ABSTRACT
Notes on Eurysthea Thomson, 1861, Smodicum Haldeman, 1847, Smodicum confusum Martins, 1985, and Smodicum clancularium Martins, 1975 are provided. Eurysthea vandenberghei sp. nov. is described from Nicaragua. Enaphalodes antonkozlovi Lingafelter & Santos-Silva, 2018 is recorded for the first time from Panama, and chromatic variations in this species are reported. Smodicum parandroides Bates, 1884 is recorded for the first time from Nicaragua and three Mexican states. Eupogonius sonorensis Wappes & Santos-Silva, 2020 is recorded for the first time from two Mexican states.
Introduction
Cerambycidae is among the largest and best-studied families of Coleoptera. Currently, it includes over 38,000 known species [Citation1]. Of these, 11,636 species occur on the American continent [Citation1]. Cerambycinae and Lamiinae are the greatest subfamilies with about ca. 11,000 and over 20,000 species, respectively [Citation1]. Despite the large number of species already known from the America, new species are still routinely described. As usual, some of those new species are somewhat similar to species already known and, not infrequently, are not easily recognized by researchers without experience on the group. However, some others are so different from all known species that they can be easily recognized as new species. Often, it is not an easy task to recognize the genus, and, sometimes, it is not possible to include them in any known genus. It is true that many of these new species are small or medium in size, but some large or relatively large species have been described.
The main aim of this work is to describe a new species found during the process of identifying part of the specimens in the second author’s collection.
By the first author: in the past two decades, we have lost some of the most active researchers of American Cerambycidae, in some cases due to death (Edmund F. Giesbert, John A. Chemsak, Frank T. Hovore, James E. Wappes from the USA; Renato Contin Marinoni, Ubirajara R. Martins de Souza, Dilma Solange Napp, from Brazil; Osvaldo R. Di Iorio, from Argentina), in others due to retirement. Despite the irreparable losses, a new generation has emerged as a hope for the study of the taxonomy and systematic of American Cerambycidae. I place all my hope in these new researchers. Unfortunately, most (probably all) did not have the pleasure of personally meeting our great bulwarks in the study of American Cerambycidae or, at most, only knew one or two of them. Even so, these new researchers have shown enormous willingness and love for the study of the family. I know that many editors and researchers do not approve of this type of manifestation in scientific works, but this paragraph is intended to encourage them to never give up. I well know that all these people I am talking about are well aware of my yearnings about them because I have told them all of that. However, I would like to keep this on record so that they will always remember even when I am no longer here. Ubirajara Ribeiro Martins de Souza always said to me: “taxonomists are an endangered species.” I hope he was wrong.
Material and methods
Photographs were taken in the MZSP with a Canon EOS Rebel T3i DSLR camera, Canon MP-E 65 mm f/2.8 1–5X macro lens, controlled by Zerene Stacker AutoMontage software. Measurements were taken in “mm” using measuring ocular Hensoldt/Wetzlar – Mess 10 in the Leica MZ6 stereomicroscope, also used in the study of the specimens.
The acronyms used in the text are as follows:
ACMT – American Coleoptera Museum (James Wappes), San Antonio, Texas, USA (currently, this collection is waiting for transference for another institution in the USA due to the death of James Wappes)
DHCO – Daniel Heffern Collection, Houston, Texas, USA
JMMC – Jean-Michel Maes Collection, León, Nicaragua
MZSP – Museu de Zoologia, Universidade de São Paulo, São Paulo, Brazil
TAMU – Texas A&M University, College Station, Texas, USA
The specimens were identified by the authors by consulting the original descriptions and by comparison with the specimens of the MZSP Cerambycidae collection.
