ABSTRACT
Studies on model organisms such as butterflies are useful tools for conservation decision-making. However, in tropical ecosystems with an intrinsic high diversity a full understanding of biotic communities is difficult to obtain. Bait trap samplings have traditionally been used for community appraisals related to ecological and conservation issues. Nonetheless, in the Andes Mountains, there is little knowledge related to the effectiveness of bait traps for butterfly sampling. In this study, we tested the success of fermented fruits and rotten fish baits for butterfly sampling in four land-cover types (páramo, cloud forests, mixed, and pasture) in the upper Rio Chico basin of the northern Central Cordillera of the Colombian Andes. A butterfly survey was conducted between 2011 and 2014, in an elevation range of 2650 to 3300 masl, within a total of 132 field days. Three sampling units for each land cover were established with four standard Van Someren-Rydon traps (VSR) per sampling unit. Traps were baited alternatively with fermented fruits and carrion (rotten fish). All 57 recorded species were captured using rotten fish, while approximately 65% (37 species) were collected from fermented fruit. Moreover, species richness was higher in all sampled land covers using rotten fish bait, but the dominant species in the land covers differed between baits. The rotten fish bait proved to be highly effective for butterfly sampling in páramo and cloud forest, although the combination of traps baited with fermented banana and rotten fish, allowed the collection of data suitable for comparison among all studied land cover.
Introduction
In recent years, there has been further research in the butterfly fauna of high-altitude neotropical areas [Citation1–7], and the search for standardized sampling protocols and methods to compare independent samples is a priority. The use of bait traps has proven to be one of the most reliable methods for comparing representative samples of fruit-feeding butterfly assemblages, although this method has had little success in montane habitats, where traps generally capture fewer butterflies than premontane and lowland tropical areas [Citation8]. Fruit-feeding butterflies obtain most of their nutritional requirements from fermented fruits, feces, carcasses, decomposing organic matter, and fermented sap [Citation9]. The group is non-monophyletic and is represented in the neotropical region by species of four Nymphalidae subfamilies: Satyrinae, Charaxinae, Biblidinae, and Nymphalinae (the latter to a lesser extent) (sensu Wahlberg et al. 2009) [Citation8,Citation10]. The tropical high Andean mountains are mainly represented by Satyrinae, particularly the subtribes Pronophilina (more than 600 species in 45 genera, Pyrcz in prep) and Euptychiina (mainly Forsterinaria sp.), followed by Biblidinae (Epiphile, Perisama, and Orophila), and some species of Charaxinae and Nymphalinae [Citation11–14].
One of the most salient characteristics of fruit-feeding butterflies is that they can be sampled in a standardized manner to avoid human collector biases [Citation15]. However, standardized butterfly sampling in neotropical montane habitats presents several difficulties when compared to lowland sampling. The vegetation of this region offers a reduced availability of fleshy fruits [Citation16] and slower decomposition rates of organic material due to the low temperatures [Citation17,Citation18]. Consequently, fruit-feeding butterflies’ assemblages at higher elevations are adapted to acquire nutrients from alternative sources, such as carrion and feces, or exchanged their food source completely to become nectar feeders (e. g. [Citation19]). Accordingly, fermenting fruits are not a viable study base in montane habitats, requiring the search for novel sampling strategies to obtain a representative picture of the local assemblages. Previous studies in these habitats have successfully used traps baited with the excrement of carnivorous mammals [Citation5–7]. Although this bait was successful in sampling butterflies in high-altitude habitats, it is difficult to standardize feces to allow for comparative studies in different localities and in long-term projects. For biological assessment and monitoring projects it is necessary to use standardized methods that provide rigorous comparable data. This allows to combine results from various studies and facilitates the comparisons of species richness, composition and abundance within and among habitat types [Citation15,Citation20]. Therefore, the present study was designed to compare the performance of two different baits, fermented fruits and rotting fish, for the standardized sampling of fruit-feeding butterflies in a high mountain site.
