Figure 2. Two living adult males of Lynchius waynehollomonae sp. nov. (a-c) Dorsolateral, dorsal and ventral views of holotype, CORBIDI 20765, 27.3 mm SVL. (d-f) Dorsolateral, dorsal and ventral views of the paratype, CORBIDI 20778, 24.9 mm SVL. Photographs by Axel Marcheli

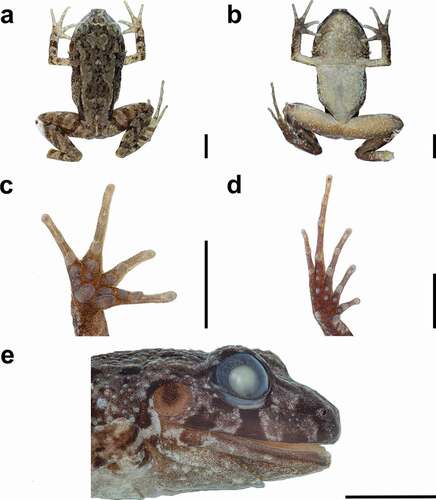
Figure 3. Preserved Lynchius waynehollomonae sp. nov. (holotype) in dorsal view (a), ventral view (b), palm (c), sole (d), and head in lateral view (e). scale 5 mm. Photographs by LAGA
Publication LSID: http://zoobank.org/39599AD9-ADC9-44F1-AF08-311A2931E00F
Taxonomic act LSID: http://zoobank.org/285994BC-891E-4F1B-89FE-2AA4B0E2B622
Holotype
CORBIDI 20765, an adult male, from Uriarte (−5.490655, −78.368660; 1981 m), Aramango district, Bagua province, Amazonas department, Peru, collected by P.J. Venegas, L.A. García-Ayachi, J.C. Chávez and A. Marchelie on 13 August 2019.
Paratypes
CORBIDI 20755, 20767, 20770, 20778, adult males, collected with the holotype; CORBIDI 22583, an adult female, from Las Higueras (−5.669842, −78.32336; 1939 m), Cajaruro district, Utcubamba province, Amazonas department, Peru, collected by J. D. Ramos-Sandoval on December 2019.
Diagnosis
A moderately large Lynchius (adult males SVL = 24.9–35.3 mm, n = 5; adult female SVL = 38.3 mm, n = 1) characterized as follows: (1) skin on dorsum of head, body, and limbs covered by conical tubercles heterogeneous in size and subconical and rounded tubercles on flanks; occipital folds absent; middorsal fold thin, complete or incomplete when present; dorsolateral fold segmented formed by connected granular warts, when is present; venter smooth to weakly areolate on the posterior third; groin with minute tubercles; discoidal fold absent and thoracic folds present; (2) tympanic membrane prominent and annulus distinct, its diameter 51–58% of eye diameter; supratympanic fold thin; postrictal tubercles present, subconical; (3) head as wide as long or slightly wider than long (HW/HL = 1.1–1.2); snout round in dorsal and lateral views; canthus rostralis weakly concave in dorsal view; loreal region concave, sloping gradually toward the lips; lips not flared; (4) cranial crests absent; upper eyelid covered by small conical tubercles; (5) dentigerous process of vomers prominent, oblique, situated posteromedial to choanae, each process bearing 5–7 teeth; (6) males with vocal slits on each side of the tongue and no nuptial pads; (7) fingers long and slender, first finger longer than second; subarticular tubercles prominent, subconical; supernumerary tubercles, prominent, round, smaller than subarticular tubercles; tips of fingers narrowly round; pads absent; circumferential grooves absent, narrow lateral fringes present, and hand webbing absent; (8) ulnar tubercle present, very low and rounded; (9) palmar tubercle prominent, slightly squarish, long as thenar tubercle and two times wider than it; thenar tubercle prominent, elliptical; (10) foot length 48–57% of SVL, narrow lateral fringes present and webbing absent; Toe III longer than Toe V; Toe III usually reaching the middle of second subarticular tubercle of Toe IV, Toe V reaching the proximal border of second subarticular tubercle of Toe IV; tips of toes narrowly rounded, lacking pads and circumferential grooves; (11) inner metatarsal tubercle ovate, prominent; outer metatarsal tubercle smaller, as the half of inner metatarsal tubercle, round, prominent; subarticular tubercles conical, prominent; supernumerary tubercles present, evident; (12) heel and tarsal tubercles present, conical and very small; (13) in life, dorsum rufous with dark brown markings; flanks brown with dark brown irregular stripes or blotches and a suprainguinal blotch; axilla, groins, and posterior surface of thighs brown without pattern; venter pale brown on the throat and chest with irregular brownish cream blotches and/or whitish irregular flecks; belly yellowish cream or dirty cream with pale brown reticulations; thighs yellowish cream or brownish cream with pale irregular flecks; shanks brown; tarsus brown with creamy brown flecks; iris black sprinkled with turquoise minute flecks and a golden ring around pupil; in preservative, dorsum grayish brown with dark brown markings and venter pale brown on throat with cream irregular flecks; belly and forelimbs cream with pale brown reticulations on belly; thighs creamy tan forward and brown back; shanks, tarsus and feet dark brown.
