ABSTRACT
We provide documentation of a population of feral domestic Asian water buffalo (Bubalus bubalis) that has persisted in the wetlands of the North Rupununi Region of Guyana for more than 75 years. Historical records indicate that water buffalo were incidentally introduced to the Rupununi by the colonial administration of British Guiana. Personal observations, camera trap photos, and reports of opportunistic encounters populate our maximum-entropy model, which projects a total of 2,489 km2 of suitable water buffalo habitat across the region, including 1,076 km2 of the RAMSAR-nominated North Rupununi wetlands (~11% of total land area). Key informants indicate that populations appear to have slowly increased and expanded their range over the past 30 years but fall far below projections using even the lowest population densities at ecologically analogous sites published in peer-reviewed literature. We discuss potential ecological impacts of water buffalo persistence and inhibitors of population growth by drawing on case studies from ecologically analogous locations.
Introduction
Valued for their milk, meat, hides, dung, and power as a beast of burden, Asian water buffalo (Bubalus arnee) were domesticated ~5,000 years ago [Citation1]. Domestic Asian water buffalo (Bubalus bubalis) have since experienced rapid population growth and expansion across the humid tropics [Citation2] facilitated by wide-ranging introductions [Citation3], a generalist diet that is well adapted to nutrient-poor grasses [Citation4], and high fitness (known factors that facilitate the establishment of non-native species [Citation5]).
Water buffalo are somewhat limited by a compromised ability to dissipate heat through evaporation [Citation6] and as a result they must graze open grasslands at night and spend daylight hours under the shade of the forest canopy or fully submerged in a muddy wallow or shallow pond [Citation7]. Tropical savanna-wetland habitats offer particularly suitable conditions to meet their needs, containing high-levels of anthropogenic and natural disturbances caused by cattle grazing, fire and seasonal flooding, high rainfall, abundant herbaceous vegetation, sufficient surface water for wallowing, and available uplands for resting and birthing of young [Citation2].
Neotropical savanna-wetlands depend on seasonal fluctuations in rainfall (eg, drought, fire, inundation) to provide ecological stability to a system that evolved in the absence of large herbivores – an attribute that makes them especially susceptible to non-native grazers who upset this balance [Citation8–10]. Today, the range of domestic Asian water buffalo includes records of feral and captive populations from southern Europe to Transcaucasia, North Africa, Southwest Asia, Indochina, Southeast Asia, northern Australia, Trinidad & Tobago, and South America [Citation3].
In the Neotropics, feral water buffalo are known from Argentina’s Iberá wetlands, the Peruvian and Brazilian Amazon, Bolivian chaco, Venezuelan llanos, Brazilian cerrado and Pantanal, and the coastal plains of Suriname, French Guiana, and Guyana [Citation3]. Here we focus on the re-discovery of a population of feral domestic Asian water buffalo that persists in the North Rupununi wetlands of Guyana, its potential for range expansion, and potential ecological impact on this intact savanna-wetland complex.
Asian water buffalo have a long history in Guyana, used as beasts of burden on colonial British Guiana sugar estates on the country’s coast since the 1800s [Citation7,Citation11]. As sugar operations became fully mechanized after the turn of the century, attention shifted to their potential value as a meat product [Citation7]. As a result, a small herd (~10 breeding pairs) was transported to a government station in the Rupununi (administrative Region 9) near Annai Village in the 1930s ([Citation11]).
Figure 1. Map of the North Rupununi wetlands including water buffalo observations, indigenous communities, private ranches, protected areas, and major habitat types [Citation45]
![Figure 1. Map of the North Rupununi wetlands including water buffalo observations, indigenous communities, private ranches, protected areas, and major habitat types [Citation45]](/cms/asset/6913cf63-911d-464b-b2dc-aac80b9e4b7c/tneo_a_1967661_f0001_oc.jpg)
Within 10 years of introduction, all individuals had escaped and formed free-roaming reproductive herds [Citation11]. A second introduction occurred around the same time, when a boat carrying two breeding pairs destined for Good Hope Ranch () capsized in the Rupununi River near the mouth of the Simoni River (1945 from the unpublished diary of T. McTurk; unreferenced). Water buffalo were initially protected from hunting in the Rupununi [Citation9], but protections were removed and hunting was encouraged in the 1950s [Citation7,Citation11] as growing feral herds became a menace to local people and property [Citation11]. This shift in management strategy may have ultimately pushed feral buffalo into the inhabited wetlands east of the Rupununi River where they remain today. Details of how water buffalo populations fluctuated between the late 1940s and today are unknown.