Materials and methods
Study site and fieldwork
The study was conducted in the conservation area of the “Páramo de Belmira” (also known as “Páramo de Santa Inés”), located in the northernmost part of the Cordillera Central of the Colombian Andes (6°35ʹ–6°51ʹ N and 75°47ʹ–75°38ʹ W). The landscape of the region is dominated by four types of land cover: cloud forest, páramo (a native open vegetation typical of high altitude in the neotropics, dominated by grasses and other herbaceous plants; see [Citation21,Citation22]), cattle pastures, and mixed vegetation (a mosaic of regeneration vegetation in various successional stages, interspersed with cloud forest, and abandoned cattle pastures without a clear prevalence of any vegetation cover). The sampling was carried out essentially once a month from June 2011 to April 2014 in 12 localities at an elevation range of 2650 to 3300 m a.s.l. In each field trip, samplings were performed for 3 to 4 consecutive days, for a total of 132 field days. The design consisted of three sampling units for each habitat type, in which four standard Van Someren-Rydon traps (VSR) were used per sampling unit. Traps were located 1.5 to 2 m above the ground and separated by 100 m, following Hamer and Hill [Citation23] and Benedick et al. [Citation24]; those studies estimated the area of influence of a bait trap to be at a radius between 50 m and 100 m (). The traps were baited alternately with 150 g of one of two feeds, fermented bananas or rotten fish (comercial avaliable trout). Baits were prepared in the butterfly house of the Laboratorio de Fisiología de Insectos of the Universidad Nacional de Colombia in Medellín, at an average temperature of 23.5 °C, a minimum of 21 °C and a maximum of 26.1 °C. The fermented bananas bait was a mixture of mashed mature banana with sugar and rum, fermented for four days, and the rotten fish bait consisted of pieces of trout, decomposed in a closed bag for five days. Bait replacement was done every 48 h during each sampling session, following Santos et al. [Citation25]. For each field trip at the same site, the types of bait were rotated at all locations.
Figure 1. (a–c). Installation of bait traps; (d). fermented fruit bait; (e). Butterflies attracted to feces of local Puma concolor Linnaeus, 1771

The collected specimens were identified based on the most recent updated faunal lists, revisions, and species accounts for the region [Citation26–29]. The website of Butterflies of America [Citation30] was also consulted, since it contains photographs of the type material for most neotropical butterflies, and also reflects the current state of knowledge on the taxonomy of the group. Specimens were deposited in the collections of the Museo Entomológico Francisco Luis Gallego of the Universidad Nacional de Colombia Sede Medellín and in the collection of the Instituto Alexander von Humboldt in Villa de Leyva, Boyacá, Colombia.
Data analysis
A Venn diagram was used to compare the number of shared and exclusive species collected by each bait type. Diversity profiles based on Hill numbers through the q statistic [Citation31,Citation32] were calculated to measure the proportion of the diversity sampled for each method: q = 0 being the order based only on the species richness of each assemblage, q = 1 represents the exponential of Shannon entropy concerning equitability, and q = 2 represents the inverse of the Gini–Simpson dominance index. To evaluate differences in diversity, generalized linear models (GLMs) were developed according to the method proposed by Checa et al. [Citation33]. GLMs were used to evaluate if the respose variables of abundance, observed species richness, or species diversity, differed with respect to sampling technique (predictor variables, fruit and rotten fish). The selection of possible models was based on the type of data: discrete (abundance, richness) or continuous (diversity), as well as Akaike Information Criterion (AIC) values and residual variance. The negative binomial distribution was the superior model over the Poisson or normal distribution for our data, in relation to abundance and species richness; the diversity of order q1 and q2 fit a Gaussian distribution and were tested with a standard linear model (LM). Analyses were performed in R version 3.6.3 [Citation34] using the package vegan 2.5–6 [Citation35].