Results
CERAMBYCINAE Latreille, 1802
ELAPHIDIINI Thomson, 1864
Notes on Eurysthea Thomson, 1861
Eurysthea Thomson, 1861 is a polymorphic genus but, apparently, it is not possible to divide it. Paramallocera Aurivillius, 1912, is currently regarded as a synonym. According to Fonseca-Gessner [Citation2] (translated): “Eurysthea Thomson, 1860 [sic, 1861] is the genus that comes closest to Paramallocera. Eurysthea, is represented by E. obliqua Thomson, 1860 [sic, 1861], which can, at first glance, be confused with P. nicolai (Aurivillius, 1908 [sic, 1909]) or with P. hirta (Kirby, 1818), since it presents the same pattern of color and pilosity. It is immediately distinguished by the absence of spines in the antennae.” Lingafelter [Citation3] reported on Eurysthea: “Eurysthea resembles greatly Paramallocera with respect to elytral maculations and sparse pubescence with long flying hairs. It differs in lacking antennal spines (present in Paramallocera), having tibial carinae (absent in Paramallocera) and clavate femora (gradually enlarged in Paramallocera)”; and on Paramallocera: “The acute lateral pronotal tubercles in most specimens [sic, species] and the pale patterns of maculations of the elytra, covered with long, erect hairs, make Paramallocera a distinctive taxon. It is similar to Eurysthea, but Eurysthea lacks the pronotal tubercles [sic, lateral tubercles of the prothorax] and mesal antennal spines. Eurysthea also differs in having tibial carina (absent in Paramallocera) and clavate femora (gradually enlarged in Paramallocera).” Martins [Citation4] synonymized Paramallocera with Eurysthea based on the extreme variability of the species: basal antennomeres with or without dorsal sulcus; spines on the basal antennomeres present or absent and, when present, with variable number and length (sometimes males with more antennomeres spined than in females); lateral tubercles of the prothorax present or absent; squamiform elytral setae present or absent; elytral pubescence variable; number of erect setae on elytra variable; femora pedunculate-clavate, with intermediate shape (femoral clubs not strongly widened), or meso- and metafemora sublinear. We believe that Martins [Citation4] was correct when reporting that the tibial carina mentioned by Lingafelter [Citation3] in species lacking a spine on the apex of the basal antennomeres may or may not be present, and species with a spine on the antennomeres may or may not have lateral tubercle on prothorax.
On some species of Eurysthea
According to Martins [Citation4], E. cribripennis Bates, 1885 only has white, hard and sparse setae on the elytra. Fonseca-Gessner [Citation2] affirmed that the elytra have whitish and decumbent setae uniformly distributed, and long and oblique setae interspersed. The information by Martins [Citation4] suggests that there is only one type of setae. However, the information by Fonseca-Gessner [Citation2] is the accurate one, as it is possible to see in the photograph of the lectotype, see [Citation5].
Martins [Citation4] reported that the elytra in E. ilinizae (Kirsch, 1889) () have sparse white setae, not interspersed by long setae. However, according to Fonseca-Gessner [Citation2], the elytra have yellowish-white decumbent setae, and longer setae interspersed on the apex. In fact, there are long and sparse erect setae on the anterior half, and decumbent setae with erect setae interspersed on the posterior half.
Figure 1. (a) Eurysthea ilinizae (Kirsch, 1889), female, dorsal habitus. (b) Eurysthea koepckei (Franz, 1956), female, dorsal habitus. (c) Enaphalodes antonkozlovi Lingafelter & Santos-Silva, 2018, female, dorsal habitus

According to Martins [Citation4], the elytra in E. koepckei (Franz, 1956) () have short, sparse setae. According to Fonseca-Gessner [Citation2], the elytra of this species have whitish, hard, short and decumbent setae, and long and erect setae interspersed. The information by Fonseca-Gessner [Citation2] is the correct one.
Martins [Citation6] pointed out that E. magnifica Martins, 1985 has elytral setae short, not very abundant. According to Martins [Citation4], the setae are short, erect and sparse. However, the holotype () has both, minute and long erect setae inserted in punctures (short setae absent toward apex).
Figure 2. (a–e) Eurysthea vandenberghei, holotype female: (a) Dorsal habitus; (b) Ventral habitus; (c) Lateral habitus; (d) Head, frontal view; (e) Antennomeres III–V. (f–g) Eurysthea sordida (Erichson, 1847), lateral habitus: (f) Female; (g) Male. (h) Eurysthea martinsi (Fonseca-Gessner, 1990), holotype female, dorsal habitus. (i) Eurysthea magnifica Martins, 1985, holotype male, dorsal habitus
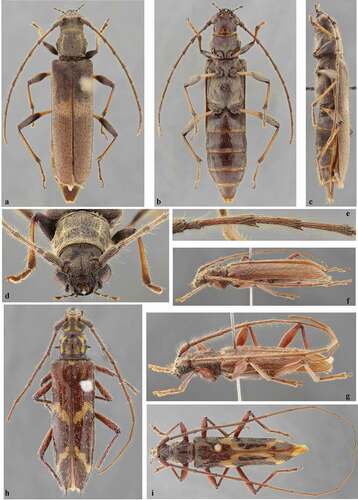
According to Martins [Citation4], E. martinsi (Fonseca-Gessner, 1990) has the elytral setae short and white. However, according to Fonseca-Gessner [Citation2], the elytra have decumbent whitish setae, and long and erect setae interspersed, more abundant on the apex. In fact, the erect setae are distinct throughout in the holotype (), and there are minute setae inserted in part of the punctures on the anterior half.
