ABSTRACT
Bon Accord Lagoon (BAL), Tobago is home to a Thalassia testudinum (K.D. Koenig, 1805) dominated seagrass community and polychaetes usually show an affinity to seagrass beds compared to other environments. The objectives of this study were to investigate the polychaete community associated with the Thalassia beds in BAL, determine seasonal variation, and compare with the neighbouring hard bottom polychaete community in Mt. Irvine Bay (MIB). Benthic macroinvertebrates were sampled using a 15 cm diameter corer at a depth of 10 cm. Six stations were sampled during the wet and dry seasons of 2018. Samples were sieved in the field using a 0. 5 mm mesh screen. They were stained and preserved with a 10% formalin-seawater mixture, sorted and macrofaunal species identified to the lowest possible taxonomic level. Thirty – one polychaete families were ranked according to trophic categories as described by Fauchald and Jumars (1979) and updated where possible. Average family density for the dry season was recorded at 206.89 ± 307.53 ind/m2 and 129.55 ± 227.23 ind/m2 for the wet. Maldanidae and Syllidae recorded highest densities for dry and wet seasons respectively. Deposit feeders were the largest trophic group represented across both seasons in the BAL with Maldanidae being the most dominant. Carnivorous polychaetes dominated MIB with Syllidae being the most dominant. BAL showed higher diversity and richness compared to MIB. Based on functional traits of the community the environment at BAL can be regarded as healthy. This study establishes a much-needed baseline for future research and management of marine biodiversity in southwest Tobago.
Introduction
Seagrass systems are generally associated with increased richness of benthic communities in terms of species and individuals [Citation1,Citation2]. The structural complexity created by the seagrass canopy provides refuge and breeding grounds for numerous invertebrates and fishes [Citation3–7] leading to increased species abundance and diversity compared to areas without seagrass vegetation [Citation4,Citation5,Citation8,Citation9]. Shoot density, leaf surface and biomass influence the composition and abundance of fauna associated with seagrass beds [Citation10,Citation11]. Polychaetes in particular thrive in seagrass beds [Citation12] such as the Thalassia testudinum (K.D. Koenig, 1805) dominated seagrass community found in Bon Accord Lagoon (BAL), Tobago and often have the highest species richness and abundance of all invertebrates found in seagrass beds [Citation4,Citation12–15].
While there are thriving seagrass beds in BAL, the majority of Tobago’s coastline is comprised of coral and rocky ecosystems which provide different habitats suitable for other polychaete taxa. Previous work has shown that the polychaete family Syllidae is highly successful in inhabiting hard bottom substrate habitats [Citation16], making their dominance in Tobago expected [Citation17].
In a review of the literature on polychaetes of the Caribbean Sea, Trinidad and Tobago was listed as one of the more species rich polychaete environments [Citation17]. The most species rich family was Syllidae with 159 recorded species followed by Eunicidae (98), Nereididae (73), Polynoidae (66), Sabellidae (64), Serpulidae (64), Terebellidae (62), and Spionidae (56). Dean (2012) [Citation17] identified 1,205 species for the Caribbean Sea which is more than double the 546 described by Gobin in Miloslavich et al., (2010) [Citation18]. This suggests that there is still plenty to learn about the regional fauna, and highlights the importance of these organisms to regional marine biodiversity.
Most of the polychaete studies conducted in Trinidad and Tobago have been off the west coast of Trinidad, and in close proximity to the Point Lisas Industrial Estate [Citation19]. Gobin (1990) [Citation20] created a polychaete checklist from a number of soft-bottom sediment benthic surveys carried out primarily on the west coast of Trinidad. Agard (1984) [Citation21] and Agard et al. (1993) [Citation22] described the soft-bottom macrobenthic intertidal communities on the northwest and southwest peninsulas of Trinidad. Kanhai & Juman (2018) [Citation23] investigated benthic communities within the Caroni Swamp. This study indicated that anthropogenic pollutants influenced the polychaete communities at various sites. Another recent study described the polychaete population on Trinidad’s southeastern tip at the Port of Galeota [Citation24] which found the benthic communities to be relatively unaffected by human activities.
Few attempts have been made to describe the polychaete populations in Tobago. An unpublished study entitled “An Investigation of the Benthic Macrofauna of Thalassia beds of Trinidad and Tobago” by Daniel in 2010 found 130 different benthic macrofaunal species with 20 polychaete families accounting for 42% of all species represented in BAL. Gobin (2010) [Citation25] described the hard bottom polychaetes found at MIB, Tobago as part of a wider national study.