Results
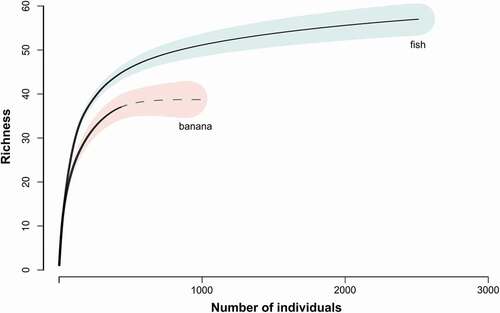
A total of 2939 fruit-feeding butterflies belonging to 57 species were collected. Among these, 430 individuals representing 37 species were captured in traps baited with fermented fruits, and 2509 butterflies included in all 57 species were found in the rotten fish traps (, ). Traps baited with rotten fish captured a higher number of species in all sampled habitats (), with a different dominant species for each bait type: Panyapedaliodes drymaea (Hewitson, 1858) represented 25% of the individuals captured with fermented banana, while Pedaliodes obstructa Pyrcz and Viloria, 1999, represented 24% of those captured with rotting fish. Significant differences were observed in abundance (GLM Estimate = 1.7639, p < 0.001), richness (GLM estimate = 0.8315, p = 0.002), and q1 diversity (GLM Estimate = 3.766, p = 0.041). Abundance was higher in traps baited with rotten fish in the cloud forest, mixed vegetation, and páramo regions, while pastures showed a higher abundance in traps baited with fermented bananas ().
Table 1. Occurrence of fruit-feeding butterfly species in each type of bait and habitat
Table 2. Butterfly diversity: abundance, observed species richness, and diversity (q1 and q2) recorded, comparing traps baited with fermented banana and rotten fish in the different studied habitats. Result of the GLMs test, significant <0.01 **, <0.05 *, determining whether butterfly diversity differed with respect to sampling technique
Figure 3. Venn diagrams, showing the exclusive and shared species registered in each bait in the different habitats
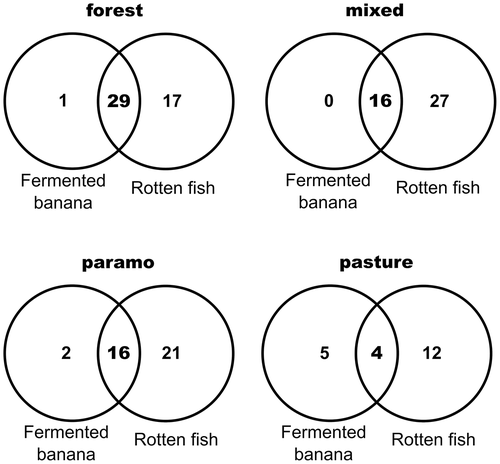
With a sample coverage higher than 91% in all sample sites, 47 species were captured in the cloud forest, comprising half of the collected specimens. In contrast, the pasture regions showed the lowest values for both parameters, with only 151 captured individuals of 21 species. All vegetation types except the pasture registered exclusive species: (1) Eretris ocellifera (C. Felder & R. Felder, 1867), Lymanopoda obsoleta (Westwood, 1851), Manerebia germaniae Pyrcz & J. Hall, 2006, Mygona irmina (E. Doubleday, [1849]), and Proboscis propylea (Hewitson, 1857) were exclusively captured in the cloud forest; (2) Morpho sulkowskyi Kollar, 1850, Panyapedaliodes jephtha (Thieme, 1905), and Perisama yeba (Hewitson, [1857]) were exclusively found in mixed vegetation; and (3) Lymanopoda casneri Pyrcz & Clavijo, 2016, Pedaliodes griseola Weymer, 1912, and Pedaliodes negreti Pyrcz, 1999 were collected solely in the páramo habitat.