According to Fonseca-Gessner [Citation2], the elytra in E. sordida (Erichson, 1847) have two types of yellowish setae uniformly distributed: decumbent, very short and thin, and setae slightly longer and inclined. According to Martins [Citation4], the elytra have two types of setae: in male, with short and abundant setae, and long setae interspersed, more abundant on posterior half; in female, the setae are more abundant. This information by Martins [Citation4] may lead to an incorrect interpretation. In fact, both males () and females () have decumbent setae with erect setae interspersed on the elytra, although the decumbent setae are often more abundant in females than in males.
Eurysthea vandenberghei sp. nov.()
Description
Holotype female
Integument mostly dark brown, almost black on some areas; mouthparts mostly reddish brown with irregular dark brown areas, except palpomeres dark brown with apex reddish brown; anteclypeus and labrum dark brown anteriorly, reddish brown posteriorly; antennae gradually lighter toward apex. Elytra with large, transverse orangish-brown macula dorsally just before middle (anterior margin slightly projected forward along suture); posterior half slightly lighter than basal area, except dark margins. Femoral peduncles orangish brown. Tibiae brown on narrow basal area, orangish-brown toward dark brown apex. Apex of tarsomere V dark reddish brown. Apex of abdominal ventrites I–IV reddish brown.
Head
Frons moderately depressed on each side of median groove, except triangular area close to clypeus; sides forming wide carina from antennal tubercles to clypeus; coarsely, confluently punctate (general appearance rugose), except obliquely striated triangular central area close to clypeus; with abundant yellowish-brown pubescence not obscuring integument, slightly shorter laterally, except glabrous triangular area close to clypeus. Area between antennal tubercles and beginning of upper eye lobes finely, densely punctate, except smooth median groove; with abundant yellowish-brown pubescence not obscuring integument. Remaining surface of vertex distinctly lower than area between antennal tubercles; finely, densely punctate, except glabrous median groove (median groove widened between upper eye lobes); with short, abundant yellowish-brown pubescence not obscuring integument, except glabrous median groove. Area behind upper eye lobes finely, densely punctate; with moderately abundant yellowish-brown pubescence not obscuring integument, slightly denser close to eye, and a few long, erect yellowish-white setae close to eye. Area behind lower eye lobes almost smooth close to eye; remaining surface finely, moderately abundantly punctate close to upper eye lobe, longitudinally striate-punctate toward ventral surface; with short, suberect, moderately sparse yellowish-brown setae close to eye, sparse yellowish-brown pubescence on punctate area, glabrous on striate-punctate area; with a few long, erect yellowish-white setae close to eye toward inferior area. Median groove distinct from clypeus to prothoracic margin. Antennal tubercles with sculpturing as on frons, except smooth apex; with yellowish-brown pubescence not obscuring integument, except glabrous apex. Genae finely, abundantly punctate on posterior half close to eye, smooth on anterior half; with yellowish-brown pubescence not obscuring integument on punctate area, glabrous on smooth area. Gulamentum smooth, glabrous on center of posterior half, transversely striate, with sparse yellowish-brown pubescence on sides of posterior 2/3 and central area close to smooth posterior half; anterior area depressed, opaque, with minute, sparse tubercles, and both, short and long yellowish setae, distinctly sparser centrally. Wide central area of postclypeus moderately concave; finely, abundantly punctate, punctures coarser, sparser centrally; wide central area with yellowish-brown pubescence not obscuring integument, denser, with long, erect setae of same color interspersed laterally; sides smooth, glabrous. Labrum coplanar with anteclypeus at posterior half, inclined at anterior half; sides of posterior half with small, minutely, densely punctate depression, each one with erect, both short and long yellowish-brown setae; center of anterior half with transverse band of long brownish setae directed forward. Distance between upper eye lobes 0.83 times length of scape (0.36 times distance between outer margins of eyes); in frontal view, distance between lower eye lobes 1.25 times length of scape (0.54 times distance between outer margins of eyes). Antennae 1.15 times elytral length, reaching posterior seventh of elytra; with yellowish-brown pubescence partially obscuring integument; with long, erect yellowish setae ventrally on scape, pedicel, and antennomeres III–VII (setae shorter and sparser toward VII); dorsal surface of scape, pedicel and apex of antennomeres III–X with long, erect, sparse yellowish setae (setae shorter toward X); inner apex of antennomeres III–VI with spine (spine distinctly short in V and VI); spine of antennomeres III–IV shorter than diameter of apex of the antennomere. Antennal formula (ratio) based on length of antennomere III (without spine): scape = 0.70; pedicel = 0.23; IV = 0.86; V = 0.94; VI = 0.94; VII = 0.90; VIII = 0.76; IX = 0.69; X = 0.57; XI = 0.55.