Comparison with other species
Among the seven known species of Lynchius, only L. megacephalus and L. simmonsi possess a dorsum with conical tubercles as L. waynehollomonae. However, L. megacephalus can be readily distinguished from L. waynehollomonae (characters in parenthesis) by having: dermal ridges on dorsum (absent), cranial crest (absent), finger I and II equal in size (finger I longer than II), palmar supernumerary tubercles low (prominent), plantar supernumerary tubercles absent (present), subarticular tubercles round (subconical), and light-blue sclera (gray sclera). Moreover, L. waynehollomonae possess dorsolateral folds, which are absent in L. megacephalus. The new species differs from L. simmonsi (characters in parenthesis) by having: conical tubercles on dorsal surface heterogenous in size (uniformly sized), palmar tubercle not bifid (bifid), suprainguinal blotch (absent), and in life, venter pale brown on the throat with irregular brownish cream blotches or whitish irregular flecks and belly yellowish cream or dirty cream with pale brown reticulations (brown with small cream flecks).
Description of holotype
Head as wide as long (HW/HL 1.1); snout rounded in dorsal view and short, rounded in lateral profile; nostrils not protuberant, oriented laterally; canthus rostralis weakly concave in dorsal view, round in frontal profile; loreal region concave; lips not flared; upper eyelid with small conical tubercles of similar size; cranial crests absent. Supratympanic fold distinct; tympanic membrane prominent and tympanic annulus distinct; tympanic membrane nearly round, its length more than half of eye length; postrictal tubercles present, subconical. Choanae not concealed by palatal shelf of the maxillary arch when roof of mouth is viewed from below; choanae small, round, separated by a distance equal to six times the diameter of choana; dentigerous process of the vomers prominent, oblique, situated posteromedial to choanae (posterior margin at level of choanae), their width approximately two times diameter of choanae, bearing five or seven vomerine teeth; vocal slits present. Skin of dorsal surfaces shagreen, sprinkled with conical tubercles on dorsum; flanks covered by subconical and conical tubercles; ventral surfaces smooth with the third posterior part weakly areolate; thighs areolate posteriorly in ventral view; occipital fold absent; middorsum folds present, thin; dorsolateral folds present, formed by connected granular warts, fragmented; discoidal fold absent; thoracic fold present. Ulnar tubercle present, small and rounded; palmar tubercle prominent, slightly squarish, two times wider than thenar tubercle; thenar tubercle prominent, elliptical; supernumerary tubercles prominent, rounded; subarticular tubercles subconical, slightly larger than supernumerary tubercles; finger tips narrowly rounded; pads and circumferential grooves absent; fingers with narrow lateral fringes and lacking webbing; nuptial pads absent; relative length of fingers: I > II < III > IV (). Foot length 50% of SVL; heel and tarsus bearing small and low tubercles, lacking folds; inner metatarsal tubercle rounded, prominent, two times larger than outer metatarsal tubercle; outer metatarsal tubercle round, prominent; supernumerary tubercles present, weakly defined proximally and prominent distally; subarticular tubercles prominent, larger than supernumerary, subconical; toes with narrow lateral fringes and lacking webbing; toe tips narrowly rounded; pads and circumferential grooves absent; relative length of toes: I < II < III > V < IV (); Toe V reaching proximal margin of second subarticular tubercle of Toe IV, and Toe III reaching to its middle.
Measurements of the holotype (in mm). SVL, 27.3; TL, 13.1; FL, 13.7; HL, 10.2; HW, 11.1; ED, 3.5; TY, 2.0; IOD, 2.8; EW 2.2; IND, 2.9; E–N, 3.2.
Color of holotype in life (). Dorsal surface rufous with brown blotches, irregular flecks and top of head brown with a brown interorbital bar with the anterior margin rufous; flanks brown with a grayish hue, dark brown irregular stripes and blotches, including a suprainguinal blotch; ventrolateral region with yellowish cream blotches; sides of head brown suffused with whitish-cream, and bearing dark brown labial bars with creamy white margins and a dark brown blotch in the loreal region; supratympanic fold dark brown posteriorly; tympanic membrane brown in the middle with a paler ring; jaw brown with whitish speckles; forelimbs and hindlimbs with dark brown transversal bars and tarsus brownish cream with dark brown bars; axilla, groins and posterior surfaces of thighs uniformly brown. Ventral surface brown on the throat and chest with irregular brownish cream blotches and irregular whitish flecks; belly yellowish cream with pale brown reticulations; thighs yellowish cream with many paler irregular flecks; shanks brown; tarsus brown with creamy brown flecks; palms and soles brown. Iris black sprinkled with turquoise minute flecks and a golden ring around pupil.
Color of holotype in ethanol 70% (). Dorsal surface grayish brown with all its markings darker than the background; limbs brownish cream with dark brown bars. Ventral surface pale brown with creamy tan irregular flecks and speckles; belly cream with pale brown reticulations; forelimbs cream with brown speckles; thighs creamy tan forward and brown back; shanks, tarsus and feet dark brown.
Intraspecific variation
The four males and one female of the type series of Lynchius possess sexual dimorphism in size (), and males possess vocal slits. Dorsal background coloration and markings are similar in all specimens, but the dorsum can be rufous or brown (CORBIDI 20778; ).
Table 1. Variation of measurements (in mm) and proportions of the type series of Lynchius waynehollomonae. See text for abbreviations
Distribution and natural history observation
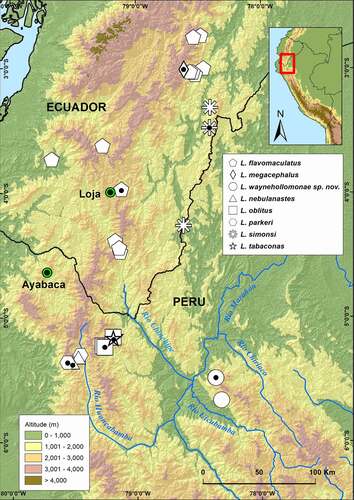
Lynchius waynehollomonae is only known from two localities in the Río Utcubamba basin, the northern extreme of Cordillera de Colán at elevations from 1,977 to 2,006 m a.s.l. (Amazonas Department, Peru) (). All specimens were collected by night in zones of abundant leaf litter on a mountain slope covered by humid montane forest (type of vegetation following Duellman and Pramuk [Citation2]) near a cattle ranching pasture. The two known localities of L. waynehollomonae lay in the Peruvian Yungas ecoregion (sensu Olson et al. [Citation38]). Other species anurans collected with L. waynehollomonae included Noblella sp., Pristimantis avicuporum, P. galdi, P. serendipitus, and Pristimantis sp.