Camera trap photos presented here re-confirm the existence of a population of feral Asian water buffalo in the North Rupununi and interviews with buffalo hunters and their families provide insights into the biology, ecology, and behavior of this population. We provide a theoretical niche estimation using known locations, along with projections of suitable habitat and population density based on key biological needs, and discuss the potential ecological and management implications of the presence of this non-native ungulate in the RAMSAR-nominated North Rupununi wetlands.
Methods
Study Area
The northern portion of the Rupununi administrative district (Region 9, SW Guyana, South America) is home to a >9,400 km2 wetland complex composed of >750 lakes, ponds, and creeks, freshwater floodplains, seasonally inundated savannas, and fragments of savanna woodlands, which connect the Rio Branco and Essequibo watersheds and are bordered by large, unbroken tracts of tropical forest in the Pakaraima and Kanuku mountains, and Iwokrama forest ( [Citation12]). Tree-grass ratios indicate that the moist savannas of the North Rupununi are most analogous to the cerrado savannas of Brazil [Citation13].
With dual rainy seasons (May–September, December–January), an average rainfall of 1780 mm [Citation14], high habitat diversity, and low human population density, the Rupununi wetlands support >400 species of freshwater fish [Citation15], as well as populations of threatened species such as arapaima (Arapaima arapaima), giant river otter (Pteronura brasiliensis), giant river turtle (Podocnemis expansa), lowland tapir (Tapirus terrestris), black caiman (Melanosuchus niger), giant anteater (Myrmecophaga tridactyla), and jaguar (Panthera onca).
Camera Traps
Camera trap photos were obtained as part of a landscape-scale study of medium- and large-bodied mammals [Citation16–18] of the Rupununi Region. Bushnell Trophy Cams #119636C, #119447C, #119734C, #119736C, and #119837C (Bushnell®, KS, USA) were set 2–3 km apart at a height of 30–40 cm, using well-established methods for research on large felids [Citation19]. Between 2012 and 2020, camera-traps were set at 720 trap locations across ~52,000 km2 of the Rupununi Region, accumulating >72,000 total trap nights. Feral Asian water buffalo were documented in a cluster of 35 camera-traps spread across Karanambu Ranch and Yupukari Village lands that accumulated >17,000 trap nights. Water buffalo were photographed at a single trap location, where the camera was operational from 12 March 2013 to 19 April 2014 and 28 September 2014 to 16 March 2015 for a total of 572 trap nights.
Semi-structured Interviews
Local experts were identified through a snowball sampling approach [Citation20]. We visited villages where buffalo hunting occurs and identified individuals who have had successful hunts by word of mouth. Local experts who confirmed first-hand encounters (n = 47) were asked to provide verbal consent to share information and then were screened based on their ability to identify water buffalo by describing its physical characteristics (Appendix I) and differentiate them from species with similar morphological characteristics (Appendix II). The large, wide-set horns are considered the key attribute for differentiating this species from other bovids.
Informants who were able to accurately identify water buffalo (n = 44) were asked to provide information on when (date, season, time of day) and where (geographic or relative location) the encounter occurred and what they observed (Appendix III). Each informant was asked if they had heard of any other locations where buffalo were present and the names of potential informants. Interviews were conducted in each village until leads were exhausted. Sightings were independently verified by other parties when possible and the locations of opportunistic encounters were marked at the nearest location with local experts present using Garmin eTrex 20 handheld GPS units.
Theoretical Niche Estimation
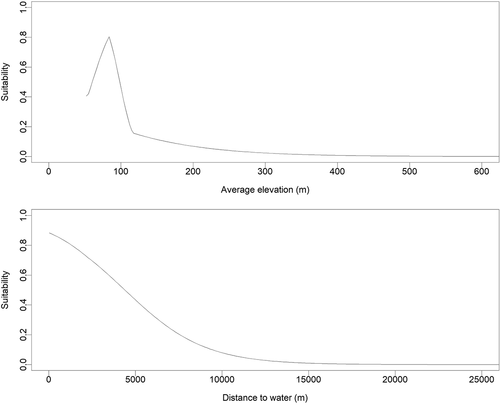
We used a maximum-entropy approach for modeling suitable habitat for B. bubalis in the Rupununi Region (Maxent version 3.3.3k [Citation21]). Occurrence locations (n = 45) were based on camera-trap detections, visual sightings, or animal tracks recorded using a GPS. Abundant permanent water sources for wallowing, nearby uplands for resting and reproduction, and abundant vegetation for grazing/browsing are necessary for their survival [Citation2,Citation7,Citation22]. We implemented these biological requirements into our model by selecting corresponding covariate layers, which included distance to water, slope, elevation, land cover type, and percent tree cover. As this species is locally harvested, we incorporated hunting pressure into our model using a distance to village layer. We held back 20% of the data for cross-validation and used default parameters in MaxEnt. We assessed model fit using the receiver operating characteristic (ROC) curve and calculating the area under the curve (AUC). The 10 percentile training presence logistic threshold [Citation23] was used to determine suitable habitat.