Thorax
Prothorax wider than long, sides moderately tuberculate centrally, divergent from anterolateral tubercles to middle, convergent toward posterolateral angles. Pronotum with median longitudinal carina from anterior fifth to posterior fifth (carina more distinct from apex of anterior third); with large, almost M-shaped carina on posterior 2/3; coarsely, moderately abundantly punctate; with abundant yellowish-brown pubescence not obscuring integument, denser on lateral “arms” of M-shaped carina, slightly dense on anterior third and sides of posterior third; with long, erect, moderately abundant yellowish setae on anterior third and sides of posterior third. Sides of prothorax coarsely, sparsely punctate; with abundant yellowish-brown pubescence not obscuring integument, denser, yellower around lateral tubercle; with long, erect, sparse yellowish setae interspersed. Prosternum finely, sparsely punctate on posterior third, transversely striate on anterior third; with yellowish-brown pubescence not obscuring integument, distinctly sparser anteriorly, and with long, erect, abundant yellowish setae interspersed. Prosternal process distinctly narrowed toward apex; with pubescence and setae as on posterior area of prosternum. Ventral surface of mesothorax with moderately abundant yellowish-brown pubescence not obscuring integument, distinctly sparser on center of anterior area of mesoventrite. Mesoventral process gradually narrowed toward emarginate apex; with both, short and long, decumbent yellowish-brown pubescence partially obscuring integument. Metanepisternum and sides of metaventrite with abundant yellowish-brown pubescence partially obscuring integument, and long, suberect yellowish setae interspersed (suberect setae almost absent on metanepisternum); central area of metaventrite with both, short and long yellowish setae distinctly not obscuring integument, except glabrous area of metathoracic discrimen. Scutellum with dense golden pubescence. Elytra. Moderately coarsely, abundantly punctate, with coarser punctures interspersed, especially on posterior 2/3; apex rounded, with sutural angle slightly projected; with abundant yellowish-brown pubescence almost obscuring integument (pubescence not obscuring punctures), and long, erect, sparse yellowish setae interspersed. Legs. Procoxae slightly tab-shaped close to trochanter. Femora pedunculate-clavate (profemora less so); apex of femora with short rounded lobe; peduncles with both, short and long erect yellowish-brown setae distinctly not obscuring integument (short setae more decumbent); clubs with abundant yellowish-brown pubescence not obscuring integument, and long, erect setae interspersed. Tibiae with both, short and long yellowish-brown setae not obscuring integument (shorter setae denser on apex of dorsal and lateral surfaces), except posterior 2/3 of ventral surface of protibiae with dense, bristly yellowish-brown pubescence; sides of tibiae not longitudinally carinate. Metatarsomere I distinctly longer than II–III together (about as long as 0.75 times length of II–V).
Abdomen
Ventrites with yellowish pubescence not obscuring integument, sparser centrally, and long, erect setae of same color interspersed (erect setae more brownish on posterior area of V). Apex of ventrite V truncate.
Dimensions in mm (female)
Total length, 23.85; prothorax length, 3.20; anterior width, 3.10; posterior width, 3.30; widest prothoracic width, 3.95; humeral width, 5.15; elytral length, 15.30.
Type material
Holotype female from NICARAGUA, Jinotega: Cerro Diablo, 1300–1500 m, 9–10.IV.2005, E. van den Berghe leg. (TAMU, formerly DHCO).
Etymology
It is a pleasure to name this species for Dr. Eric van den Berghe, collector of the holotype, who has provided the second author and others in the scientific community with numerous specimens from Nicaragua and Honduras for over 30 years.
Remarks
Eurysthea vandenberghei sp. nov. differs from all other species of the genus, except E. sordida (Erichson, 1847), and E. llinasi Taboada-Verona & Botero, 2018, by the outer apical angle of the elytra rounded. It differs from E. sordida ( and g) by the elytra with distinctly shorter pubescence (longer in E. sordida), presence of large orangish-brown macula on elytra (absent in E. sordida), and femora bicolorous (unicolorous in E. sordida). Eurysthea vandenberghei can be easily separated from E. llinasi, see photographs of the holotype on Bezark [Citation5], by the elytra with transverse large orangish-brown macula about middle (with inverted V-shaped band, from about apex of anterior quarter to about middle prolonged along suture to about posterior third, and almost V-shaped yellow band in posterior quarter in E. llinasi).
Enaphalodes Haldeman, 1847Enaphalodes antonkozlovi Lingafelter & Santos-Silva, 2018 ()
Enaphalodes antonkozlovi Lingafelter and Santos-Silva [Citation7,p.2]; Monné [Citation8,p.335] (cat.).
This species was described based on a single female from Costa Rica (Cartago). Now we examined a second female from Panama, which shows the following variations: pubescent maculae on head and elytra mostly yellowish-white (mostly yellow in the holotype); scape dark reddish brown except black base and apex (black in the holotype); femora reddish brown with apex black (dark brown with apex and base black in the holotype); tibiae slightly dark reddish brown toward apex (black in the holotype).