Etymology
The specific epithet waynehollomonae is a noun in the genitive case and is a matronym for Wayne Hollomon Price (1943–2011), an American artist, licensed professional counselor and philanthropist, who was passionate about protecting the Earth’s natural environments and wildlife. During her lifetime, Hollomon Price generously supported the protection and preservation of the rainforest, the natural environment, trees and endangered species, and she founded the Hollomon Price Foundation 12 years before her death. Since then, the foundation has continued Hollomon Price’s work and legacy through its ongoing support of organizations focused on preserving people, biodiversity and ecosystems upon which all life depends, in ways that advance the lasting welfare of present and future generations. The Hollomon Price Foundation grew out of her love for the rainforest and its inhabitants, and the name waynehollomonae honors her enduring legacy as a champion of protecting the rainforest.
Oreobates colanensis sp. nov.
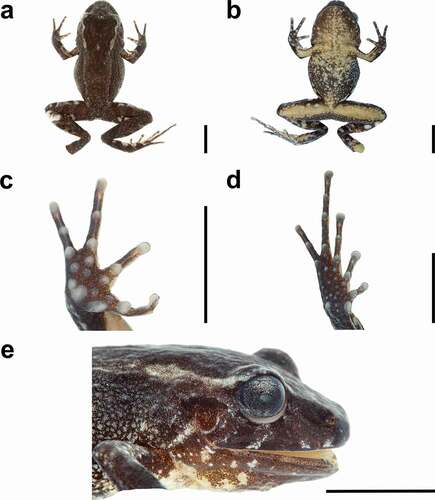
Figure 5. Two living adult males of Oreobates colanensis sp. nov. (a) Dorsolateral view, (b) lateral view showing the pattern on hidden surfaces, (c) dorsal view showing the pattern on the posterior surface of thighs, and (d) ventral view of holotype, CORBIDI 21191, 21.9 mm SVL. (e-f) Dorsolateral and ventral views of the male paratype, CORBIDI 21295, 22.5 mm SVL. Photographs by Axel Marcheli

Figure 6. Preserved Oreobates colanensis sp. nov. (holotype) in dorsal view (a), ventral view (b), palm (c), sole (d), and head in lateral view (e). Scale 5 mm. Photographs by LAGA
Publication LSID: http://zoobank.org/39599AD9-ADC9-44F1-AF08-311A2931E00F
Taxonomic act LSID: http://zoobank.org/38BED60A-FD6A-485D-82B6-C0A1107B6AAC
Holotype
CORBIDI 21191, an adult male, from Lechucita (−5.631091, −78.256952; 2608 m), Cajaruro district, Utcubamba province, Amazonas department, Peru, collected by J.R. Ormeño on 23 November 2019.
Paratypes
CORBIDI 21295–96, adult males, from Lechucita (−5.630999, −78.256935; 2611 m), Cajaruro district, Utcubamba province, Amazonas department, Peru, collected by J.R. Ormeño and S. Bullard on 2 December 2019.
Diagnosis
A small species of Oreobates (SVL of adult male 21.9–22.5 mm, n = 3; adult females SVL unknown) characterized as follows: (1) skin of dorsum shagreen with many conical turbercles, flanks with many subconical tubercles on dorsolateral region but becoming rounded toward to ventrolateral region; occipital\/-shaped fold present; dorsolateral fold present, formed by connected granular warts; venter smooth; posterior surfaces of thighs areolate, groin smooth; discoidal fold absent; postrictal tubercles present, rounded; (2) tympanic membrane and annulus distinct, large, about 36%–43% of length of eye; supratympanic fold present, ill-defined; (3) head as wide as long or slightly wider than long (HW/HL = 1.1–1.2); snout short, round in dorsal view and in lateral view; canthus rostralis concave in dorsal view, round in profile; (4) cranial crests absent; upper eyelid with scattered round tubercles; (5) dentigerous process of the vomers large, prominent, oval in shape, situated posteromedial to choanae (posterior margin at level of choanae), their width about 1.2 times diameter of choanae, bearing 4 to 7 vomerine teeth; (6) males without vocal slits and vocal sacs; (7) hands with long and slender fingers, first finger barely longer than second; subarticular tubercles large and prominent, conical; supernumerary tubercles prominent, round, smaller than subarticular tubercles; fingertips round, barely enlarged, lacking circumferential grooves; lateral fringes and keels on fingers absent; (8) ulnar tubercles absent; (9) heel and tarsus bearing very low conical tubercles; (10) inner metatarsal tubercle ovate, prominent; outer metatarsal tubercle smaller than the inner metatarsal tubercle, subconical, prominent; subarticular tubercles, conical, prominent, supernumerary tubercles smaller than subarticular tubercles, prominent, round; (11) toes long and slender (foot length 48%–49% SVL), lateral fringes and webbing absent; toe V reaching to the middle of second subarticular tubercle of Toe IV, and Toe III reaching to its distal margin; tips of toes slightly enlarged, rounded, circumferential groove absent; (12) axillary glands absent; (13) in life, dorsum dark brown or reddish brown with brown flanks, ventrolateral region grayish brown with minute white flecks; dorsal markings not contrasted, occipital fold can be reddish brown; tiny whitish labial flecks or blotches present; axilla, groin, and hidden surfaces of hindlimbs with yellowish cream or white blotches; ventrally, throat brown with a reddish patch covering the middle, chest and ventral surface of forelimbs red, belly dark brown suffused with red with many white irregular flecks and small blotches, thighs red, shanks dark brown with cream blotches; iris gold with a brown stripe on the middle. In preservative, dorsum grayish brown; occipital and dorsolateral folds paler than the background color; blotches on axilla, groin, anterior and posterior surface of thighs white; ventrally, dark brown with dirty cream blotches and white flecks.