The total study area was calculated using a polygon fit to the extent of camera-trap surveys conducted across the Rupununi Region (). We identified 12 of the level eight hydroBASIN polygons that drain from the North Rupununi into the Rupununi River [Citation24] to delineate the North Rupununi wetlands. The range of water buffalo population densities were estimated using the highest (34 individuals/km2, Kakadu National Park, Australia [Citation5]) and lowest (3.8 individuals/km2, Ruhuna National Park, Sri Lanka [Citation25]) densities published in peer-reviewed literature from ecologically analogous sites. We expressed estimated densities as a range because water buffalo are known to exhibit higher population densities in areas considered to be “core habitat” [Citation25].
Figure 2. (top) Asian water buffalo caught on camera traps set in the North Rupununi wetlands (©Karanambu Trust/Rupununi Wildlife Research Unit); (middle left) an adult male water buffalo killed by hunters near Kwaimatta Village (©Kevin Edwards); (middle right) track of an adult Asian water buffalo in a dry creek bed near Taraqua Lake (©Gerard Pereira); (bottom left) North Rupununi wetlands during the rainy season (©Matt Hallett); (bottom right) dry season fire in the Rupununi savannas (©Matt Hallett)

Results
Asian water buffalo were documented by a total of 21 camera trap photos from two occasions at a single trap location and descriptions of 44 opportunistic encounters by local experts who had personally encountered water buffalo.
Camera-trap Photos
Camera-trap photos of Asian water buffalo were obtained on two occasions at a trap location () set east of the Rupununi River (N 3.764544°, W −59.28015°, ). This site is composed of gallery (mixed) forest set along a gently sloping creek bed that connects the Simoni River and Taraqua Lake in the rainy season (May – September), while remaining dry the rest of the year (October – April).
Two individuals (one male and one female) were photographed at 23:20 on 24 December 2013 (12 photos) and one individual (a mature male) at 19:26 on 25 January 2014 (nine photos) (). We could not determine whether the male photographed is the same individual in both sets of photos, if these individuals were part of a larger herd, or if the number of individuals photographed represents the true number of animals present at the time the photographs were taken. Camera-trap photos from across Karanambu Ranch and Yupukari village lands indicate that water buffalo co-exist with at least 38 mammal (including six non-native domestics), 34 bird, and seven reptile species, including potential competitors, predators, and variety of syntopic species (Appendix IV & V).
Habitat Preference, Behavior, and Herd Size based on Encounters
Data collected from interviews with informants (n = 44) (Appendix VI) indicate that water buffalo are most often observed along water edges (n = 25) or submerged in water (n = 19) during the dry season (n = 39). Water buffalo were most often observed in association with “Oxbow Lakes” (n = 18), compared to “Flooded Forest” (n = 11), “Flooded Savanna” (n = 7), “Savanna Ponds” (n = 5), and “Open Water in Rivers” (n = 3). Water buffalo were most often observed grazing (n = 25), followed by wallowing (n = 16), walking (n = 9), resting (n = 6), swimming (n = 5), browsing (n = 4), and running (n = 4). Buffalo are generally believed to range widely across the savanna in search of sufficient water and forage during the dry season (September–November, January–April), while congregating in patches of savanna and gallery forest situated on small hills during rainy season (May–August, December).
Herd sizes were most often reported as a range of values, with the minimum number observed used for calculations. Observed herd sizes ranged from 1 to 30 individuals, with a mean of 10.3. Using observations of large groups that accumulate near water sources in the dry season, informants indicate that the Rupununi water buffalo population likely consists of ~40–70 individuals divided among one large herd (a matriarchal herd that may split into smaller herds in the dry season), bachelor herds (composed of younger males), and rogue bulls.
Water Buffalo Hunting in the Rupununi
Feral Asian water buffalo are seasonally hunted for subsistence purposes by indigenous Makushi people from Massara, Kwaimatta, and Yakarinta villages. Hunting activity specifically targeting buffalo was described in 26% (n = 11) of opportunistic encounters in this study. Yearly harvest generally consists of one or two individuals/village, but some years are unsuccessful. Hunting most often occurs during the Rupununi’s short December rainy season (also associated with Christmas feasts) but may also occur during the longer rainy season (May–August) given optimal water levels. Buffalo are generally considered very elusive and extremely aggressive – it may take hunting parties consisting of more than 20 people over a week to locate buffalo.