In addition to the insights polychaetes can provide on community distribution patterns [Citation26], trophic structure analysis is also useful in assessment of the distribution of benthic communities and as a tool for ecological and environmental benthic studies [Citation27]. Taxonomic studies may not always highlight all relevant information that can be uncovered by taxonomic groupings [Citation28–30]. Multiple feeding modes of polychaetes enables them to provide information on trophic structure of macrobenthic communities [Citation31].
To establish a much-needed baseline for future research and management interventions in BAL given that the seagrass beds are being progressively disturbed by coastal development and other human activities [Citation32–35] we undertook to survey the characterised the polychaete communities of Tobago and highlight their trophic groups. This work also adds new information to the polychaete records of species found in Trinidad and Tobago. The objectives of this study were: (i) Assess the polychaete community in Southwest Tobago and group them into trophic categories (ii) Compare the polychaete community across wet and dry seasons in BAL (iii) Compare the polychaete communities between a seagrass dominated site and a hard bottom unvegetated site (iv) Establish the status of the environment in BAL based on the functional traits of the polychaete community and (v) Establish a baseline for future environmental assessment of the benthic environment in southwest Tobago.
Methodology
Site Description
Trinidad and Tobago (T&T) is located on the continental shelf of South America, and immediately adjacent to the outflow of the Orinoco River. The country is less exposed to tropical storms and hurricanes than most of the Caribbean nations because of its southerly location, and has a tropical climate with two distinct seasons [Citation36]. The dry season occurs between January and April, while the wet season extends from June to November. May and December are considered transitional months between the two seasons. Its marine ecosystems are influenced by discharge from South American Rivers, mainly the Orinoco River, while its terrestrial biota is largely South American.
Bon Accord Lagoon (BAL)
BAL lies in the southwestern region of Tobago at latitude 11°10ʹand 11°11ʹN and longitude, 60°49ʹ and 60°51ʹ E ().
Figure 1. The location of seagrass sample sites in Bon Accord Lagoon (BAL) with sampling stations labelled A-F and the hard bottom sample site taken from Gobin [Citation25] in Mt. Irvine Bay (MIB).
![Figure 1. The location of seagrass sample sites in Bon Accord Lagoon (BAL) with sampling stations labelled A-F and the hard bottom sample site taken from Gobin [Citation25] in Mt. Irvine Bay (MIB).](/cms/asset/4dfb7ce0-1695-4890-8a53-71e1027cdc35/tneo_a_2021051_f0001_b.gif)
The seagrass community in BAL covers an area of approximately 102 ha.2 and extends in some places to a depth of approximately 6 m [Citation37]. The seagrass beds are found in three main areas: north of Sheebird’s Point in the back reef area, south of Sheebird’s Point and in the Lagoon extending from east of Gibson’s jetty straight toward the southeastern end Pigeon Point [Citation37]. Turtle grass, T. testudinum is the dominant seagrass species in the Lagoon while smaller areas of Halophila decipiens (Ostenfeld, 1902) and Halodule wrightii (Ascherson, 1868) are interspersed among the turtle grass [Citation37]. The dominant algal genera are Acanthophera, Padina, Bryopsis, Dictyota, Halimeda and Caulerpa while corals such as Porites porites (Pallas, 1766) also occur in the lagoon among the seagrass beds [Citation37]. Grain size analysis performed at sampled stations by Daniel (2010, unpublished) showed the substrate to be comprised of gravelly sand (GS) with a sand range of 85.06–94.90 %; gravel range of 5.00–13.75 % and a mud range of 0.1–2.31 %. Salinity averaged 36.20‰ for the dry season and decreased to 29.02‰ for the wet. Temperature averaged 27.10 °C for the dry season and increased to 29.02 °C for the wet. Dissolved oxygen averaged 6.87 mg/l for the dry season, and 5.84 mg/l for the wet. pH averaged 7.77 for the dry season and 7.95 for the wet.
Mt. Irvine Bay (MIB)
MIB is located at 60°79ʹ E 11°18ʹN in the southwestern region of Tobago ().
The bay consists of two sections separated by an outcrop of coral-algal limestone with one section extending east-west and the other north-south. The east-west section of the bay is 550 m long with a sea wall protecting the road. Rough seas are experienced between November and April at this section of the bay. Waves of moderate energy approach from the northwest with 30 cm average height for the dry season and 16 cm for the wet season. Weak longshore currents flow to the southwest at an average speed of 6 cm/s.
The sediment is comprised of light-brown, medium-grained sand composed mainly of quartz. The north-south section of the beach is 600 m long and usually calmer for sea bathing [Citation38]. There were no available environmental parameters for MIB.