Comparison with other species
Among the 25 species of Oreobates currently known, O. colanensis can be readily distinguished by the absence of axillary and inguinal glands, and by the presence of white or cream blotches on the dark brown hidden surfaces of body as axilla, groins, anterior and posterior surfaces of thighs, and shanks. Oreobates colanensis also possess distinct dorsolateral folds, which are only present in O. ayacucho, O. gemcare, and O. lehri. However, O. gemcare and O. lehri differ by lacking conical tubercles on dorsum, while O. ayacucho differs by having finger I shorter than II (finger I barely larger or equal in size to finger II in O. colanensis). Furthermore, O. gemcare and O. lehri lack blotches or spots in the axillary region and groins (present in O. colanensis).
Oreobates colanensis and O. lundbergi are the only species in the genus that lack vocal slits, but O. lunbergi has smooth dorsum (bearing conical tubercles in O. colanensis) and lacks dorsolateral folds (present).
Description of holotype
Head wider than long (HW/HL 1.1); snout rounded in dorsal view and short, rounded in lateral profile; nostrils not protuberant, oriented laterally; canthus rostralis concave in dorsal view, round in frontal profile; loreal region concave; lips not flared; upper eyelid with few small granules; cranial crests absent. Supratympanic fold diffuse; tympanic membrane and annulus prominent; tympanic membrane nearly round, its length less than half of eye length; postrictal tubercles present, round. Choanae not concealed by palatal shelf of the maxillary arch when roof of mouth is viewed from below; choanae small, round, separated by a distance equal to five times the diameter of choana; dentigerous process of the vomers prominent, oblique, situated posteromedial to choanae (posterior margin at level of choanae), their width approximately one and a quarter times the diameter of choanae, bearing 5 or 6 vomerine teeth. Skin on dorsum shagreen, with many low subconical tubercles, especially on the posterior half; flanks covered by small rounded tubercles; ventral surfaces smooth; thighs tuberculate posteriorly in ventral view; occipital fold\/-shaped; middorsum bearing one thin fold, slightly visible; dorsolateral folds present, evident, fragmented; discoidal fold absent; thoracic fold present, weakly defined. Ulnar tubercle absent; palmar tubercle rounded, prominent, slightly larger than thenar tubercle; thenar tubercle prominent, elliptical; supernumerary tubercles prominent; subarticular tubercles rounded, larger than supernumerary tubercles; finger tips narrowly rounded; pads absent, circumferential grooves absent; fingers lacking lateral fringes and webbing; relative length of fingers: I > II < III > IV (). Foot length 48% of SVL; heel and tarsus bearing very low conical tubercles, tarsus lacking folds; inner metatarsal tubercle oval, prominent, a bit larger than outer metatarsal tubercle; outer metatarsal tubercle round; supernumerary tubercles present, distinct; subarticular tubercles prominent, subconical; toes lacking lateral fringes and webbing; toe tips rounded, barely expanded; pads and circumferential grooves absent; relative length of toes: I < II < III > V < IV (); Toe V reaching to the middle of second subarticular tubercle of Toe IV, and Toe III reaching to its distal margin.
Measurements of the holotype (in mm): SVL, 21.9; TL, 10.8; FL, 10.6; HL, 7.6; HW, 8.4; ED, 2.6; TY, 1.1; IOD, 2.2; EW 1.8; IND, 2.5; E–N, 2.1.
Color of holotype in life ((a-d)). Dorsal surface dark brown; rostrum with a white vertical stripe in the middle; top of snout paler than dorsum; canthal stripe broad, darker than background; labials bars broad, darker than background with thin white borders; jaw dark brown with some white speckles; supratympanic fold darker than background; occipital fold reddish brown; dorsolateral folds slightly paler than background; flanks grayish brown with white flecks on the ventrolateral region; axillary region, groin, and anterior and posterior surface of thighs with cream blotches; forearm reddish brown; tibia with darker bars; tarsus brownish cream with dark brown bars; dorsal surface of feet and toes pale brown. Ventrally, throat brown with a reddish patch covering the middle, chest and ventral surface of forelimbs; belly dark brown suffused with red with many white flecks, thighs red, shanks dark brown with cream blotches, palms and soles dark brown. Iris gold with a brown stripe in the middle.
Color of holotype in ethanol 70% (). Dorsal surface grayish brown; top of head turns paler than the background (we use “turn” for referral to the change in colour due to preservation in ethanol), head markings darker than the dorsum as in life, occipital folds turn brownish cream and dorsolateral folds grayish brown; flanks dark brown, hidden surfaces brown and the blotches turn white; forearms turn brownish cream, tarsus and feet turn dirty cream with dark brown bars, and toes dirty cream. The ventral surface is dark brown, but reddish colorations turn creamy tan, flecks and blotches remain white, and shanks dark brown with white blotches.