Material examined
PANAMA (new country record), Bocas del Toro: Fortuna Cabins, 8.7814ºN, 82.1909ºW, 1 female, 30.VII-5.VIII.2019, E.G. Riley leg. (DHCO).
SMODICINI Lacordaire, 1868Notes on Smodicum Haldeman, 1847
Smodicum Haldeman [Citation9,p.38]; Monné [Citation8,p.809] (cat.).
For complete references on Smodicum, see Monné [Citation8] and Tavakilian and Chevillotte [Citation1].
Martins [Citation10] used the elytral shape to separate some species of Smodicum in his key: “Elytra distinctly plane at apical region,” leading to S. torticolle Martins, 1975, S. pacificum Linsley, 1934, S. parandroides Bates, 1884, S. clancularium Martins, 1975, S. cucujiforme (Say, 1827), and S. texanum Knull, 1966; and “Elytra not as above; epipleurae not strongly reduced to apex; dorsal curve regular,” leading to S. recticolle Martins, 1975, S. angusticolle Aurivillius, 1919, and S. longicorne Martins, 1975. However, the shape of the elytral apex is often somewhat variable intraspecifically, with specimens having the posterior area distinctly plane, curved, or intermediate between these two extremes. Accordingly, this feature is not really useful to separate species in the key. Another feature used in the key was the shape of the prosternal process: “Prosternal process wider than an anterior coxa,” leading to S. pacificum, and S. parandroides; and “Prosternal process narrower than an anterior coxa,” leading to S. clancularium, S. cucujiforme, and S. texanum. This feature is usually useful to separate species. However, the width of the prosternal process is somewhat variable intraspecifically (for example, see ). Furthermore, it was not specified where the width of the prosternal process was measured. This is important because frequently, the prosternal process is distinctly narrower centrally than apically.
Smodicum parandroides Bates, 1884
()
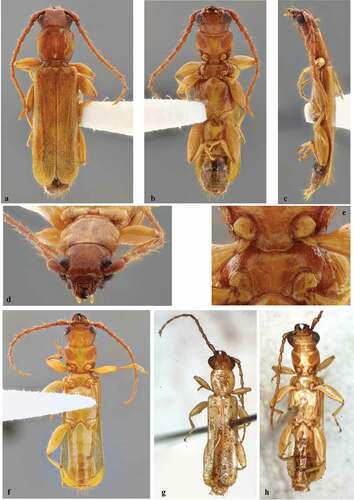
Figure 3. Smodicum parandroides Bates, 1884. (a–e) Male from Mexico (Chiapas): (a) Dorsal habitus; (b) Ventral habitus; (c) Lateral habitus; (d) Head, frontal view; (e) Prosternal and mesoventral processes. (f) Male from Honduras, ventral habitus. (g–h) Male from Nicaragua: (g) Dorsal habitus; (h) Ventral habitus. Images g–h by Jean-Michel Maes
Smodicum parandroides Bates [Citation11,p.241]; Aurivillius [Citation12,p.12] (cat.); Blackwelder [Citation13,p.558] (checklist); Chemsak [Citation14,p.79] (lect.); Martins [Citation10,p.352]; Damoiseau and Cools [Citation15,p.17] (types); Chemsak et al. [Citation16,p.26] (checklist); Monné [Citation17,p.3] (cat.); Monné and Giesbert [Citation18,p.25] (checklist); Noguera and Chemsak [Citation19,p.396] (checklist); Turnbow et al. [Citation20,p.5] (distr.); Monné [Citation21,p.539] (cat.); Hovore [Citation22,p.371] (distr.); Devesa et al. [Citation23,p.30, 100] (distr.); Monné [Citation8,p.811] (cat.).
Smodicum sp.; Maes et al. [Citation24,p.13].
Smodicum parandroides was originally described based on at least a couple of specimens from Mexico (Veracruz) and Guatemala (Escuintla). Chemsak [Citation14] designated a male from Mexico (Veracruz) as lectotype. Currently, the species is known from Mexico (Veracruz, Yucatán), Guatemala (Escuintla), Honduras (El Paraíso, Olancho, Francisco Morazán, Yoro), and Cuba [Citation1]. The material examined allows expansion of the geographic distribution of the species to three Mexican states. Furthermore, the photographs in Maes et al. [Citation24], as well as the photographs sent by Jean-Michel Maes () allows confirmation of the presence of this species in Nicaragua.