Sampling Methods
Field Sampling
Benthic macrofauna were collected among seagrass beds within the BAL during two sampling periods, dry and wet season 2018 (March and September, respectively) using a hand held PVC corer. The National Geography in Shore Areas (NaGISA) protocol [Citation39] for sampling seagrass infauna was used which involved immersing a 15 cm diameter corer to a depth of 10 cm and using a rubber bung at the top of the device to create a vacuum.
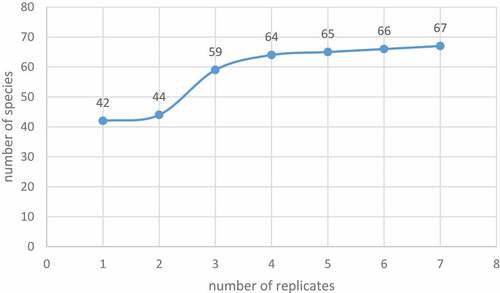
A pre-sampling experiment was conducted to determine the number of replicates required to accurately determine benthic composition per site. The cumulative frequency curve suggested five replicates would be adequate ().
Core samples were sieved in the field using a 0.5 mm mesh screen. Samples were stained with Rose Bengal and preserved with a 10% formalin-seawater mixture. The benthic organisms studied were polychaetes, which were sorted and identified to the lowest possible taxonomic level using the key: The Polychaete worms [Citation40]. Accepted taxa were checked at World Register of Marine Species www.marinespecies.org [Citation41].
Seagrass shoots were sampled using the CARICOMP Methodology [Citation42]. At each seagrass location, six 10 × 20 cm (0.2 m2) PVC quadrats were randomly deployed. All the seagrass shoots in each of the quadrats were counted.
Analysis of results
Species constancy and the Biological Dominance Index were calculated based on McCloskey (1970) [Citation43]. Shannon’s species diversity H’Σ− = i2iplogp, and evenness E = H’/log2S [Citation44] were calculated using PRIMER 6 along with Analysis of Similarity (ANOSIM) and cluster analysis [Citation45]. These indices are commonly used when conducting benthic population studies. To achieve homogeneity of variances, densities were transformed to log10 (n + 1). One-way ANOVA [Citation46] and Paired-t tests [Citation47] were performed to ascertain differences between the seasons sampled.
Comparison with Gobin (2010)
The macrobenthic population at MIB, Tobago recorded in Gobin (2010) [Citation25] was compared with the soft bottom benthic seagrass communities in the present study. These hard bottom communities were sampled using five Artificial Substrate Units (ASUs) () as part of a wider study of hard bottom benthic communities throughout varying sites in Trinidad and Tobago.
Figure 3. Artificial Substrate unit used by Gobin (2010) to investigate the hardbottom communities of MIB.
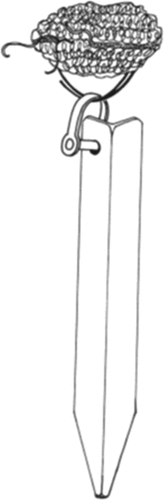
The area sampled by the ASUs was not given. This meant it was not possible to calculate abundance per square metre for comparisons with the present study. Trophic categories of the polychaetes sampled in MIB and BAL were established using the framework provided by Fauchald & Jumars [Citation48], and later updated by Jumars et al. [Citation49]
Results
Seasonal comparison
Polychaete families, abundance per square metre, biological index of dominance, trophic categories and constancy of occurrence in BAL are presented in . The full species table is presented in Appendix A1.
Table 1. Polychaete families collected in Bon Accord Lagoon, (Total BAL) Tobago, Trophic Category (TC), (D) density average (ind/m2), Standard Deviation (SD), Biological Index of Dominance (Bid), DF: Deposit Feeder, C: Carnivore, FF: filter feeder, O: Omnivore and family counts collected at Mt.Irvine Bay (MIB) For full species table please see Appendix 1
One thousand and twelve (1,012) individuals (56,226 ind/m2) belonging to 30 families and 68 species (51 to the generic level and 17 to the specific level), were collected across both seasons in BAL. Six hundred and sixty-five (665) individuals were found during the dry season and 347 found during the wet season. Average family density for the dry season was recorded at 206.89 ± 307.53 ind/m2. This decreased during the wet season to 129.55 ± 227.23 ind/m2. Maldanidae recorded the highest density during the dry season (1241.33 ± 20.72 ind/m2) while Syllidae recorded the highest density during the wet season (683.20 ± 23.97 ind/m2).
Deposit feeders were the largest trophic group represented across both seasons with 422 and 195 individuals recorded for dry and wet seasons, respectively ().