Intraspecific variation
All specimens have similar sizes and proportions (). Coloration is identical in all specimens, but specimen CORBIDI 21295 has a reddish brown dorsum ().
Table 2. Variation of measurements (in mm) and proportions of the type series of Oreobates colanensis. See text for abbreviations
Distribution and natural history
Oreobates colanensis is only known from the Refugio Lechucita, in the eastern slope of Cordillera de Colán, in the Río Utcubamba basin at an elevation of 2,608 m a.s.l. (Amazonas Department, Peru) (). The Refugio Lechucita lays in the Peruvian Yungas ecoregion (sensu Olson et al. [Citation38]). All individuals were collected by night in the leaf litter of a slope covered by primary humid montane forest [types of vegetation following Duellman and Pramuk [Citation2]]. The other syntopic species of anurans were Noblella sp., Pristimantis corrugatus,Pristimantis sp., and Rhinella arborescandens.
Etymology
The specific epithet “colanensis” refers to the mountains where we discovered the species, the Cordillera de Colán.
Discussion
The clade containing Lynchius, Oreobates, and Phrynopus represents an interesting radiation of frogs in both their morphology and distribution. The within-genus morphological diversity also occurs across genera. For example, in their external morphology, the high Andean species O. ayacucho and O. pereger are more similar to the species of Phrynopus and Andean species of Lynchius than to their Oreobates relatives, and were initially considered to be members of Phrynopus (see Lynch [Citation39]; Cannatella [Citation40]; Lehr [Citation41]). Similarly, Lynchius simmonsi from the cloud forests on the easternmost ridges of the Amazonian Andes was initially considered to be an Oreobates, due to its similarity to species of Oreobates from montane forests and the Amazon lowlands [Citation42]. Interestingly, when we collected specimens of Lynchius waynehollomonae, we believed they represented a new species of Oreobates, since it also resembles Oreobates species from montane forests and the Amazon lowlands. Instead, the new species is sister to L. simmonsi, and this clade supports the hypothesis of convergent evolution along elevation gradients in Lynchius, Oreobates, and Phrynopus [Citation13].
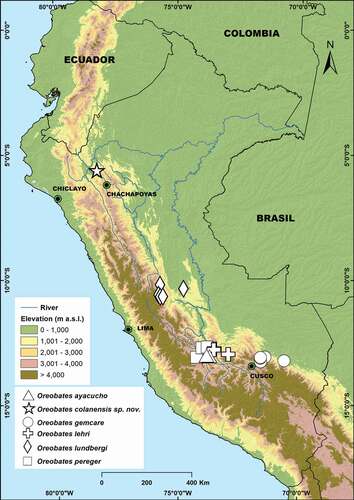
The two new species improve our understanding of the distribution of Lynchius and Oreobates in the Andes. The Huancabamba Depression has long been considered a major biogeographic barrier to the north–south dispersal of some Andean organisms [Citation43–45] and a migration corridor for others [Citation46,Citation47]. The Huancabamba Depression may have influenced the radiation of several Andean genera of lizards, such as Andinosaura, Macropholidus, Petracola, Pholidobolus, Riama, and Stenocercus [Citation43,Citation45,Citation48–53]. For amphibians, the Huancabamba Depression is the southernmost limit for the radiation of Lynchius [Citation13] and may be the northernmost limit for Phrynopus (see Chávez et al. [Citation54]; Venegas et al. [Citation21]). However, with the discovery of L. waynehollomonae, we extend the distribution of the genus to the south of Huancabamba Depression. In addition, with the discovery of O. colanensis, the Huancabamba Depression is now the northernmost limit for Andean species of Oreobates. According to Quintana et al. [Citation46], the Huancabamba area was at sea level by the Eocene, while other portions of the Andes were already uplifted, and it was not until the Middle Miocene that the Andes emerged to form the Huancabamba Depression. With most species of Lynchius restricted to the Huancabamba Depression, it is plausible that the rise of the Andes along the Huancabamba Depression influenced the evolution and diversification of this genus, probably acting as a center of origin and diversification.
The relatively low-elevation mountains at the Huancabamba Depression created a mixture of montane environments [Citation2], which terrestrial-breeding frogs such as Lynchius could easily colonize, promoting its diversification. Except for L. simmonsi, a species restricted to the Cordillera del Condor in Ecuador, the species of Lynchius reach elevations above 2000 m, where bush-like elfin forests and paramos gradually replace montane forests [Citation13]. Lynchius waynehollomonae is the first species of Lynchius found south of the Huancabamba Depression and occurring exclusively in the Peruvian Yungas (see Olson et al. [Citation38]).
Species of Oreobates are broadly distributed, and occur from the dry Atlantic forests in eastern Brazil, across the Cerrado of central Brazil and the western Amazonian lowland forests, as well as the Andean montane forests from northern Argentina to northern Peru [Citation23]. Oreobates quixensis and O. saxatilis were the only species known to occur in northern Peru, along the Amazonian lowlands and Andean hills up to 1000 m (see Padial et al. [Citation23]). Only five species of Oreobates possess an Andean distribution mostly above 2000 m (O. ayacucho, O. gemcare, O. lehri, O. lundbergi, and O. pereger), and they are restricted to a region between 10º and 14º of latitude of the Amazonian versant of the Andes in the Cordillera Oriental of Peru [Citation23,Citation24]. Oreobates colanensis represents the northernmost high-Andean species of Oreobates, extending the distribution of the genus in the high Andes by more than 600 km north ().