Material examined
MEXICO, Chiapas (new state record): El Aguacero N. Park, 1 male, 23.V.1987, E.G. Riley leg. (DHCO). Campeche (new state record): 10 mi N Hopelchen Camp, 1 female, 16.IV.1962, F.D. Parker & L.A. Stange leg. (MZSP). Durango (new state record): Sierra de Durango, 1 female, no more data (MZSP). Veracruz: Córdoba, 1 male, 1 female, no date, A. Fenyes leg. (MZSP); Jalapa, 1 female, no date, F. Schneider leg. (MZSP). HONDURAS, Francisco Morazán: Res. Bio. Uyuca, 1500–1800 m, 1 male, 1–9.VI.2018, 14º03ʹN, 87º07ʹW, E. van den Berghe, J. Vlasak & D. Heffern leg. (MZSP). NICARAGUA (new country record), Jinotega: Reserva Datanli- El Diablo, La Esmeralda, bosque de neblina, 1483 m, 13º06ʹ20”N, 85º51ʹ28”W, 1 male, IV.2005, J.M. Maes leg. (JMMC).
Smodicum confusum Martins, 1985 ()
Figure 4. (a–c) Smodicum confusum Martins, 1985, paratypes female: (a) Specimen 1, dorsal habitus; (b) Specimen 2, ventral habitus; (c) Specimen 3, lateral habitus habitus. (d) Specimen illustrated by Galileo et al. (2011). (e–g) Smodicum clancularium Martins, 1975 sensu Maes et al. (2010): (e) Dorsal habitus; (f) Ventral habitus; (g) Lateral habitus

Smodicum confusum Martins [Citation6,p.169]; Monné [Citation17,p.2] (cat.); Monné and Giesbert [Citation18,p.25] (Checklist); Martins & Galileo [Citation25,p.35]; Monné [Citation21,p.538] (cat.); Galileo et al. [Citation26,p.9, 14]; Monné [Citation8,p.809] (cat.).
Smodicum confusum shares with S. clancularium Martins, 1975 the elytra with a few erect setae on the posterior area. Although the original description of S. confusum did not mention if the metaventral process reaches or not the middle of the mesocoxae, it reaches only the base of the mesocoxae in the three paratypes female () deposited in the MZSP collection. According to Martins [Citation10], in the male of S. clancularium, the metaventral process reaches the middle of the mesocoxae.
Although the redescription of S. confusum by Galileo et al. [Citation26], agrees with the species, the female illustrated () cannot belong to S. confusum because the upper eye lobes are much further apart than in the paratype females. Unfortunately, no information about the origin or depository institution of the specimen was provided, and it was not possible to identify the species of that female. This type of variation was not observed in any of the other species of the genus.
Smodicum clancularium Martins, 1975 ()
Smodicum clancularium Martins [Citation10,p.351]; Maes et al. [Citation27,p.22] (distr.); Maes [Citation28,p.886] (distr.); Monné [Citation21,p.538] (cat.); Swift et al. [Citation29,p.29] (distr.); Maes et al., 2010: 10 (distr.); Monné [Citation8,p.809] (cat.).
Maes et al. [Citation27] recorded S. clancularium for Nicaragua. It seems evident that the record was based on the male identified by Frank T. Hovore (), which was illustrated in Maes et al. [Citation24], with the following information: “Nicaragua: León, III-90, col. B. Garcete, det. ‘Smodicum sp. poss. clancularium’ por F.T. Hovore 91 (1 ex. col. Museo Entomológico de León).” However, this specimen cannot be S. clancularium because the metaventral process does not reach the middle of the mesocoxae. Furthermore, the prothoracic shape and sculpturing does not agree with the original description: “Prothorax constricted at base, scarcely angulated at sides. Disk plane and smooth; sides punctured.” We examined a couple also from Nicaragua perfectly agreeing with the specimen illustrated in Maes et al. [Citation24]. After comparing the female with the paratypes female of S. confusum, it was not possible to separate them. However, without examining a male of S. confusum from Venezuela, it is not possible to affirm that the specimen identified by Maes et al. [Citation24,Citation27] as S. clancularium is really S. confusum. However, it is possible to exclude S. clancularium from the Nicaraguan fauna.
LAMIINAE Latreille, 1825DESMIPHORINI Thomson, 1860
Eupogonius sonorensis Wappes & Santos-Silva, 2020 ()
Figure 5. Eupogonius sonorensis. (a–d) Male from Jalisco (Mexico): (a) Dorsal habitus; (b) Ventral habitus; (c) Lateral habitus; (d) Head, frontal view. (e) Holotype male, dorsal habitus

Eupogonius sonorensis Wappes and Santos-Silva [Citation30,p.9]; Monné [Citation31,p.613] (cat.).
This species was recently described based on three males and three females from Mexico (Sonora).
