ABSTRACT
Studies have been devoted to describing the diet of D. monticola. However, knowledge of how D. monticola utilizes food resources through ontogeny remains unknown. We characterized D. monticola general dietary patterns and tested for ontogenetic diet shifts. We also inferred on juvenile recruitment patterns. We examined the stomach contents of 120 specimens ranging in size from 20 to 154 mm in standard length (SL). To infer on recruitment periodicity, we examined the size of specimens deposited in museum collections to compile size frequencies by month. We assume that the occurrence of smaller-size class categories will shed light on their recruitment time. Our results show that D. monticola is an omnivore that largely feeds on aquatic insects but also includes detritus, terrestrial material, fishes and crustaceans. Our analysis did not detect strong changes in diet through ontogeny, instead some changes were detected but mainly on the percentage of some food items included at different size category classes. In regards to the recruitment periodicity, we found younger D. monticola (20–40 mm SL) throughout most of the year, suggesting that they may be reproducing for longer periods of time than those previously reported from Honduras.
Resumen
Varios estudios se han dedicado a describir la dieta de D. monticola. Sin embargo, el conocimiento de cómo D. monticola utiliza los recursos alimenticios a través de la ontogenia sigue siendo desconocido. Caracterizamos los patrones generales de la dieta de D. montícola. Tambien estudiamos los cambios ontogenéticos en dieta e inferimos sobre los patrones de reclutamiento de juveniles. Con el fin de comprender los cambios ontogenéticos en la dieta, examinamos el contenido estómacal de 120 especímenes que varían en tamaño de 20 a 154 mm en longitud patrón (SL). Para inferir sobre la periodicidad de reclutamiento, examinamos el tamaño de los especímenes depositados en las colecciones de los museos para recopilar las frecuencias de tamaño por mes. Suponemos que la aparición de categorías de clase de menor tamaño arrojará luz sobre su tiempo de reproducción. Nuestros resultados muestran que D. monticola es un omnívoro que se alimenta en gran medida de insectos acuáticos, pero también incluye detritus, material terrestre, peces y crustáceos. No detectamos cambios fuertes en la dieta a través de la ontogenia, sino que se detectaron algunos cambios, pero principalmente en el porcentaje de algunos alimentos incluidos en diferentes categorías de tamaño. Con respecto a la periodicidad de reclutamiento, encontramos D. monticola juveniles (20 - 40 mm SL) durante la mayor parte del año, lo que sugiere que pueden estar reproduciéndose por períodos de tiempo más largos que los reportados previamente en Honduras.
Introduction
Most fishes show ontogenetic dietary shifts related to changes in ecology or morphology. Various studies have linked diet to morphology [Citation1], behavior and physiological demands [Citation2], changes in habitat [Citation3], prey availability and risk of predation [Citation4]. These and other factors will result in changing quality and quantity of consumed prey [Citation5] throughout their life history. Documenting ontogenetic shifts in diet is fundamental to understanding the ecology of a species and for conservation or management of broader ecosystem services.
Dajaus monticola (Bancroft, 1834), the mountain mullet, is an amphidromous mullet [Citation6] with a distributional range that extends from the southern United States through Venezuela and Ecuador and the West Indies [Citation7–9]. In Central America, D. monticola is commonly found in all major river basins [Citation7,Citation10–14]. Where D. monticola is found in freshwater environments, the species has been reported to prefer habitats with faster moving water with rapids, riffles and water falls [Citation12,Citation15,Citation16]. In some areas, D. monticola is an important species for local fisheries [Citation17]. Along the Honduran Caribbean coast, D. monticola is an important part of an artisanal-subsistence fishery, especially in economically depressed communities near rivers [Citation15].