Subsistence hunting is conducted using traditional bow and arrow. Informants indicate that some communities have come to depend on this non-native species to supplement their diet or support large celebrations because overharvest, conversion of gallery and savanna forest to agriculture, or forest loss due to catastrophic savanna fires has driven local declines in native fish and game species. Rupununi water buffalo also have caught the attention of sport hunters who target this species for their horns [Citation7]. Although contemporary interest in sport hunting is not widespread in Guyana, recently sport hunters hired local guides, targeted a rogue bull from the air, and took its horns as a trophy (). This seems to be an isolated incident, as the vast majority of buffalo hunting is limited to substance use by indigenous communities.
Theoretical Niche Estimation
Our top model exhibited excellent fit (AUC = 0.983) and indicated that distance to water was the most important factor determining habitat suitability followed closely by elevation (). Low-lying areas close to water were the most suitable habitats. Land cover, slope, and tree cover were of negligible importance in the models (). Distance to village was removed from the variable list as sightings were opportunistic and inherently close to villages, which introduced bias into the model. Interviews with local informants suggest that feral Asian water buffalo inhabit the wetland complex east of the Rupununi River between Yupukari and Rewa Villages (); however, opportunistic encounters are generally limited to areas easily accessed by informants.
Table 1. Variables used in the top MaxEnt model and their permutation importance
Total study area size (extent of camera traps) was 52,669 km2. The area of suitable habitat as determined by the 10 percentile training presence logistic threshold (0.164) for Rupununi water buffalo was 2,489 km2 (5% of the total study area) (). The proportion of the North Rupununi wetlands supporting suitable water buffalo habitat was 11% (1,076 km2 out of 9,483 km2). Based on the range of known densities, the Rupununi Region could support approximately 9,458–84,626 water buffalo, with 4,089–36,584 individuals in the North Rupununi wetlands alone. We used a post-hoc assessment of 717 camera-trap locations set across the study area between 2012 and 2020 that did not detect buffalo to further validate our model. We found that 93% (664 out of 717) of these cameras were not in habitat deemed suitable by the model, which suggests that our delineation of suitable habitat is accurate.
Figure 4. Map of the theoretical niche of feral Asian water buffalo in the Rupununi Region, Guyana [Citation42]
![Figure 4. Map of the theoretical niche of feral Asian water buffalo in the Rupununi Region, Guyana [Citation42]](/cms/asset/1f1350e0-a406-4266-8ff8-280a0c40ce82/tneo_a_1967661_f0004_oc.jpg)
Discussion
Data on the distribution of species are critical for effective management [Citation26]. Rupununi water buffalo have proven particularly difficult to detect using conventional research techniques due to their elusive nature and remote habitats. While camera trap photos presented here provide proof that a population of feral domestic water buffalo continues to persist in the North Rupununi wetlands, these data alone are limited when it comes to management applications.
Application of Data from Opportunistic Encounters
Concern persists in academic circles that data collected by “non-scientists” are subject to misconceptions, exaggerations, and assumptions that should preclude them from inclusion in peer-reviewed research. However, studies that properly structure data collection and rigorously vet information increasingly show that the incorporation of indigenous ecological knowledge (IEK) into the scientific process has considerable potential for improving research and natural resource management [Citation27], particularly when focused on species and their distribution [Citation28]. Additionally, in an age where communities have become the focus of conservation, research provides an opportunity to engage resource users in a shared learning process, thus increasing the potential that results are applied to local resource management. Informants who contributed to this study were vetted to ensure that their descriptions of water buffalo biology, ecology, and behavior accurately reflect what has been previously documented in the literature, allowing us to confidently apply this data to projections of the theoretical niche, suitable habitat, and population density of feral Asian water buffalo in the North Rupununi wetlands.
Potential for Population Growth and Range Expansion
When introduced under adequate environmental conditions, explosive water buffalo population growth is made possible by relatively high rates of fertility and calf survival [Citation5], as well as a propensity for living in high-density herds (up to 10 individuals/km2 [Citation8]), even when founder populations are relatively small. Buffalo populations at Kakadu National Park in northern Australia grew from a population of <100 individuals in 1826 to an estimated 340,000 in 1985 (before eradication campaigns [Citation6]). Although the Rupununi water buffalo population has remained relatively small (likely <100), we believe that it warrants attention because the tropical savanna-wetland complex found in the North Rupununi provides suitable habitat for population growth.