Our findings also improve our understanding of the morphological variation and phylogenetic relationships of Lynchius and Oreobates. Lynchius simmonsi has been considered remarkably distinct from the other species of the genus in its morphology, and represented an early divergent radiation in the genus. The recovery of Lynchius waynehollomonae as sister species to L. simmonsi corroborates that the lowland species of Lynchius indeed represent a distinct evolutionary lineage, with very different morphology and habitat from its congeners. Oreobates colanensis represents morphological and phylogenetic novelties for Oreobates. For example, Oreobates colanensis is the only species of the genus that has flash marks. Moreover, its phylogenetic relationship can also be considered an unexpected finding. The highland species of Oreobates have consistently been recovered as part of the same clade [Citation13, Citation21, Citation55–57], which represents one event of diversification [Citation57]. However, our analyses did not recover Oreobates colanensis as part of this clade and the species represents an independent lineage of highland species of Oreobates.
Our knowledge of species diversity for the genera Lynchius, Oreobates, and Phrynopus has greatly improved as a result of recent systematic researches focusing on this group [Citation13,Citation18,Citation21,Citation22,Citation25]. More than one third of the 67 species of this clade were described in the last decade [Citation9], and many more are awaiting to be named [Citation13, Citation23, Citation25]. However, the diversity of species is still underestimated, and many areas of the Andes and the adjacent lowlands suitable for the existence of species of Lynchius, Oreobates, and Phrynopus remain to be surveyed. Future surveys in other poorly sampled areas in the cordilleras of Peru will certainly reveal further new species in this clade.
Author contribution
PJV, LAGA, AC, and APM conceived and designed the study. PJV wrote the manuscript. AC, APM and LAGA reviewed and improved the manuscript. LAGA gathered morphological data. JROB and SB collected the type series and field data of Oreobates colanensis. APM made the molecular analysis and phylogenetic trees.
Acknowledgments
We are especially grateful to the Servicio Nacional de Áreas Naturales Protegidas por el Estado (SERNANP), especially with the professional personnel of the Santuario Nacional Cordillera de Colán: Christian Olivera, Jhonny D. Ramos, Gerlys Fernandez, and Abner García for its logistic support. We thank A. Marchelie for his photographs of Bosque Quemado. We also thank Axel Marchelie and Juan C. Chávez for the company and field assistance. APM thanks São Paulo Research Foundation (FAPESP) for her PhD scholarship (grant #2017/08488-3).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Peñaherrera del Aguila C. Atlas del Perú. Lima (PE): Instituto Geográfico Nacional; 1989.
- Duellman WE, Pramuk JB. Frogs of the genus Eleutherodactylus (Anura: leptodactylidae) in the Andes of Northern Peru. Nat Hist Mus, the Univ of Kans. 1999;13:1–78.
- Sillitoe RH. Tectonic segmentation of the Andes: implications for magmatism and metallogeny. Nature. 1974;250(5467):542–545.
- Duellman WE. Two new species of marsupial frogs (Anura: hylidae) from Peru. Copeia. 1987;1987(4):903–909.
- Duellman WE. Frogs of the genus Colostethus (Anura; Dendrobatidae) in the Andes of northern Peru. Nat Hist Mus, the Univ of Kans. 2004;35:1–49.
- Wiens JJ. Systematics of the Leptodactylid Frog genus Telmatobius in the Andes of northern Peru. Occas Pap Mus Nat Hist Univ Kans. 1993;162:1–76.
- Rivero JA. New Colostethus (Amphibia, Dendrobatidae) from South America. Breviora. 1991;493:1–28.
- Rivera–Correa M, Garcia–Burneo K, Grant T. A new Red-Eyed of stream treefrog of Hyloscirtus (Anura: hylidae) from Peru, with comments on the taxonomy of the genus. Zootaxa. 2016;4061(1):29–40.
- Frost DR Amphibian species of the world: An Online Reference. Version 6.1: American Museum of Natural History, New York, USA.; 2021 [ cited 2021 Apr 27]. Available from: https://amphibiansoftheworld.amnh.org/index.php
- Padial JM, Grant T, Frost DR. Molecular systematics of terraranas (Anura: brachycephaloidea) with an assessment of the effects of alignment and optimality criteria. Zootaxa. 2014;3825(1):1–132.
- Elmer KR, Cannatella DC. Three new species of leaflitter frogs from the upper Amazon forests: cryptic diversity within Pristimantis “ockendeni” (Anura: strabomantidae) in Ecuador. Zootaxa. 2008;1784(1):11–38.
- Ortega-Andrade HM, Venegas PJ. A new synonym for Pristimantis luscombei (Duellman and Mendelson 1995) and the description of a new species of Pristimantis from the upper Amazon basin (Amphibia: craugastoridae). Zootaxa. 2014;3895(1):31–57.
- Motta AP, Chaparro JC, Pombal JJP, et al. Molecular phylogenetics and taxonomy of the Andean genus Lynchius Hedges, Duellman, and Heinicke 2008 (Anura: craugastoridae). Herpetol Monogr. 2016;30(1):119–142.
- Rodríguez LO, Catenazzi A. Four new species of terrestrial-breeding frogs of the genus Phrynopus (Anura: terrarana: craugastoridae) from Río Abiseo National Park, Peru. Zootaxa. 2017;4273(3):381–406.
- Páez NB, Ron SR. Systematics of Huicundomantis, a new subgenus of Pristimantis (Anura, Strabomantidae) with extraordinary cryptic diversity and eleven new species. Zookeys. 2019;868:1–112.