Table 2. Number of polychaete individuals belonging to different trophic categories found in stations A – F. (DF) deposit feeders, (O) omnivores, (C) carnivores, (FF) filter feeders
This was followed by carnivores (172 dry; 95 wet), omnivores (67 dry; 52 wet) and filter feeders (4, dry; 5, wet). Highest abundance was recorded at Station F for the dry season while B had the lowest. Wet season abundance was highest at Station A and lowest at F ().
Table 3. Species Abundance, Richness, Evenness and Shannon’s Diversity for sampled stations across the wet and dry seasons in Bon Accord Lagoon
The paired t-test comparing polychaete populations in the wet and dry seasons gave a P-value of greater than 0.05 failing to reject the null hypothesis, and finding no significant difference between the seasons (Paired T-test, P > 0.05). (See Appendix for values).
Species richness was highest at Station F and lowest at Station B for the dry season whereas during the wet season, B was highest and F was lowest (). There was a significant difference between the seasons (Paired T-test, P > 0.05).
Species diversity was highest at Station F and lowest at Station B for the dry season. Station B was highest and F was lowest for the wet season (). Species diversity showed significant difference between seasons (Paired T-test, P > 0.05).
For the dry season evenness was highest at Station C and lowest at Station E. Station C recorded the highest value for the wet season again while F recorded the lowest (). There was a significant difference between the seasons for evenness (Paired T-test, P > 0.05).
The highest number of deposit feeders were recorded at Station F for the dry season and this fell to 0 during the wet season (). Station F is located on the eastern side of the lagoon near Pigeon Point, a sandy beach.
Figure 4. Polychaete individuals belonging to different trophic categories found in stations A – F where “d” denotes dry season and “w” denotes wet season.
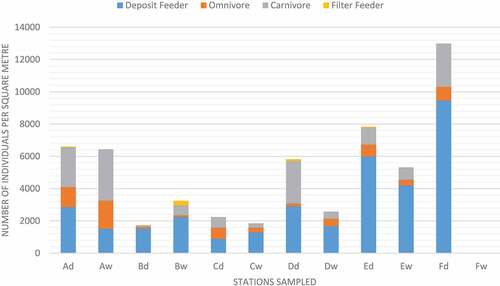
The largest number of omnivores (31) and carnivores (57) were recorded at station A during the wet season. Station B recorded the most filter feeders during the wet season (5). Station A and B are located north of Sheerbird’s Point in the back reef area.
Thalassia shoot density ranged from 270 to 340 shoots/m2 for the dry season and 325 to 400 shoots/m2 for the wet season. A significant positive Pearson Correlation was found between the number of Thalassia shoots and Shannon’s diversity index for polychaetes (r = 0.13, P < 0.01) (See Table Appendix 2 for values). Thalassia shoot density and species richness also showed a positive Pearson Correlation (r = 0.43, P < 0.01); species richness and Shannon’s diversity (r = 0.82, P < 0.05) were also positively correlated across both seasons. During the dry season, Station E recorded the highest shoot density with a value of 408 shoots/m2, followed by station D (340) and Station C (270). For the wet season station D recorded the highest shoot density with a value of 400 shoots/m2, followed by Station E (341) and Station C (325).
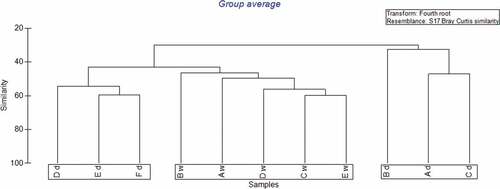
Cluster analysis produced three groups (). Stations A, B and C for the dry season produced one group with low similarity levels and the remaining stations D, E and F producing another group with similarity of greater than 50%. All the wet season stations with the exception of F, which was excluded, produced one group with similarity levels of less than 50%.
Polychaete species composition in BAL and MIB
The polychaete community in BAL was more diverse than the community in MIB. In BAL, 68 species from 30 families were recorded while at MIB, the hard bottom polychaete population comprised 39 species from 12 families (). The Shannon’s diversity index was 2.53 for the soft bottom seagrass community and 1.44 for the hard bottom polychaete community. Species evenness for the hard bottom community was calculated as 0.58 whereas for the seagrass community it was 0.74. The seagrass population sampled with the corer comprised 1,012 individuals whereas the ASUs counted 401 individual polychaetes in the hard bottom substrate (). Fifteen species were found in common at both sites.
Syllidae was the most dominant family in the hard bottom population with 216 individuals, accounting for 55% of all species found. This was followed by Serpulidae (94 individuals, 24%), and Sabellidae (29 individuals, 8%). The most dominant family in the seagrass bed was the Maldanidae with 218 individuals, accounting for 22% of the population followed by Syllidae (145 individuals, 14%) and Nereididae (99 individuals, 10%).