Theoretical niche estimates presented here show that the Rupununi Region of Guyana hosts ~2,500 km2 of suitable Neotropical wetland habitat capable of supporting herds of ~9,500–85,000 individuals. All known occurrences to date are limited to the RAMSAR-nominated North Rupununi wetlands, which our models suggest hosts >1,000 km2 of suitable habitat that may support herds of ~4,000–35,000 individuals. Suitable habitat in the North Rupununi represents >10% of an ecologically significant wetland complex that supports unique floristic diversity and provides critical habitat for a wide variety of conservation-dependent species of fish, amphibians, crocodilians, river turtles, wading birds, and semi-aquatic and terrestrial mammals. Potential ecological impacts in the Rupununi were surmised based on those seen in ecologically analogous habitats.
Potential Ecological Impacts on Native Species
Water buffalo shift between grazing and browsing in an effort to take advantage of seasonally available resources. Feeding activity has shown to decrease biomass, diversity, and cover of grassy and woody vegetation, facilitate the spread of exotic plants, increase soil erosion and siltation [Citation8], reduce infiltration capacity of soils [Citation29], cause shifts in species composition (including triggering trophic cascades [Citation30–32]), destabilize natural fire regimes [Citation33], and remove food and cover used by smaller vertebrates [Citation6].
The North Rupununi wetlands are a mosaic of savanna grassland, wetland, and tropical forest habitats that support a diverse aggregation of plant species with high degrees of endemism and floristic turnover [Citation34] that may be altered by water buffalo feeding activity. Water buffalo graze on emergent, floating, and aquatic vegetation in the dry season and browse on trees and shrubs, forage for herbs, bark, and fruits, and graze along water edges during the rainy season [Citation35]. Adult water buffalo consume 6–30 kg of rank dry matter/day [Citation22], including a greater abundance and variety of grasses [Citation29] when compared to cattle [Citation30]. Grazing reduces biomass and diversity of grasses, while browsing reduces vegetation diversity, alters species composition, and affects natural succession in gallery forests and savanna woodlands [Citation31].
Introduced water buffalo may serve as direct competitors for food resources to native herbivores, such as capybara (Hydrochoerus hydrochaeris), lowland tapir (Tapirus terrestris), and red brocket (Mazama americana) and white-tailed (Odocoileus cariacou) deers. Buffalo feeding behavior also reduces vegetative cover used by native mammals, such as giant anteater (Myrmecophaga tridactyla), crab-eating fox (Cerdocyon thous), deer, rodents (Oryzomys spp., Sigmodon alstoni), and opossums (Didelphis spp., Caluromys spp., Marmosa spp.), as well as grassland birds (Oryzoborus spp., Arundinicola leucocephala, Sturnella spp., Ammodramus humeralis). Water buffalo also trample nests and alter nesting habitat utilized by crocodilians (Melanosuchus niger, Caiman crocodilus, Paleosuchus palpebrosus) [Citation36], turtles (Podocnemis expansa, P. unifilis), and birds (Cairina moschata, Colinus cristatus, Riparia spp., Tinamus spp., Crypturellus spp.) [Citation6], as well as grooming, basking, and latrine sites used by giant river and neotropical (Lontra longicaudis) otters. Growing domestic water buffalo populations are known reservoirs for disease (i.e. Mycobacterium bovis and Trypanosoma vivax) that may affect both livestock and wildlife [Citation37,Citation38].
Wallowing and regular movement between grazing and resting sites alters the hydrology [Citation39] and chemistry [Citation6] of freshwater wetlands. The succession of wallows, channels, and gullies creates worn paths lined with compacted soils, facilitating erosion and runoff, and contaminating adjacent waterways with suspended sediments and nutrients (ie, nitrogen, phosphorus) [Citation6]. Reduced freshwater retention and interruptions in the flow of seasonal floodwaters may reduce spawning habitat, reproductive success and, ultimately, populations of a wide variety of seasonally migratory fish species (Arapaima arapaima, Hydrolycus scomberoides, Osteoglossum bicirrhosum, Cichla ocellaris, Pseudoplatystoma fasciatum). Interrupting the hydrology of this seasonally flooded savanna-wetland would undermine the ecological integrity of this intact system, while also jeopardizing a primary source of protein for many riverine communities.
Management Implications
Despite seemingly sufficient suitable habitat and time, Rupununi water buffalo have not exhibited the type of explosive population growth observed elsewhere. While it is possible that additional individuals inhabit parts of the Rupununi Region that are not frequented by subsistence hunters, it does not seem feasible considering the sampling effort of camera–trap studies, the frequency and breadth of subsistence hunting activities, and the conspicuous nature of water buffalo sign. Targeted sampling in future camera–trap studies, however, may increase detection rate and provide a more accurate projection of space use, as data presented here originate from surveys designed for jaguars and their prey. The majority of camera locations (93%) fell outside of suitable water buffalo habitat and the 53 camera-traps that did not detect buffalo within suitable habitat may not have been set at optimal height, proximity to water, or season for surveying water buffalo.