- Santa-Cruz R, von May R, Catenazzi A, et al. A new species of terrestrial-breeding frog (Amphibia, Strabomantidae, Noblella) from the upper Madre de Dios watershed, Amazonian Andes and lowlands of southern Peru. Diversity. 2019;11(9):145. .
- De la Riva I. Unexpected beta-diversity radiations in highland clades of Andean Terraranae frogs. In: Rull V, Carnaval AC, editors. Neotropical Diversification: patterns and Processes. Switzerland (SW): Springer; 2020. p. 741–764.
- Hedges SB, Duellman WE, Heinicke MP. New World direct-developing frogs (Anura: terrarana): molecular phylogeny, classification, biogeography, and conservation. Zootaxa. 2008;1737(1):1–182.
- De la Riva I. Bolivian frogs of the genus Phrynopus, with the description of twelve new species (Anura: brachycephalidae). Herpetol Monogr. 2007;21(1):241–277.
- Catenazzi A, Mamani L, Lehr E, et al. A new genus of terrestrial-breeding frogs (Holoadeninae, Strabomantidae, Terrarana) from southern Peru. Diversity. 2020;12(5):184.
- Venegas PJ, Barboza AC, Riva I, et al. A new species of Phrynopus from the northeastern Andes of Peru, its phylogenetic position, and notes on the relationships of Holoadeninae (Anura: craugastoridae). Zootaxa. 2018;4446(4):501–524.
- Beaupre SJ, Jacobson ER, Lillywhite HB, et al. Guidelines for use of live amphibians and reptiles. In: The Am Soc of Ichthyol and Herpetol, editor. field and laboratory research. 2th. United States (US): The American Society of Ichthyologists and Herpetologists; 2004. 1–43.
- Padial JM, Chaparro JC, Castroviejo-Fisher S, et al. A revision of species diversity in the neotropical Genus Oreobates (Anura: strabomantidae), with the description of three new species from the Amazonian Slopes of the Andes. Am Mus Novit. 2012;3752(3752):1–55.
- Duellman WE, Lehr E. Terrestrial breeding frogs (Strabomantidae) in Peru. Münster: Natur und Tier - Verlag GmbH; 2009. 1-382
- Sanchez-Nivicela JC, Urgiles VL, Navarrete MJ, et al. A bizarre new species of Lynchius (Amphibia, Anura, Strabomantidae) from the Andes of Ecuador and first report of Lynchius parkeri in Ecuador. Zootaxa. 2019;4567(1):1–24.
- von May R, Lehr E, Rabosky DL. Evolutionary radiation of earless frogs in the Andes: molecular phylogenetics and habitat shifts in high-elevation terrestrial breeding frogs. PeerJ. 2018;6:e4313.
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780.
- Wheeler WC, Aagesen L, Arango CP, et al. Dynamic homology and phylogenetic systematics: a unified approach using POY. United States (US):Am Mus Nat Hist; 2006. 1-365.
- Wheeler W. Optimization alignment: the end of multiple sequence alignment in phylogenetics? Cladistics. 1996;12(1):1–9.
- Wheeler WC. Iterative pass optimization of sequence data. Cladistics. 2003;19(3):254–260.
- Varón A, Vinh LS, Wheeler WC. POY version 4: phylogenetic analysis using dynamic homologies. Cladistics. 2010;26(1):72–85.
- Farris JS, Albert VA, Källersjö M, et al. Parsimony jackknifing outperforms neighbor-joining. Cladistics. 1996;12(2):99–124.
- Katoh K, Kuma K-I, Toh H, et al. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucl Acids Res. 2005;33(2):511–518.
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinform. 2014;30(9):1312–1313.
- Tamura K, Dudley J, Nei M, et al. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596–1599.
- de Queiroz K. The general lineage concept of species, species criteria, and the process of speciation. In: Howard DJ, Berlocher SH, editors. Endless Forms: species and Speciation. New York: Oxford University Press; 1998. p. 57–75.
- de Queiroz K. Species concepts and species delimitation. Syst Biol. 2007;56(6):879–886.
- Olson DM, Dinerstein E, Wikramanayake ED, et al. Terrestrial ecoregions of the world: a new map of life on earth. Biosci. 2001;51(11):933–938.
- Lynch JD. A review of the Andean leptodactylid frog genus Phrynopus. Occas Pap Mus Nat Hist. 1975;35: 1–51. The Univ of Kans, Lawrence, Kans.
- Cannatella DC. Two new species of the leptodactylid frog genus Phrynopus, with comments on the phylogeny of the genus. Occas Pap Mus Nat Hist. 1984;113: 1–16. The Univ of Kans, Lawrence, Kans.
- Lehr E, DE LA RIVA I, PADIAL JM. A new species of Phrynopus from Departamento Cusco, southern Peru(Anura: brachycephalidae). Zootaxa. 2007;1618(1):61–68.
- Caramaschi U, Canedo C. Reassessment of the taxonomic status of the genera Ischnocnema Reinhardt and Lütken, 1862 and Oreobates Jiménez-de-la-Espada, 1872, with notes on the synonymy of Leiuperus verrucosus Reinhardt and Lütken, 1862 (Anura: leptodactylidae). Zootaxa. 2006;1116(1):43–54.
- Cadle JE. Systematics of lizards of the genus Stenocercus (Iguania: tropiduridae) from Northern Perú: new species and comments on relationships and distribution patterns. Proc Acad Nat Sci Phila. 1991;143:1–96.
- Duellman WE. The South American Herpetofauna: its Origin, Evolution, and Dispersal. Monogr of the Mus of Nat Hist, the Univ of Kans. 1979;7:1–485.