Previous studies describing diet [Citation17,Citation18], reproduction, e.g. [Citation19] or both [Citation15,Citation20,Citation21] of D. monticola have not come to consistent conclusions regarding trophic placement of the species. Some have concluded the species is an insectivore [Citation20,Citation21], while others have classified it as an ‘opportunistic feeder’ [Citation17,Citation18]. Cruz [Citation15] classified the species as a carnivore. Regardless of trophic position, little is known about juvenile diet and ontogenetic shifts in D. monticola (but see [Citation18]). Similarly, ambiguity surrounds the reproductive biology of the species. There is no general consensus on when D. monticola reproduces or how long the reproductive season lasts. Timing and length of the spawning season has been reported to vary geographically from one month in Puerto Rico (August [Citation22–24]:), two months in Honduras (November–December [Citation15]), three months in Jamaica (May–July [Citation21]) and up to five months on the Island of Trinidad (June–October [Citation20]). In contrast, it was concluded that D. monticolaspawns year-round based on the presence of young of the year (e.g. < 50 mm standard length [SL]) in coastal tidal [Citation25].
We investigated the diet and recruitment periodicity of D. monticola found within the rivers of the Honduran Caribbean coast. We predicted that the diet of D. monticola will change through ontogeny and that recruitment periodicity may be different from what has been previously reported. Recent intensive hydroelectric development along the Honduran Caribbean coast has raised concern about the conservation status and long term viability of populations. There is a particular need for more knowledge of the autecology and life history characteristics of D. monticola as an abundant migratory species of economic interest.
Materials and methods
Study area and data collection
Samples for this study are from the Honduran Caribbean coast, an area that spans from the Motagua River drainage (Honduras-Guatemala border) to the Coco River drainage (Honduras-Nicaragua border) and the Honduran Bay Islands (). For the stomach content analysis, D. monticola were collected from 2005 to 2006 () using seine, electrofishing and spear fishing. These specimens and associated by-catch were deposited into The University of Southern Mississippi Ichthyological Museum (USM, Hattiesburg). To complement our field collections in assessing recruitment periods, we reviewed specimens from Honduran lots deposited at other museums, including the University of Florida Museum of Natural History (UF), United States National Museum (USNM), University of Michigan Museum of Zoology (UMMZ), and the Los Angeles County Natural History Museum (LACM). The inclusion of these data allowed for a better representation of different size classes of D. monticola throughout the year in Honduras.
Ontogenetic shift in diet
We measured the standard length (SL, mm) of each fish (120 individuals) before removing the entire digestive tract and preserving it in 70% ethanol until processing. The stomach contents were then removed, and examined under a Nikon SMZ 1500 light dissecting microscope (60–100 x) coupled with a Sony HD video camera and a Samsung LCD Video. Identification of prey was based on sclerotized body parts, particularly head capsules, mouth parts and leg fragments [Citation26]. Stewart & Stark [Citation27] stated that the count of sclerotized fragments (i.e. head capsules or legs) can give a reasonably accurate count of prey consumed. Prey items were identified to the lowest possible taxonomic level (e.g. order, ) and enumerated; prey items per sample were retained for analyses. Previous studies in D. monticola life history have determined that adulthood is reached at sizes between 122 mm SL (Eslava and Diaz 2011) and 1655 mm SL (Aiken 1998). This huge discrepancy on size at first maturity and the fact that a vast majority of our samples are < 100 mm in SL, lead us to divide our data set in three size categories: 20–59 mm SL (small-size), 60–100 mm SL (medium-size) and >100 mm SL (large-size). Base on existing life history studies (e.g Eslava and Diaz 2011; Aiken 1998); most of our samples may represent juveniles.
Table 1. Summary of % contribution based on abundances of food items (e.g. functional groups) found in stomachs of 120 D. monticola in three standard length size classes categories
To visualize patterns of prey usage, we used a Nonmetric Multidimensional Scaling (NMDS [Citation28,Citation29];) procedure implemented with a Bray-Curtis distance measure. The NMDS is an ordination method that is well-suited to data that are non-normal or are on arbitrary, discontinuous, or otherwise questionable scales [Citation28]. As a result, the technique is commonly used with ecological data [Citation30]. To identify the food items that were important contributors in each size class, we used an indicator species analysis (ISA [Citation31];). The ISA calculates an index based on the abundance and rate of occurrence for each prey type in each size class of fish [Citation32]. A high ISA value (1.0) indicate that prey type was both abundant and exclusive (found in all stomachs) to a particular size class. Alternatively, an ISA value of 0 indicate prey that are less abundant or found ubiquitously among the three size classes [Citation31]. To test the significance of ISA values, we used a Monte Carlo test with 1000 random permutations of diet data to generate a distribution of expected values. In order to test for significance differences of food item composition between the three size groups, we applied a one-way analysis of similarity (ANOSIM) [Citation33]. The test calculates a test statistic (R), which reflects the observed differences between groupings, contrasted with differences within groupings [Citation33]. The R statistic ranges between 0 and 1: if R = 1 then all sites within a group are more similar to each other than any sites from different groups, and if R = 0 then the similarities between and within groups are the same on average [Citation33]. The NMDS, ISA and ANOSIM analyses were implemented with the statistical software R 2.8.1 [Citation34], with the R packages vegan [Citation35] and indicspecies [Citation36].