However, let us consider an ecological explanation for the current status of water buffalo populations in the North Rupununi wetlands. The water edges and shallow flooded grasslands that water buffalo prefer contain a relatively high diversity and biomass of herbaceous species, but cover only a small proportion (11%) of the North Rupununi wetlands. Poor soils (highly weathered, acidic, nutrient deficient [Citation40]), vegetation communities dominated by fibrous, nutrient poor perennial bunch grasses (Trachypogon sp., Andropogon sp.) [Citation41], extreme seasonal variation (drought, fire, inundation), and high evapotranspiration (high temperature, low humidity, strong winds) across most of the Rupununi savannas may reduce potential dispersal ability and carrying capacity. A stocking density of one animal unit (AU) per 17–25 ha has been suggested for the free-roaming cattle [Citation42], which would result in a maximum of 32,000–47,059 individuals. Stocking rates for water buffalo would most likely be even lower because of their need to remain close to water and their tendency to graze or browse more intensively, likely on the lower end of the range of densities projected within the suitable habitat using our top model.
Another key question remaining is whether this population is capable of explosive growth, considering their potential genetic limitations. Previous studies have documented genetic bottlenecks in portions of the water buffalo population introduced to Australia [Citation43,Citation44] with larger founder populations than are known for the Rupununi. The reduction in genetic variation associated with bottlenecks in a small population can reduce reproductive success, survival, and ultimately population growth. Further research into the genetic structure of Rupununi water buffalo would help managers understand the status of a population that may still be in recovery.
History indicates that invasive species management tends to be reactionary, with action taken only after populations have reached, or are near to reaching, their biological potential. Water buffalo eradication campaigns in Northern Australia that included focused aerial gunning were effective in dramatically reducing water buffalo populations but cost the government >$450 million USD [Citation31]. Key informants indicate that the total population of feral Asian water buffalo in the North Rupununi wetlands is <100 individuals, making eradication feasible and cost-effective at this point if research into their genetic structure suggests that they may be capable of dramatic population increases in the future.
We recommend the development of a Rupununi water buffalo management plan while populations, costs, and ecological impacts remain low. If further research indicates that the population size is larger than previously thought, or that the genetic structure of this population could support a dramatic increase under the right conditions, steps should be taken to control or eradicate water buffalo before it is too late or costly to do so. Regardless of the status of water buffalo, management actions that address observed local declines in native fish and game species reported by key informants are needed to reduce dependence on this introduced species, while improving the livelihoods of subsistence hunters in our study area.
Author Contributions
MH determined study design, designed data collection tools, co-facilitated implementation of camera-trap data collection, processed and maintained camera-trap and interview databases, informed statistical analysis, worked with the team to interpret results, and served as primary author and editor of manuscript. GP informed study design, co-facilitated implementation of camera-trap data collection, primary facilitator of semi-structured interviews, worked with the team to interpret results, and provided feedback to the draft manuscript. OA contributed to design and implementation of camera-trap data collection and reviewed the draft manuscript. DM informed design and implementation of the camera-trap survey and interviews and provided historical documents, insights, and context. BB contributed to design and implementation of camera-trap data analysis, running Maxent models, wrote portions of the methods and results sections pertaining to these models, produced of figures, worked with the team to interpret results, and contributed to writing and editing of the manuscript.
Geolocation Information
Karanambu Ranch (N 3.750063°, W −59.309421°)
Supplemental Material
Download MS Word (887.5 KB)Acknowledgments
The authors would like to thank the participants in this study, especially Kenneth, Cecil, Beverly and Manuel Mandook, Fitzroy, Nadia, Rickford and Rawlings Batholomew, Tichie Roberts, Mary Ambrose, Simpson Moses, Valentine and Charles Roberts, Micah Davis, Melanie and Eddie McTurk, Mike Williams, Andrew Jackson, Lakeram Haynes, Cain Edwards, Tommy Kenyon, Alex Mendes, and Ashley Holland. We appreciate the permission to carry out this study provided by the Environmental Protection Agency Guyana, Ministry of Indigenous People’s Affairs, North Rupununi District Development Board, and Kanuku Mountains Community Representative Group. We are very grateful to John Blake and June Hardy for providing guidance along the way, as well as Karanambu Trust and Lodge, and the leadership and residents Yupukari, Quattata, Markanata, Kwaimatta, Massara, Yakarinta, Kwatamang, Annai, and Rewa villages for their overwhelming kindness, hospitality, generosity, cooperation, and support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Roth J Bubalus bubalis [Internet]. Animal Diversity Web. 2004 [cited 2017 May 1]. Available from: http://animaldiversity.org/accounts/Bubalus_bubalis/.