Trophic categories
For the seagrass beds, deposit feeders were the most represented trophic group accounting for 61% of all individuals recorded (616 individuals) () This was followed by carnivores at 26% (268 individuals), omnivores at 12% (119 individuals) and filter feeders at 1% (9 individuals) (). For the hard bottom communities, carnivores were the most represented trophic group accounting for 61% (246 individuals) and dominated by the Syllidae family followed by deposit feeders with 28% (47 individuals) and herbivores at 8% (14 individuals) ().
Figure 6. Number of polychaete individuals and their trophic categories found in Bon Accord Lagoon (BAL) and Mt. Irvine Bay (MIB).
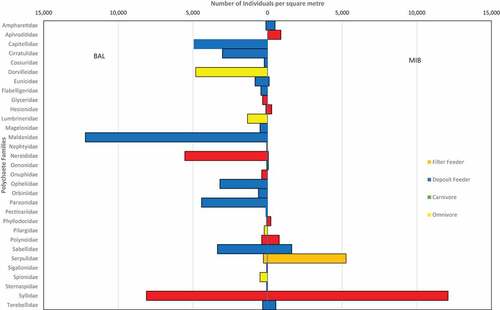
Of the five most abundant species at the hard bottom site, three were carnivores: Pinosyllis sp.1, Pontogenia sp.1 and Syllis sp1., one was a filter feeder Pseudovermilia occidentalis (McIntosh, 1885) and one was a deposit feeder Paradialychone americana (Day, 1973). Of the five most abundant species at the seagrass site, two were deposit feeders: Maldane sp, and Capitella capitata (Fabricius, 1780) two were carnivores Syllis sp.1 and Nereididae sp1, and one was an omnivore, Dorvillea sp.1.
Fifteen species from 10 families were common to both sites: Ampharete sp 1., Eunice websteri Fauchald, 1969, Nereididae sp1, Arabella iricolor (Montagu, 1804), Mystides borealis Théel, 1879, Harmothoe sp1., Lepidonotus sublevis Verrill, 1873, Bispira melanostigma (Schmarda, 1861), Sabella sp1., Sabella sp2., P. occidentalis, Syllis prolifera Krohn, 1852, Syllis Sp1, Trypanosyllis sp 1 and Terebellidae Sp 1. (8 Carnivores, 6 Deposit feeders and 1 Filter feeder).
A two-tailed t-test conducted between the families at both sites failed to reject the null hypothesis, indicating that there is no difference between families at both sites (P > 0.05). ANOSIM conducted for substrate type also confirmed this finding (P = 0.1, R = 0.46).
Community analysis was performed for the polychaete families found at both sites. A description of the functional traits and whether these families are indicators of pollution can be seen in .
Table 4. Functional traits and pollution characteristics of the polychaete families found at BAL and MIB
Discussion
Of the five most abundant polychaete species in the dry season in BAL, two were deposit feeders (C. capitata and Maldane sp.1.), one was a carnivore (Syllis sp.1) and two were omnivores (Dorvillea sp.1 and Lumbrineris sp.1). This is similar to a study conducted in the Gulf of Mexico which reported Capitellidae and Maldanidae among the dominant polychaete families, and the majority of polychaete families being either suspension or deposit feeders from soft-bottom seagrass communities [Citation56]. Capitellidae can be used as indicators of organic enrichment [Citation57] along with the presence of Nereididae [Citation57]. Capitellidae also impact the biogeochemistry by burrowing and ingesting of sediments which influence particle distribution [Citation58]. The absence of Maldanidae on the other hand is normally an indicator of poor environmental quality [Citation59] ie. Their presence indicates good environmental quality.
The wet season showed dominance by deposit feeders with four deposit feeders (Maldane sp.1, Armandia sp.1, Euclymene sp.1 and Aricidea sp.1) being the most abundant, with only one omnivore (Dorvillea sp.1(Appendix A1).
Trophic structure of polychaete communities in the Caribbean do not show much variation from one season to the next. Studies conducted in Colombia at a higher frequency than the present study (3 to 4 times per year) [Citation60,Citation61] showed no significant changes between the dry and wet periods indicating no seasonal variability. The results of this study are consistent with other studies conducted in tropical soft-bottom and rocky shore communities, where neither number of taxa nor their abundances significantly changed during the year [Citation62,Citation63].