Reproductive periodicity
We inferred reproductive periodicity of D. monticola by measuring the SL of 616 A. monticola that were then divided into five size classes: ≤ 20 mm SL, 21–30 mm SL, 31–40 mm SL, 41–50 mm SL, and ≥ 51 mm SL. Our records included samples throughout the year except for the months of September and December. Size frequency data were then transformed using a natural logarithm +1 transformation to normalize monthly samples with disparate abundance.
Results
Ontogenetic shift in diet
We analyzed the stomach contents of 120 D. monticola of which 35 did not contain any food items. Of the remaining 85 individuals, 17 were small-sized, 53 were medium-sized and 15 were large-sized. Items were partitioned into 14 functional groups (e.g. order; ). Overall, D. monticola diet was dominated by aquatic insects, with dipterans representing the most dominant prey items in all three size classes (Juveniles = 42.27%; Adults = 34.65%; Large Adults = 46.83%; , ).
Figure 2. Results of the NMDS (stress 20.7%). Small-size = individuals between 20 and 59 mm in SL, medium-size = individuals between 60 and 100 mm SL and large-size = individuals larger than 100 mm Sl. Labels depict the 14 functional groups in which food items were clustered
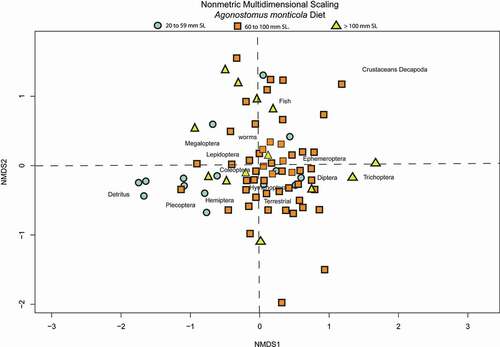
Figure 3. Dajaus monticola museum and field collection size frequency data. Monthly abundance data was ln+1 transformed to normalize for abundance disparity
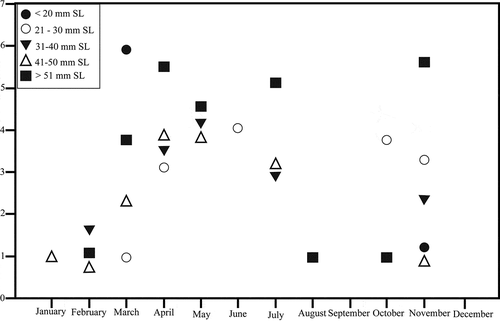
Figure 4. Pie chart depicting the porcentage of food items by fuctional group utilize per D. monticola size caterogy
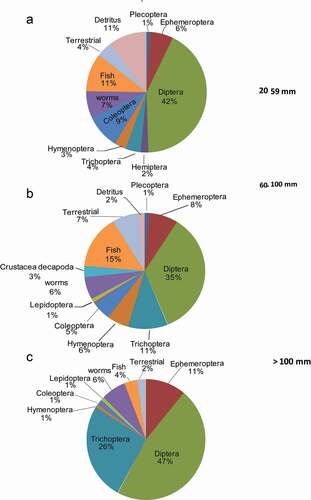
The NMDS results (; 20.7% stress) showed that the three size classes overlapped in multivariate space, but were significantly different in ANOSIM (P= 0.001; R = 0.2135). The low ANOSIM R values in the global, as well as in each of the pairwise comparisons (small-sized vs. medium-sized = R 0.2135; small-size vs. large-size: R = 0.1958; large-size vs. medium-size: R = 0.212), suggested high levels of shared food items between groups [Citation33].