Some additional information on the male from Jalisco (Mexico): distance between upper eye lobes 0.38 times length of scape (0.24 times distance between outer margins of eyes); in frontal view, distance between lower eye lobes 0.94 times length of scape (0.59 times distance between outer margins of eyes). Antennae 1.7 times elytral length, reaching elytral apex at apex of antennomere VIII. Antennal formula (ratio) based on antennomere III (without spine): scape = 0.63; pedicel = 0.17; IV = 1.07; V = 0.50; VI = 0.48; VII = 0.45; VIII = 0.41; IX = 0.36; X = 0.30; XI = 0.35.
Dimensions in mm (male from Jalisco/ male from Nayarit)
Total length, 6.30/6.55; prothorax length, 1.15/1.25; anterior width, 1.00/1.05; posterior width, 1.05/1.15; widest prothoracic width, 1.35/1.40; humeral width, 1.65/1.70; elytral length, 4.15/4.45.
Material examined
MEXICO, Jalisco (new state record): Pinar de la Venta, 1684 m, 20º43.25ʹN/103º31.78ʹW, 27.VI.2019, 1 male, Nogueira & Cunningham leg. (DHCO). Nayarit (new state record): Volcán Ceboruco, 1946 m, 1 male, 1–2.VII.2019, R. Cunningham leg. (MZSP formerly ACMT).
Remarks
The original description compared Eupogonius sonorensis with E. arizonensis Knull, 1954. This species is also similar to E. affinis Breuning, 1942, see photograph of the holotype on Bezark [Citation5], but differs by the elytra proportionally narrower and longer, with longitudinal pubescent band and punctures finer and denser on the anterior third. In E. affinis, the elytra are proportionally wider and shorter (elytral length 4.8 times humeral width), lacking a longitudinal pubescent band, and with punctures on the basal third coarser and sparser. It is also somewhat similar to E. major Bates, 1885, see photograph of the holotype on Bezark [Citation5], differing by the upper eye lobes wider and not strongly separated from each other (narrower and strongly separated from each other in E. major), and to E. scutellaris Bates, 1885, see photograph of the holotype on Bezark [Citation5], but differs especially by the antennomeres X–XI not yellowish (yellowish in E. scutellaris).
Acknowledgments
We express our thanks to Jean-Michel Maes for sending photographs of two specimens illustrated in Maes et al. [24]. The second author wishes to thank Eric van den Berghe (Zamorano University, Honduras), Edward Riley (College Station, Texas) and Richard Cunningham (Show Low, Arizona) for providing specimens. The third author is grateful to the “Fundação de Amparo à Pesquisa do Estado de São Paulo” (FAPESP) for a postdoctoral fellowship (process number 2017/17898-0).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Tavakilian G, Chevillotte H. Titan: base de données internationales sur les Cerambycidae ou Longicornes. Version 3.0. France; 2021 [cited 2021 Mar 20]. Available from: http://titan.gbif.fr/
- Fonseca-Gessner AA. Revisão taxonômica do gênero Paramallocera Aurivillius, 1912 (Coleoptera, Cerambycidae, Cerambycinae, Sphaerionini). Rev Bras Ento. 1990;34(4):817–856.
- Lingafelter SW. The genera of Elaphidiini Thomson, 1864 (Coleoptera: Cerambycidae). Mem Ent Soc Wash. 1998;20:1–118.
- Martins UR. Tribo Elaphidionini. In: Martins UR, editor. Cerambycidae Sulamericanos (Coleoptera) Taxonomia. Vol. 7. Curitiba: Sociedade Brasileira de Entomologia; 2005. p. 1–394.
- Bezark LG. A photographic catalog of the cerambycidae of the world. New world Cerambycidae catalog. Sacramento; 2021 [cited 2021 Mar 20]. Available from: http://bezbycids.com/byciddb/wdefault.asp?w=n
- Martins UR. Novos táxons, sinonímias, notas e nova combinação em Cerambycidae (Coleoptera) neotropicais. Rev Bras Ento. 1985;29(2):169–180.
- Lingafelter SW, Santos-Silva A. New central American and Mexican Enaphalodes Haldeman, 1847 (Coleoptera: Cerambycidae) with taxonomic notes and a key to species. Pap Avulsos Zool. 2018;58:1–17.
- Monné MA. Catalogue of the Cerambycidae (Coleoptera) of the neotropical region. Part I. Subfamily cerambycinae. 2021 [cited 2021 Mar 20]. Available from: https://cerambycids.com/catalog/
- Haldeman SS. Material towards a history of the Coleoptera Longicornia of the United States. Trans Amer Philos Soc. 1847;10:27–66.
- Martins UR. A taxonomic revision of the world Smodicini (Coleoptera, Cerambycidae). Arq Zool. 1975;26(4):319–359.
- Bates HW. Supplement. In: Godman FD, Salvin O, editors. Biologia centrali-Americana, insecta, coleoptera. Vol. 5. London: Taylor and Francis; 1884. p. 225–248.