The hard bottom site at MIB was sampled using an entirely different methodology compared to BAL therefore focusing on abundance numbers could potentially be misleading. However, increased productivity, abundance and biodiversity between seagrass beds and unvegetated sites is well documented [Citation5,Citation64–69]. Seagrass beds support higher abundance and richness of faunal assemblages when compared to unvegetated sites [Citation70]. It is therefore reasonable to suggest the polychaete assemblages at BAL are higher in abundance and diversity when compared to those found at MIB. The carnivorous polychaete family, Aphroditidae, was the only family not also found at the BAL study site. It is possible hard bottom seafloors would favour carnivorous polychaetes as prey are unable to burrow into seafloor to escape.
The significant correlation found between Thalassia shoot density and Shannon’s Diversity and species richness supports the view that seagrass beds harbour a greater number and diversity of species due to increased shelter, food resources and protection from predation [Citation10,Citation11]. Two polychaete families in particular are found associated with seagrasses. The family Eunicidae are found among Thalassia beds [Citation58] and the family Pectinariidae are found associated with the shoots of Zostera [Citation58].
Gobin (2010) [Citation25] stated that the hard bottom polychaete community was typical of hard bottom substrates found in New Zealand and the southwest coast of England [Citation71]. The dominance, diversity and abundance of Syllidae among hard bottom, shallow water substrates is well-documented [Citation72–75]. It has been suggested that Syllidae will generally dominate in any 3-dimensional environment where adequate microhabitats are present for settlement [Citation76,Citation77]. Syllidae will also be the first to colonise artificial substrate panels similar to those used by Gobin (2010) [Citation25], however their long-term viability on those panels in terms of seasonal and environmental variation remains to be tested [Citation78]. The composition of this population in general is in keeping with that found by Dean (2012) [Citation17] for the Caribbean where Syllidae were the most dominant polychaete family along with a high number of Serpulidae. Syllidae are normally indicators of good environmental quality as their numbers tend to decrease with increasing organic material [Citation59].
Given the high amount of coral reefs located around the coastlines of Tobago [Citation79], the presence of large numbers of Serpulidae is unsurprising. Serpulidae can sometimes be regarded as indicators of stressed conditions, typically hypoxia [Citation59]. In this case, the presence of Serpulidae appears to be more of a function of the substrate rather than water quality as there are numerous other polychaete families which serve as indicators of good environmental quality such as Sabellidae [Citation59].
Sabellidae and Serpulidae comprised the main components of the filter-feeding group found at the hard bottom site in Tobago [Citation25]. The hard bottom community showed marked differences with respect to trophic categories when compared to the soft bottom seagrass site with Syllidae (carnivore), Sabellidae (deposit feeder) and Serpulidae (filter feeder) being the most dominant families.
The range in the number of polychaete species recorded in Thalassia beds from the Atlantic American coast in previous studies is relatively wide, varying from 21 to 51 species, with an average of 35.71 ± 10.71; the range of densities is even wider, between 60 and 4,409 ind/m2 (X = 1036.43 ± 1554.99) [Citation80]. The number of species found at MIB (41) falls within this range. The number of polychaete species found at BAL- 68 is higher than this range but similar to communities sampled along the coastline in Rio de Janeiro Brazil [Citation81] where 68 species were found among the Halodule wrightii (Ascherson, 1868) dominated seagrass beds. Species richness and average density values obtained in BAL are within the mean of the respective interval provided by Arana & Díaz (2006) [Citation80].
Bell et al., (1993) [Citation82] reported that polychaete seagrass populations studied in Tampa Bay, Florida were dominated by deposit-feeders. Omena & Creed (2004) [Citation81] also reported H. wrightii seagrass beds in Rio de Janeiro Brazil were dominated by the deposit feeder Magelona papillicornis (Müller, 1858). Similar results were reported by Wan Shi et al., (2014) [Citation83] in Johor, Malaysia where seagrass beds sampled were dominated by deposit-feeding polychaete families Spionidae and Capitellidae, as well as the carnivorous Glyceridae. Deposit- feeders were the most dominant trophic group accounting for 60% of all polychaetes followed by carnivores at 25% abundance in BAL.
Conclusion
This is the first study that describes the polychaete communities in BAL, Tobago. BAL was dominated by deposit-feeding polychaetes across both seasons while MIB was dominated by carnivorous polychaetes. The different in substrate type, namely, sandy vs hard bottom can explain this phenomenon as deposit feeders will be unable to feed in hard bottom conditions which would favour carnivorous polychaetes. Both sites can be characterised as being in good environmental standing as several polychaete families, characteristic of good environmental conditions are present. Both populations are typical of their characteristic substrate type. This study provides an important baseline, which can be used in the future to assess environmental quality and biodiversity changes in these areas.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abbott IA. Biology of Seagrasses. A treatise on the biology of seagrasses with special reference to the Australian Region. Aquatic Botany. 1990;36(3):297–298. Aquatic Botany.