Discussion
The present results clearly indicate that the two types of baits, resulted in different performances of sampling in fruit-feeding butterflies, with significant differences in abundance, richness, and diversity. Traps baited with rotting fish were more effective for obtaining a representative picture of the local assemblages. Considering all species of fruit-feeding butterflies in the region with 61 species [Citation36], fermented bananas sampled 61% of the local assemblage, while rotting fish returned 93% of the total local species richness (including all species sampled with fermented bananas). The performance of the baits showed differences among the different land covers: a higher performance of the rotten fish was reported for páramo, cloud forest and mixed land covers, while the fermented bananas baits had a higher performance in pastures (with a 62% of the registered specimens), contributing significantly to the sampling coverage in this land cover. The difference in abundance performance of the baits in pastures is possibly related to a high abundance of one specific species, Panyapedaliodes drymaea (Hewitson, 1858), that apparently present a high preference by this kind of bait (). In short, the present results showed that the use of fermented fruit baits is not sufficient for collect a representative sample of the assemblage of fruit-feeding butterflies in high mountainous regions. Conversely, the use of rotting fish or a combination of both bait types was more effective for obtain a great sample coverage of comparable data for high mountain butterfly assemblages among different land covers.
Unlike previous studies, where different baits were used to inventory all butterfly communities [Citation33], the present study aimed to provide a standardized protocol for sampling and monitoring butterflies in montane habitats, similar to those proposed for other forest types (see [Citation8,Citation10,Citation37–39]). The present proposal of the use of trout represented a clear methodological advantage for the following reasons: (1) trout is an introduced freshwater species now present in many neotropical montane sites, including the Andean region, the mountains from Southeastern Brazil and Central America; (2) trout are not as expensive as squid and shrimp and are available in inland regions; (3) the more common species of trout are available throughout the neotropical region, which allows for a relatively standardized bait (which is extremely difficult in the case of feces). Rotten fish and feces have shown to be particularly useful for short-term inventories in high Andean environments [Citation4,Citation6,Citation7]. However, for environmental community comparison and long-term diversity monitoring studies, is necessary to use standardized and replicable sampling methods. The present study shows that comparative standardized studies in Neotropical montane habitats can be made by the combined use of traps baited with fermented banana and rotten fish, allowing the collection of robust ecological data suitable for statistical analysis, concerning general patterns of diversity and structuration of fruit-feeding butterflies assemblages and monitoring programs.
Author contributions
CFA, CAI, AVLF, and MAM conceived and designed the study. CFA, ACG, and MAM performed the field study. CFA and MAM wrote the first draft of the manuscript. CFA, ACG, and TWP perforemd, sample processing, taxonomy support, and specimen identification. CFA and MAM analyzed the data. SIU, TWP, CAI, and AVLF reviewed and improved the manuscript.
Geolocation information
Páramo de Belmira, Antioquia, Colombia. Elevation range of 2650 to 3,300 meters of elevation. Coordinates (N 6°35ʹ to 6°51ʹ) (W 75°47ʹ to 75°38ʹ).
Acknowledgments
We would like to thank the local community of Belmira, in particular Hector Rojas (Corantioquia-DMI Belmira) for field support, GSM members, volunteers, and all the people who helped in field work. Corporación Universitaria Lasallista and the Universidad Nacional de Colombia sede Medellín for logistic support. Specimen collection was done under license number 4 of 7 May 2011 and a resolution 503 on 24 May 2013 by ANLA (Agencia Nacional de Licencias Ambientales, Colombia).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Acevedo AA, Sanguino OA, Quiñónez CAO, et al. Potential species richness of frogs and diurnal butterflies in three biogeographical units from Northeastern Colombia: conservation implications. Acta Biol Colomb. 2017;23:151–162.
- Álvarez-Hincapié CF, Clavijo A, Rojas H, et al. Aporte del área de influencia del páramo de Belmira (Santa Inés) a la diversidad regional de pronophilina (Lepidoptera: Satyrinae) del norte de los Andes. Rev Mex Biodivers. 2017;88(2):402–409.
- Mahecha O, Garlacz R, Andrade MG, et al. Island biogeography in continental areas: inferring dispersal based on distributional patterns of pronophilina butterflies (Nymphalidae: Satyrinae) in the north Andean massifs. Rev Mex Biodivers. 2019;90:e902796.