- Werner PA. The rise and fall of the Asian water buffalo in the monsoonal tropics of northern Australia. In: Prins HHT, Gordon IJ, editors. Invasion Biology and Ecological Theory: insights from a Continent in Transformation. United Kingdom: Cambridge University Press; 2014. p. 452–496.
- Hoffpuir R. Water Buffalo. In: editor, Kiple KF. The Cambridge world history of food. Vol. 2. United Kingdom: Cambridge University Press; 2000.p. 583-606.
- Bowman DMJS, Murphy B, McMahon CR. Using carbon isotope analysis of the diet of two introduced Australian megaherbivores to understand Pleistocene megafaunal extinctions. J Biogeograph. 2010;37:499–505.
- McMahon CR, Brook BW, Bowman DMJS, et al. Fertility partially drives the relative success of two introduced bovines (Bubalus bubalis and Bos javanicus) in the Australian tropics. Wildl Res. 2011;338:386–395.
- Skeat AJ, East TJ, Corbett LK. Impact of feral water buffalo. In: Finlayson CM, von Oertzen I, editors. Landscape and Vegetation Ecology of the Kakadu Region, Northern Australia. The Netherlands: Kluvier Academic Publishers; 1996. p. 155–177.
- Brock SE. Hunting in the Wilderness – big Game Hunting North of the Amazon. London (UK): Robert Hale Ltd; 1963.
- Petty AM, Werner PA, Lehmann CER, et al. Savanna responses to feral buffalo in Kakadu National Park, Australia. Ecol Monogr. 2007;77:441–463. Available from.
- Sankaran M, Ratnam J, Hanan NP. Tree-grass coexistence in savannas revisited—insights from an examination of assumptions and mechanisms invoked in existing models. Ecol Lett. 2004;7:480–490.
- Gardner TA. Tree–grass coexistence in the Brazilian cerrado: demographic consequences of environmental instability. J Biogeograph. 2006;33:448–463.
- Baldwin R Wildlife and Me, Timehri Magazine. The Journal of the Royal Agricultural and Commercial Society of British Guiana. 1960;39:76–77.
- Mistry J, Roopsind M The North Rupununi Adaptive Management Process (NRAMP) Darwin Initiative Guyana Partnership. 2004 [cited 2018 January 15]. Available from: https://repository.royalholloway.ac.uk/file/10bd80ea-3090-dba8-9013-eea3bab0004d/7/NRAMP_2008_Full_Version.pdf
- Eden MJ, McGregor DFM. Dynamics of the forest-savanna boundary in the Rio Branco-Rupununi region of northern Amazonia. In: Furley PA, Proctor J, Ratter JA, editors. Nature and dynamics of forest-savanna boundaries. London (UK): Chapman and Hall; 1992. p. 77–88.
- Hawkes MD, Wall JRD. Site Resource Survey. The Commonwealth and Government of Guyana Rain Forest Programme, Phase I, Chatham (UK): Main Report Natural Resources Institute; 1993.
- de Souza LS, Armbruster JW, Werneke DC. The influence of the Rupununi portal on distribution of freshwater fish in the Rupununi district, Guyana. Cybium. 2012;36(1):31–43.
- Hallett MT Landscape-scale research as a tool for engaging communities in a shared learning process for conservation and management in the Rupununi, Guyana. Doctoral dissertation, University of Florida, Gainesville, Fl, USA; 2017 [cited 2018 June 26]. Available from: https://ufdcimages.uflib.ufl.edu/UF/E0/05/18/39/00001/HALLETT_M.pdf
- Hallett MT, Kinahan AA, McGregor R, et al. Impact of low-intensity hunting on game species in and around the Kanuku Mountains Protected Area, Guyana. Frontiers in Ecology and Evolution. 2019;7:412.
- Hallett MT, Guyana Large SWM Mammal Monitoring: final Implementation Report – year 2. Sustainable Wildlife Management Programme. Georgetown, Guyana; 2020.
- Silver S. Assessing jaguar abundance using remotely triggered cameras. New York (USA): Wildlife Conservation Society; 2004.
- Biernacki P, Waldorf D. Snowball sampling: problems and techniques of chain referral sampling. Sociol Methods Res. 1981;10(2):141–163.
- Phillips SJ, Dudík M, Schapire RE Maxent software for modeling species niches and distributions (Version 3.3.3k); 2016 [cited 2020 November 10]. Available from: http://biodiversityinformatics.amnh.org/open_source/maxent/.