- Tanner JE. Edge effects on fauna in fragmented seagrass meadows. Austral Ecol. 2005;30(2):210–218.
- Den Hartog C. The structural aspect in the ecology of sea-grass communities. Helgoländer Wissenschaftliche Meeresuntersuchungen. 1967. DOI:10.1007/BF01618658
- Lewis FG, Stoner AW. Distribution of macrofauna within seagrass beds: an explanation for patterns of abundance. Bull Mar Sci. 1983;30(3):537–551.
- Orth RJ, Heck KL, van Montfrans J. Faunal Communities in Seagrass Beds: a Review of the Influence of Plant Structure and Prey Characteristics on Predator: prey Relationships. Estuaries. 2006. DOI:10.2307/1351618
- Whanpetch N, Nakaoka M, Mukai H, et al. Temporal changes in benthic communities of seagrass beds impacted by a tsunami in the Andaman Sea, Thailand. Estuar Coast Shelf Sci. 2010;87(2):246–252.
- Thomson ACG, York PH, Smith TM, et al. Response to “Comment on ‘Seagrass Viviparous Propagules as a Potential Long-Distance Dispersal Mechanism’by A. C. G. Thomson et al.”. Estuaries Coasts. 2016;39(3):875–876.
- Stoner AW. The role of seagrass biomass in the organization of benthic macrofaunal assemblages. Bull Mar Sci. 1980;30(3):537–551. Retrieved fromhttp://www.ingentaconnect.com.ezproxy.library.dal.ca/search/download?pub=infobike://umrsmas/bullmar/1980/00000030/00000003/art00001&mimetype=application/pdf
- Alsaffar Z, Pearman JK, Cúrdia J, et al. The role of seagrass vegetation and local environmental conditions in shaping benthic bacterial and macroinvertebrate communities in a tropical coastal lagoon. Sci Rep. 2020;10(1):1–17.
- Hall MO, Bell SS. Response of small motile epifauna to complexity of epiphytic algae on seagrass blades. J Mar Res. 1988;46(3):613–630.
- Bostrom C, Bonsdorff E. Zoobenthic community establishment and habitat complexity - The importance of seagrass shoot-density, morphology and physical disturbance for faunal recruitment. Marine Ecology Progress Series. 2000;205:123–138. Marine Ecology Progress Series
- Box A, Martin D, Deudero S. Changes in seagrass polychaete assemblages after invasion by Caulerpa racemosa var. cylindracea (Chlorophyta: caulerpales): community structure, trophic guilds and taxonomic distinctness. Sci Mar. 2010;74(2):317–329.
- O’Gower AK, Wacasey JW. Animal communities associated with Thalassia, Diplanthera, and sand beds in Biscayne Bay I. Analysis of communities in relation to water movements. Bull Mar Sci. 1967;17(1):175–210.
- Hutchings P. Biodiversity and functioning of polychaetes in benthic sediments. Biodivers Conservat. 1998;7(9):1133–1145.
- Labrune C, Grémare A, Amouroux JM, et al. Structure and diversity of shallow soft-bottom benthic macrofauna in the Gulf of Lions (NW Mediterranean). Helgol Mar Res. 2008;62(3):201–214.
- Musco L. Ecology and diversity of Mediterranean hard-bottom Syllidae (Annelida): a community-level approach. Marine Ecology Progress Series. 2012;461:107–119. Marine Ecology Progress Series
- Dean HK. A literature review of the Polychaeta of the Caribbean Sea. Zootaxa. 2012;3596(1):1. Zootaxa.
- Miloslavich P, Díaz JM, Klein E, et al. Marine biodiversity in the Caribbean: regional estimates and distribution patterns. PLoS ONE. 2010;5(8). DOI:10.1371/journal.pone.0011916
- Gobin J 1988. The polychaete macrofauna near a large Industrial Complex at Point Lisas, Gulf of Paria, Trinidad. M.Phil. Thesis, University of the West Indies, St. Augustine, Trinidad and Tobago
- Gobin J. A checklist of marine polychaetous annelids (Polychaeta) for the Gulf of Paria, Trinidad, West Indies. Caribb Mar Stud. 1990;1:37–47.
- Agard JB 1984. A Baseline survey of the effects of Pollution on the benthos of the nearsgore Diego-Martin to Port-Of-Spain coastal area. I.M.A. Technical Report, Institute of Marine Affairs, Chaguaramas, Trinidad, West Indies.
- Agard JBR, Warwick RM, Gobin J. Analysis of marine macrobenthic community structure in relation to pollution, natural oil seepage and seasonal disturbance in a tropical environment (Trinidad, West Indies). Mar Ecol Prog Ser. 1993;92:233–243.