- Marín MA, Giraldo CE, Marín AL, et al. Differences in butterfly (Nymphalidae) diversity between hillsides and hilltop forest patches in the northern Andes. Stud Neotrop Fauna Environ. 2015;50(3):194–203.
- Pyrcz TW, and Rodríguez G. Mariposas de la tribu Pronophilini en la Cordillera Occidental de los Andes de Colombia(Lepidoptera: Nymphalidae, Satyrinae). Shil Rev Lepidopterol. 2007;35:455–489.
- Pyrcz TW. Pronophiline butterflies of the highlands of Chachapoyas in northern Peru: faunal survey, diversity and distribution patterns (Lepidoptera, Nymphalidae, Satyrinae). Genus. 2004;15:455–622.
- Pyrcz TW, Wojtusiak J, Garlacz R. Diversity and distribution patterns of Pronophilina butterflies (Lepidoptera: nymphalidae: satyrinae) along an altitudinal transect in north-western Ecuador. Neotrop Entomol. 2009;38(6):716–726.
- Freitas AVL, Iserhard CA, Santos JP, et al. Studies with butterfly bait traps: an overview. Rev Colomb Entomol. 2014;40:209–218.
- DeVries PJ. The butterflies of Costa Rica and their natural history. Papilionidae, Pieridae, Nymphalidae. Princeton: Princeton University Press; 1987.
- Santos JP, Marini-Filho OJ, Freitas AVL, et al. Monitoramento de borboletas: o papel de um indicador biológico na gestão de unidades de conservação. Biodiversidade Bras. 2016;6:87–99.
- Henao-B ER, and Stiles FG. Un inventario de las mariposas diurnas (Lepidoptera: hesperioidea-Papilionoidea) de dos reservas Altoandinas de la Cordillera Oriental de Colombia. Rev La Fac Ciencias. 2018;7(1):71–87.
- Lamas G, Grados J, and Valencia G. Las mariposas de Machu Picchu, Cuzco, Perú: Un inventario preliminar (Lepidoptera: Rhopalocera). Rev Peru Entomol. 1999 41 1 ;1–8.
- Marín MA, Álvarez CF, and Giraldo CE, et al. Mariposas en un bosque de niebla andino periurbano en el Valle de Aburrá, Colombia. Rev Mex Biodivers. 2014;85(1):200–208.
- Olarte-Quiñonez CA, Acevedo-Rincón AA, Ríos-Málaver IC, et al. Diversidad de mariposas (Lepidoptera, Papilionoidea) y su relación con el paisaje de alta montaña en los Andes nororientales de Colombia. Arx Miselania Zoológica. 2016;14:233–255.
- DeVries PJ, Hamm CA, Fordyce JA. A Standardized sampling protocol for fruit-feeding butterflies (Nymphalidae). In: Larsen TH, editor. Core standardized methods for rapid biological field assessment. (Arlington VA): Conservation International; 2016. p. 139–148.
- Tobler MW. Habitat use and diet of baird’s tapirs (Tapirus bairdii) in a montane cloud forest of the Cordillera de Talamanca, Costa Rica. Biotropica. 2002;34(3):468–474.
- Coûteaux MM, Sarmiento L, Bottner P, et al. Decomposition of standard plant material along an altitudinal transect (65–3968 m) in the tropical Andes. Soil Biol Biochem. 2002;34(1):69–78.
- Marian F, Sandmann D, Krashevska V, et al. Leaf and root litter decomposition is discontinued at high altitude tropical montane rainforests contributing to carbon sequestration. Ecol Evol. 2017;7:6432–6443.
- Rosa AHB, Ribeiro DB, Freitas AVL. Population biology, natural history and conservation of two endangered high elevation Neotropical butterflies. J Insect Conserv. 2020;24(4):681–694.