- Considine ML. Buffalo in the Top End. Ecos. 1985;44:3–12.
- Peterson AT, Soberón J, Pearson RG, et al. Ecological Niches and Geographic Distributions (MPB-49). Vol. 56. New Jersey (USA): Princeton University Press; 2011.
- Lehner B, Grill G. Global river hydrography and network routing: baseline data and new approaches to study the world’s large river systems. Hydrological Process. 2013;27(15):2171–2186. Available from: www.hydrosheds.org
- de Silva M, Dissanayake S, Santiapillai C. Aspects of the population dynamics of the wild Asiatic water buffalo (Bubalus bubalis) in the Ruhuna National Park, Sri Lanka. Journal of South Asian Natural History. 1994;1:65–76.
- Pearce JL, Boyce MS. Modelling distribution and abundance with presence‐only data. J Appl Ecol. 2006;43(3):405–412.
- Baird IG, Flaherty MS, Baird G. Mekong River fish conservation zones in Southern Laos: assessing effectiveness using local ecological knowledge. Environ Manage. 2005;36(3):439–454.
- Moller H, Berkes F, O’Brian Lyver P, et al. Traditional Ecological Knowledge: monitoring Populations for Co-Management. Ecol Soc. 2004;9(3):2.
- Braithwaite RW, Dudzinski ML, Ridpath MG, et al. The impact of water buffalo on the monsoon forest ecosystem in Kakadu National Park. Australian Journal of Ecology. 1984;9(4):309–322.
- Ohly JJ, Hund M. Floodplain Animal Husbandry in Central Amazonia. In: Junk, WJ, Ohly, JJ, Piedade, MTF, Soares, MGM, editors. The Central Amazon Floodplain: actual Use and Options for a Sustainable Management. The Netherlands: Backhuys Publishers. 2000. p. 313–343.
- Sheikh PA, Merry FD, McGrath DG. Water buffalo and cattle ranching in the Lower Amazon Basin: comparisons and conflicts. Agric Syst. 2006;87(3):313–330.
- Michels GH, Vieira EM, de Sà FN. Short- and long-term impacts of an introduced large herbivore (Buffalo, Bubalus bubalis L.) on a neotropical seasonal forest. European Journal of Forest Resources. 2011;131(4):965–976.
- Werner PA. Impact of feral water buffalo and fire on growth and survival of mature savanna trees: an experimental field study in Kakadu National Park, northern Australia. Australian Ecology. 2005;30(6):625–647.
- Silva JMC, Bates JM, Patterns B. Conservation in the South American Cerrado: a Tropical Savanna Hotspot. BioScience. 2002;52(3):225–234.
- Tulloch DG. Seasonal movements and distribution of the sexes in the water buffalo, Bubalus babalis, in the northern territory. Aust J Zool. 1970;18:399–414.
- Campos Z. Effect of habitat on survival of eggs and sex ratio of hatchlings of Caiman crocodilus yacare in the Pantanal, Brazil. J Herpetol. 1993;27(2):127–132.
- Hein WR, Tomasovic AA. An abattoir survey of tuberculosis in feral buffaloes. Aust Vet J. 1981;57(12):543–547.
- Camus E. History of Trypanosoma vivax in the New World. In: Vokaty S, Desquesnes M, editors Proceedings of the first symposium on New World Trypanosomes.Bridgetown, Barbados: Inter-American Institute for Cooperation on Agriculture Technical Cooperation Agency in Barbados; 1996. p. 1-4.
- Wharton CH An ecological study of the kouprey, Novibos sauveli (Urbain). Monographs of the Institute of Science and Technology, Manila, the Philippines. 1957;5:1–107.
- Eden MJ. Savanna vegetation in the Northern Rupununi, Guyana. Journal of Tropical Geography. 1970;30:17–29.
- Duthie DW. Mineral deficiency and cattle raising in British Guiana. Agricultural Journal of British Guiana. 1939;10:194–204.
- McCorkle JS. Range and Livestock in Tropical Savannahs of British Guiana. Journal of Range Management. 1952;5(4):259–265.
- Barker JSF, Tan SG, Selvaraj OS, et al. Genetic variation within and relationships among populations of Asian water buffalo (Bubalus bubalis). Anim Genet. 1997;28:1–13.
- Hoogensteijn R, Hoogensteijn A. Conflicts between cattle ranching and large predators in Venezuela: could use of water buffalo facilitate felid conservation? Oryx. 2008;42(1):132–138.
- ESRI. ArcGIS Desktop: release 10.4.1. Redlands, CA (USA): environmental Systems Research Institute; 2016.