- Vuilleumier F. Pleistocene speciation in birds living in the high Andes. Nature. 1969;223(5211):1179–1180.
- Quintana C, Pennington RT, Ulloa CU, et al. Biogeographic barriers in the Andes: is the Amotape—Huancabamba zone a dispersal barrier for dry forest plants? 1. Ann Mo Bot Gard. 2017;102(3):542–550.
- Doan TM. A south‐to‐north biogeographic hypothesis for Andean speciation: evidence from the lizard genus Proctoporus (Reptilia, Gymnophthalmidae). J Biogeogr. 2003;30(3):361–374.
- Doan TM, Castoe TA. Phylogenetic taxonomy of the Cercosaurini (Squamata: gymnophthalmidae), with new genera for species of Neusticurus and Proctoporus. Zool J Linn Soc. 2005;143(3):405–416.
- Kizirian D, Bayefsky-Anand S, Eriksson A, et al. A new Petracola and re-description of P. ventrimaculatus (Squamata: gymnophthalmidae). Zootaxa. 2008;1700(1):53–62.
- Sánchez-Pacheco SJ, Nunes PMS, Marques-Souza S, et al. Formal recognition of the species of Oreosaurus (Reptilia, Squamata, Gymnophthalmidae) from the Sierra Nevada de Santa Marta, Colombia. ZooKeys. 2017;691:149–162.
- Torres–Carvajal O. Phylogeny and biogeography of a large radiation of Andean lizards (Iguania, Stenocercus). Zool Scr. 2007;36(4):311–326.
- Torres-Carvajal O, Mafla-Endara P. Evolutionary history of Andean Pholidobolus and Macropholidus (Squamata: gymnophthalmidae) lizards. Mol Phylogenet Evol. 2013;68(2):212–217.
- Torres-Carvajal O, Venegas PJ, Sales Nunes PM. Description and phylogeny of a New species of Andean lizard (Gymnophthalmidae: cercosaurinae) from the Huancabamba depression. South Am J of Herpetol. 2020;18(1):13–23.
- Chávez G, Santa-Cruz R, Rodríguez D, et al. Two new species of frogs of the genus Phrynopus (Anura: terrarana: craugastoridae) from the Peruvian Andes. Amphib Reptil Conserv. 2015;9(1):15–25.
- De La Riva I, Chaparro JC, Castroviejo-Fisher S, et al. Underestimated anuran radiations in the high Andes: five new species and a new genus of Holoadeninae, and their phylogenetic relationships (Anura: craugastoridae). Zool J Linn Soc. 2018;182(1):129–172.
- Pansonato A, Motta A, Cacciali P. Haddad CFB, Strüssmann C, Jansen M. On the identity of species of Oreobates (Anura: craugastoridae) from Central South America, with the description of a new species from Bolivia. J Herpetol. 2020;54(4):393–412.
- Montero-Mendieta S, De la Riva I, Irisarri I, et al. Phylogenomics and evolutionary history of Oreobates (Anura: craugastoridae) Neotropical frogs along elevational gradients. Mol Phylogenet Evol. 2021;161:107167.
Appendix 1.
Material examined
Lynchius flavomaculatus: KU 119721–24, 119737–42, 121354, 142201–02, 202654–55, 218210, 119716–20, 119725–36, 141474–77, 142198–200, 165955–67, 202656; FMNH 197838, 197846, USNM 98929–30, 195393–424, 260788, BMNH 1947.2.16.11, 1947.2.16.12–14; Lynchius nebulanastes: KU 181407, 181392–406, 181408–14, 181841, 219806–819; Lynchius oblitus: MHNC 8598, 8599, 8601, 8602, 8606, 8614, 8625, 8626, 8652, 8672, 8673, 8674, 8675, 8676, 8677, 8689, 8690, MTD 45,954; Lynchius parkeri: KU 135278, 135279–311, 181288–91, 181393, 219820, 181292–356, 181823, 181825–27, 181829, 196581–91, LSU 32172–32239; Lynchius simmonsi: KU 147068, 147069, QCAZ 30829–30, 41639–40; Lynchius tabaconas: MHNC 8637, 8649, 8650; Oreobates antrum: CFBH 34897, 39552, 39547, 39549, 39544, 39548, 39551; Oreobates ayacucho: MUSM 37858, 35548, 35552, 35549, 35553, 35551, MCZ 24362, 24363; Oreobates berdemenos: MACN 45641, 45651, 45639, 45661, 45673; Oreobates cruralis: KU 215461, 207749; Oreobates discoidalis: KU 206435, 154526, 182814, 182813, 182815, 154522, MACN 45698; Oreobates gemcare: AMNH 157013, 153087, KU 173233, 173231 MHNCP 6687, 4564, 4602; Oreobates granulosus: AMNH 6060, 6062, 6063; Oreobates heterodactylus: MNRJ 106, MZUSP 85628; Oreobates lehri: CM 158519, 158523, 158535, 158505; Oreobates lundbergi: MUSM 17822; Oreobates machiguenga: MHNCP 6809; CM 158524; Oreobates pereger: MUSM 13980, 19982, 19983; Oreobates quixensis: AMNH 84849, 84850, 103000, 102999, 22179; Oreobates remotus: MZUSP 141708, 141709–724; Oreobates sanderi: AMNH 6063, 6066; Oreobates saxatilis: KU 212327, 212556, 212330, 217352, 217327, MUSM 8431, 8432.
Appendix 2.
Geenbank accession numbers.
Table.A1. Genbank accession numbers of species sampled in this study. Sequences produced in this study are highlighted in bold font