The results of ISA () suggests that out of 14 functional groups, just three were statistically significant (P < 0.05; large-size: Ephemeroptera and Lumbricidae; small-size: detritus), however the ISA indicator values recovered were below 0.5 (Ephemeroptera = 0.44; Lumbricidae = 0.44; Detritus = 0.46), suggesting that they are not particularly strong indicators for the groups. The ISA results are largely congruent with the NMDS, those marginaly strong indicators (~ 0.5) indicate that those items are commonly used by individuals of one sized class, but only occasionally reported in others. For instance, detritus was more important in the diet of small-sized fish (11%), but it was also present in smaller amounts (2%) in medium-size fish (, ). Epehemeropterans and Lumbricidae were significant indicators for medium-size, but they also occurred in small-size fish relative quantities in the other two sized classes. Finally, the NMDS showed a positive association between trichopterans and large-sized fish, however the ISA analysis did not find trichopterans as strong indicators allthough the overall contribution of trichopterans to the diet of D. monticola was of 26.04% and in medium-sized and large-sized individuals was of 4.12 and 10.64 % respectively ().
Table 2. Results of the indicator species analysis for the three size class categories. Small-size = 20–59 mm SL; medium-size = 60–100 mm SL; larger-size = < 100 mm SL. Significance values determined based on 1000 permutations. Statistically significant prey items (<0.05) are bolded. Note that indicators values below 0.5 are considered as poor indicators (Punchi-Manage et al. 2013)
Recruitment periodicity
Of the 616 individuals used in this study 23.4% belonged to the < 20 mm SL category, 9.2% to the 21–30 mm SL, 7.9% to the 31–40 mm SL, 8.3% to the 41–50 mm SL and 51.2% to the > 50 mm SL category. Individuals smaller than 20 mm SL were sampled in November and March (), while D. monticola between 21–30 mm SL were sampled from March to June and October to November. Larger D. monticola (31–40 mm) were found in February, April and May, July and November. Thus, smaller D. monticola (< 20 mm to 40 mm SL.; ) were consistently collected in almost every month of the year along the Honduran Caribbean coast.
Discussion
Our results indicate that the diet of D. monticola is dominated by aquatic insects and includes fish, nematodes, crustaceans, and detritus (). These results are comparable to other D. monticola dietary studies [Citation15,Citation17,Citation18,Citation20,Citation21]); however, here we conclude that D. monticola is a generalist omnivore [Citation17,Citation18] and is not a specialist insectivores [Citation21], or carnivores [Citation15] as previously suggested.
Although, the one-way ANOSIM results found significant differences between classes (, ). None of the diet items were exclusive to a specific group, instead the differences are more related to the relative abundance of items. For instance, the NMDS shows smaller-size D. monticola associated with detritus (negative NMDS axis 1 scores, ). The relative contribution of detritus in the smaller-size diet was 11% compared to 2% for the medium-size class and was not present in individuals of the larger-size class. As a result, detritus had the highest indicator value in the smaller-size size class (0.46 in smaller-size, 0.05 and 0.01 in the medium-size and large-size categories, respectively), suggesting that detritus is more important in the small-size fish diet. It is likely that small-size fish ingest large amounts of detritus targeting the epi or infauna therein while large-size fish may consume some detritus incidentally in feeding on larger benthic invertebrates. Along with detritus, the ISA found ephemeropterans and Lumbricidae as significant indicators. Gape limitation would prevent small-size fish from consuming these items, however their overall scarcity in large-size fish diets resulted in generally low indicator values (below 0.5), suggesting that they are weak indicators [Citation37].
Trophic studies in other mullet species (e.g. Liza ramada and L. aurata) using stable isotopes, have shown that young of the year and older mullet are both secondary consumers [Citation38], and at 30 mm (fork length), shift to grazing on benthic prey. Our results are consistent in showing smaller D. monticola feeding on detritus, suggesting that smaller-size D. monticola may be grazing in different sections of the water column. However, our feeding class categories were too broad to verify if smaller juveniles are actually using different resources than juveniles of larger size.