- Aurivillius C. Coleopterorum Catalogus, pars 39, cerambycidae: cerambycinae. Berlin: W. Junk, Berlin; 1912.
- Blackwelder RE. Checklist of the coleopterous insects of Mexico, Central America, the West Indies and South America. Part 4. Bull U S Nat Mus. 1946;185:551–763.
- Chemsak JA. Lectotype designations of Cerambycidae in the British Museum (Natural history). J Kans Entomol Soc. 1967;40(1):73–81.
- Damoiseau R, Cools J. Liste du material typique conserve dans les collections entomologiques de l’Institut royal des Sciences Naturelles de Belgique. Coleoptera, Cerambycoidea: Cerambycidae: Aseminae, Cerambycinae, Disteniinae, Lepturinae, Parandrinae, Prioninae et Spondylinae. Documents Travail. 1987;42:1–39.
- Chemsak JA, Linsley EG, Noguera FA. Listados faunísticos de México. II. Los Cerambycidae y Disteniidae de Norteamérica, Centroamérica y las Indias Occidentales (Coleoptera). Mexico City: Universidad Nacional Autónoma; 1992.
- Monné MA. Catalogue of the Cerambycidae (Coleoptera) of the Western Hemisphere. Part I. Subfamily Cerambycinae: tribes Erlandiini, Smodicini, Oemini, Methiini, Xystrocerini, Dodecosini, Opsimini, Achrysonini and Pleiarthrocerini. São Paulo: Sociedade Brasileira de Entomologia; 1993.
- Monné MA, Giesbert EF. Checklist of the Cerambycidae and Disteniidae (Coleoptera) of the Western Hemisphere. Burbank: Wolfsgarden Books; 1994.
- Noguera FA, and Chemsak JA. Cerambycidae (Coleoptera). Llorente Bousquets, JE, Garcia Aldrete, AN, and Gonzalez Soriano , E eds. In: biodiversidad taxonomía, y biogeografía de artrópodos de México: hacia una síntesis de su conocimiento. Vol. I. Mexico City: Universidad Nacional Autónoma de México; 1996. p. 381–409.
- Turnbow RH, Cave RD, Thomas MC. A list of Cerambycidae of Honduras, with additions of previously unrecorded species. Ceiba. 2003;44(1):1–43.
- Monné MA. Catalogue of the Cerambycidae (Coleoptera) of the neotropical region. Part I. Subfamily Cerambycinae. Zootaxa. 2005;946:1–765.
- Hovore FT. The Cerambycidae (Coleoptera) of Guatemala. In: Cano E, editor. Biodiversidad de Guatemala. Vol. 1. Guatemala: Universidad del Valle de Guatemala; 2006. p. 363–378.
- Devesa S, Fonseca E, Barro A. Longicornios de Cuba (Coleoptera: Cerambycidae). Parandrinae, Prioninae, Spondylidinae, Cerambycinae. Vol. 1. Barcelona: Greta Editores; 2015.
- Maes JM, Berghe E, Dauber D, et al. Catalogo ilustrado de los Cerambycidae (Coleoptera) de Nicaragua. Parte II - Cerambycinae. Rev Nicar Entomol. 2010;70(Suplemento 1–2):1–640.
- Martins UR, Galileo MHM. Tribo Smodicini. In: Martins UR, editor. Cerambycidae Sul-americanos (Coleoptera) Taxonomia. Vol. 4. São Paulo: Sociedade Brasileira de Entomologia; 2002. p. 9–35.
- Galileo MHM, Martins UR, Moyses E. Cerambycidae Sul-Americanos (Coleoptera). Suplemento 3. Porto Alegre: Museu de Ciências Naturais da Funcação Zoobotânica do RS; 2011.
- Maes JM, Allen A, Monné MA, et al. Catálogo de los Cerambycidae (Coleoptera) de Nicaragua. Rev Nicar Entomol. 1994;27:1–58.
- Maes JM. Catálogo de los insectos y artropodos terrestres de Nicaragua. Vol. 2. Marena: Secretaría Técnica Bosawas; 1998.
- Swift I, Bezark LG, Nearns EH, et al. Checklist of the Cerambycidae (Coleoptera) of Costa Rica. Insecta Mundi. 2010;131:1–68.
- Wappes JE, Santos-Silva A. Nomenclatural notes on some currently known species of Eupogonius LeConte, 1852 (Coleoptera: Cerambycidae: Lamiinae) and description of seven new species. Insecta Mundi. 2020;748:1–20.
- Monné MA. Catalogue of the Cerambycidae (Coleoptera) of the neotropical region. Part II. Subfamily lamiinae. 2021 [cited 2021 Mar 20]. Available from: https://cerambycids.com/catalog/