- Kral K, Harmon J, Limb R, et al. Improving our science: the evolution of butterfly sampling and surveying methods over time. J Insect Conserv. 2018;22(1):1–14.
- Cuatrecasas J. Aspectos de la vegetación natural de Colombia. Rev Acad Colomb Ciencias Exactas. 1958 10 ;221–268.
- van der Hammen T. The Pleistocene changes of vegetation and climate in tropical South America. J Biogeogr. 1974;1(1):3–26.
- Hamer KC, Hill JK. Scale-dependent effects of habitat disturbance on species richness in tropical forests. Conserv Biol. 2000;14(5):1435–1440.
- Benedick S, White TA, Searle JB, et al. Impacts of habitat fragmentation on genetic diversity in a tropical forest butterfly on Borneo. J Trop Ecol. 2007;23(6):623–634.
- Santos JPD, Iserhard CA, Carreira JYO, et al. Monitoring fruit-feeding butterfly assemblages in two vertical strata in seasonal Atlantic Forest: temporal species turnover is lower in the canopy. J Trop Ecol. 2017;33(5):345–355.
- Pyrcz TW, Clavijo A, and Uribe SI, et al. Páramo de Belmira as an important centre of endemism in the northern Colombian Andes: new evidence from Pronophilina butterflies (Lepidoptera: Nymphalidae, Satyrinae, Satyrini). Zootaxa. 2016;4179(1):77–102.
- Pyrcz TW, Rodríguez G. Notas sobre el grupo Panyapedaliodes muscosa (Thieme) con la descripción de una nueva especie y dos nuevas subespecies (Lepidoptera, Nymphalidae, Satyrinae, Pronophilini). Lambillionea. 2005;CV:187–194.
- Pyrcz TW, and Rodríguez G. Description of a new remarkable species of Lymanopoda Westwood and identification of a centre of endemism of cloud forest butterflies in Belmira, northern Central Cordillera, Antioquia, Colombia (Lepidoptera: Nymphalidae: Satyrinae). Genus. 2006;17:291–297.
- Pyrcz TW, Willmott KR, Hall JPW, et al. A review of the genus Manerebia Staudigner (Lepidoptera: Nymphalida: Satyrinae) in the northern Andes. J Res Lepid. 2006;39:37–79.
- Warren AD, Davis KJ, and Stangeland EM, et al. Illustrated lists of american butterflies [21-XI-2017]. [ Internet]. 2017 Accessed10 10 2020. Internet: http://butterfliesofamerica.com/.
- Jost L. Entropy and diversity. Oikos. 2006;113(2):363–375.
- Hill MO. Diversity and evenness: a unifying notation and its consequences. Ecology. 1973;54(2):427–432.
- Checa MF, Donoso DA, Rodriguez J, et al. Combining sampling techniques aids monitoring of tropical butterflies. Insect Conserv Divers. 2019;12(4):362–372.
- Team RC. R: a language and environment for statistical computing. (Vienna Austria): R Foundation for Statistical Computing; 2020.
- Oksanen J, Blanchet FG, and Friendly M, et al. vegan: community ecology package. R Package Version. 2019;2.5-6.298pp.
- Marín MA, Lopéz-Rubio A, Clavijo A, et al. Use of species delimitation approaches to tackle the cryptic diversity of an assemblage of high Andean butterflies (Lepidoptera: Papilionoidea). Genome. 2021;64(10):937–949.
- Freitas AVL, Santos JPD, and Rosa AHB, et al. Sampling methods for butterflies (Lepidoptera). In: Santos JC, Fernandes GW, editors. Meas Arthropod Biodivers a Handb Sampl Methods. (Cham Switzerland): Springer; 2021. p. 101–123.
- Van Swaay C, Regan E, and Ling M, et al. Guidelines for standardised global butterfly monitoring. GEO BON Tech Ser 1. 2015 32pp .
- DeVries PJ, and Walla T. Species diversity and community structure in Neotropical fruit‐feeding butterflies. Biol J Linn Soc. 2001;74(1):1–15.