The reproductive season of D. monticola in the Honduran Caribbean coast has been suggested to occur from November to December [Citation15]. Our results indicate that juvenile (e.g. individuals < 50 mm SL) D. monticola are present throughout the year. Anderson [Citation39], suggested that if D. monticola spawn in either marine or brackish waters they will be four to six weeks of age before reaching 20 mm SL. Based on the above estimates, we conclude that D. monticola 20–30 mm SL sampled in March–June and October-November, along with 31–40 mm SL individuals sampled in February, April, May, July and November, could not have been spawned only in November and December [Citation15]. Our results are consistent with the results of Chicas [Citation25], in which the authors concluded D. monticola spawn year-round in Costa Rica after finding juveniles (e.g. < 50 mm SL) year-round in coastal tidal ponds. The presence of smaller individuals sampled throughout the year is consistent with D. monticola having a longer recruitment season in Honduras than previously thought.
Acknowledgments
We are grateful to two anonymous reviewers for providing helpful comments on an earlier draft of this manuscript. We thank the Honduran Instituto de Conservacion Forestal for issuing collection permits to WAM. In addition we thank the staff at University of Michigan Museum of Zoology (UMMZ), The Florida Museum of Natural History (UF), the Los Angeles County Museum of Natural History (LACM) and the United States National Museum (USNM) for loaning specimens. Collection trips to Honduras were possible for a World Wildlife Fund (WWF) and United States Agency for International Development (USAID) grants to WAM. Additional funding was provided by a Critical Environment Partnership Fund.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wainwright PC, Barton RA. Predicting patterns of prey use from morphology of fishes. Environ Biol Fish. 1995;44(1–3):97–113.
- Luczkovich JJ, Norton SF, Gilmore RG. The influence of oral anatomy on prey selection during the ontogeny of two percoid fishes, Lagodon rhomboides and centropomus undecimalis. Environ Biol Fish. 1995;44(1–3):79–95.
- Godin J-GJ. Daily patterns of feeding behavior, daily rations, and diets of juvenile pink Salmon (Oncorhynchus gorbuscha) in two marine bays of British Columbia. Can J Fisheries Aquat Sci. 1996;38(1):10–15.
- Grutter AS. Ontogenetic variation in the diet of the cleaner fish Labroides dimidiatus and its ecological consequences. Mar Ecol Prog Ser. 2000;197:241–246.
- Weliange WS, Amarasinghe US. Seasonality in dietary shifts in size-structured freshwater fish assemblages in three reservoirs of Sri Lanka. Environ Biol Fish. 2003;68(3):269–282.
- Smith WE, Kwak TJ. Otolith microchemistry of tropical diadromous fishes: spatial and migratory dynamics. J Fish Biol. 2014;84(4):913–928.
- Greenfield DW, Thomerson JE. Fishes of the continental waters of belize. Gainesville(FL).: University Press of Florida; 1997.
- Matamoros WA, Schaefer JF, Mickle P, et al. First record of agonostomus monticola (Family: Mugilidae) in Mississippi freshwaters with notes of its distribution in the Southern United States. Southeast Nat. 2009;13:39–42.
- McMahan CD, Matamoros WA, Álvarez Calderón FS, et al. Checklist of the Inland fishes of El Salvador. Zootaxa. 2013;3608(6):440–456.
- Villa J. Peces Nicaragüenses de Agua Dulce. Managua: Fondo de Promocio´n Cultural, Banco de Ame´rica; 1982.
- Bussing WA. Peces de Las Aguas Continentales de Costa Rica/Freshwater fishes of Costa Rica. San Jose: Universidad de Costa Rica; 2002.
- Miller RR, Minckley WL, Norris SM. Freshwater fishes of Mexico. Chicago(IL): The University of Chicago, Press; 2005.
- Kihn-Pineda PH, Cano EB, Morales A. Peces de las aguas interiores de Guatemala. In: Cano EB, editor. Biodiversidad de Guatemala. Guatemala: Universidad del Valle de Guatemala; 2006. p. 457–485.
- Matamoros WA, Schaefer JF, Kreiser BR. Annotated checklist of the freshwater fishes of continental and insular Honduras. Zootaxa. 2009;2307(1):1–38.
- Cruz G. Reproductive biology and feeding habits of Cuyamel, Joturus pichardi and tepemechin, agonostomus monticola (Piesces; Mugilidae) form Rio Palatano, Mosquitia, Hondruas. Bull Mar Sci. 1987;40:63–72.
- González Murcia S, and Alvarez FS. Your place, my place, distribution of agonostomus monticola and Sicydium multipunctatum in the Acahuapa watershed. Rev Mex Biodivers. 2018;89(3):854–864.
- Torres-Navarro C, and Lyons J. Diet of agonostomus monticola (Pisces: mugilidae) in the Río Ayuquila, Sierra de Manantlán Biosphere Reserve, Mexico. Revista de Biologia Tropical. 1999;4:1087–1092.
- Cotta-Ribeiro T, and Molina-Ureña H. Ontogenic changes in the feeding habits of the fishes Agonostomus monticola (Mugilidae) and Brycon behreae (Characidae), Térraba River, Costa Rica. Revista de Biologia Tropical. 2009;57:285–290.
- Eslava Eljaiek P, Díaz Vesga R. Reproduction de Joturus pichardi y agonostomus monticola (Mugiliformes: mugilidae) en ríos de la Sierra Nevada de Santa Marta, Colombia. Revista de Biologia Tropical. 2011;59(4):1717–1728.
- Phillip DAT. Reproduction and feeding of the mountain mullet, agonostomus monticola, in Trinidad, West Indies. Environ Biol Fish. 1993;37(1):47–55.
- Aiken KA. Reproduction, diet and population structure of the mountain mullet, agonostomus monticola, in Jamaica, West Indies. Environ Biol Fish. 1998;53(3):347–352.
- Erdman DS. Inland game fishes of Puerto Rico. San Juan: Department of Agriculture, Commonwealth of Puerto Rico; 1972.
- Erdman DS. Spawning patterns of fishes from the northeastern Caribbean. FAO Fish Rep. 1977;200:145–170.
- Corujo-Flores I. 1980. A study of fish populations in the Espiritu Santo River estuary.
- Chicas FA. Peces juveniles en una poza de marea, Reserva Forestal Terraba-Sierpe, Puntarenas, Costa Rica. Revista de Biologia Tropical. 2001;49:307–314.
- Bo T, Cammarata M, Candiotto A, et al. Trophic preferences of three allochthonous fishes in Bormida River (Alessandria, NW Italy). Hidrobiologica. 2012;22:195–200.
- Steward KW, Stark BP. Nymphs of North American Stonefly Genera (Plecoptera). Lanham (MD): Entomological Society of America; 1988.
- McCune B, Grace JB. 2002. Analysis of ecological communities.
- McCune B, Mefford MJ. 1999. PC-ORD –multivariate analysis of ecological data Version 4.25.
- Kennen JG, Chang M, Tracy BH. Effects of landscape change on fish assemblage structure in a rapidly growing metropolitan area in North Carolina. Am Fish Soc Symp. 2005;47:39–52.
- Dufrene M, Legendre P. Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr. 1997;67:345–366.
- Roberts ME, Taylor CM. Using community-level analyses to identify dietary patterns for species in space and time patterns for species in space and time. J Freshwater Ecol. 2008;23(4):519–528.
- Clarke KR, Warwick RM. Similarity-based testing for community pattern: the two-way layout with no replication. Mar Biol. 1994;176(1):167–176.
- R Core Team. 2018. R: a language and environment for statistical computing. https://www.r-project.org
- Oksanen J, Blanchet FG, Friendly M, et al. 2019. vegan: community ecology package. version 2.5-5. https://github.com/vegandevs/vegan
- De Cáceres M, Jansen F, Dell N. 2020. indicspecies: relationship between species and groups of sites. https://vegmod.ctfc.cat/software/indicspecies
- Punchi-manage R, Getzin S, Wiegand T, et al. Effects of topography on structuring local species assemblages in a Sri Lankan mixed dipterocarp forest. J Ecol. 2013;101(1):149–160.
- Lebreton B, Richard P, Guillou G, et al. Trophic shift in young-of-the-year Mugilidae during salt-marsh colonization. J Fish Biol. 2013;82(4):1297–1307.
- Anderson WW. Larval forms of the frs-water mullet (Agonostomus monticola) from the open ocean off the Bahamas and south Atlantic coast of the United States. Fisheriy Bull. 1957;57:415–425.