- Kanhai A, Juman R. The effect of seasonal and human pressure on macrobenthic fauna in the Caroni Swamp Ramsar Site, Trinidad And Tobago. Revista de Biologia Tropical. 2018;66(3):1101.
- Kanhai A. Macrobenthic Baseline Survey of the Port of Point Galeota, Southeast Trinidad. Chaguaramas: Institute of Marine Affairs; 2018.
- Gobin JF. Free-living marine polychaetes (Annelida) inhabiting hard-bottom substrates in Trinidad And Tobago, West Indies. Revista de Biologia Tropical. 2010. DOI:10.15517/rbt.v58i1.5200
- Giangrande A, Licciano M, Musco L. Polychaetes as environmental indicators revisited. Mar Pollut Bull. 2005;50(11):1153–1162.
- Castanedo N, Hernández Alcántara P, Solís-Weiss V, et al. Distribution of polychaete feeding guilds in sedimentary environments of the Campeche Bank, Southern Gulf Of Mexico. Helgoland Marine Research. 2012. doi:10.1007/s10152-011-0283-y
- Cheung SG, Lam NWY, Wu RSS, et al. Spatio-temporal changes of marine macrobenthic community in sub-tropical waters upon recovery from eutrophication. II. Life-history traits and feeding guilds of polychaete community. Mar Pollut Bull. 2008;56(2):297–307.
- Pagliosa PR. Another diet of worms: the applicability of polychaete feeding guilds as a useful conceptual framework and biological variable. Marine Ecology. 2005;26(3–4):246–254. Marine Ecology
- Shuai X, Bailey-Brock JH, Lin DT. Spatio-temporal changes in trophic categories of infaunal polychaetes near the four wastewater ocean outfalls on Oahu, Hawaii. Water Res. 2014;58:38–49.
- Bianchi N, Morri C. Policheti come descrittori della strutura trofica degli ecosistemi marini. Ocbalia. 1985;11:203–214.
- Kjerfve B, G.m.e. P, Gardner L, et al. Morphodynamics of muddy environments along the Atlantic coasts of North and South America. In: Healy T, Wang Y, Healy J-A, editors. Muddy Coasts of the World: processes, Deposits and Functions. N.Y: Elsevier; 2002. p. 479–532.
- Van Tussenbroek BI, Cortés J, Collin R, et al. Caribbean-wide, long-term study of seagrass beds reveals local variations, shifts in community structure and occasional collapse. PLoS ONE. 2014;9(3):e90600. PLoS ONE
- Bonanno G, Orlando-Bonaca M. Marine plastics: what risks and policies exist for seagrass ecosystems in the Plasticene? Mar Pollut Bull. 2020;158:111425.
- Duarte B, Martins I, Rosa R, et al. Climate change impacts on seagrass meadows and macroalgal forests: an integrative perspective on acclimation and adaptation potential. Front Mar Sci. 2018. DOI:10.3389/fmars.2018.00190
- Henry E. Climate variability and climate change - the role of small Island states, for instance Trinidad And Tobago . In Trinidad And Tobago Climate Change document. Trinidad and Tobago: Meteorological Services; 1990. p. 20.
- Juman R. Seagrass monitoring programme for Trinidad And Tobago 2002-2004. Chaguaramas, Trinidad: Institute of Marine Affairs; 2006.
- Institute of Marine Affairs. A Guide to Beaches and Bays of Trinidad And Tobago. Pt. Lisas, Trinidad: Yara Trinidad Limited; 2004.
- Rigby R, Iken K, Kato T (2007). Sampling biodiversity in coastal communities NaGISA protocols for seagrasses and macroalgal habitats. A NAGISA HANDBOOK Sampling Biodiversity in Coastal Communities - NaGISA Protocols for Seagrass and Macroalgal Habitats.
- Fauchald K. Polychaete worms. Sci Ser. 1976;63:107–111.
- WoRMS Editorial Board. (2020). World Register of Marine Species. www.marinespecies.org. Date accessed: 9/3/2020
- CARICOMP. CARICOMP Methods Manual- Level I. Manual of methods for Mapping and Monitoring of Physical and Biological Parameters in the Coastal Zone of the Caribbean. UWI Mona Kingston Jamaica: CARICOMP Data Management Centre; 2001.
- McCloskey LR. The Dynamics of the Community Associated with a Marine Scleractinian Coral. Internationale Revue Der Gesamten Hydrobiologie Und Hydrographie. 1970;55(1):13–81.
- Pielou EC. The measurement of diversity in different types of biological collections. J Theor Biol. 1966;13:131–144.
- Clarke KR, Gorley R, Somerfield P, et al. Change in marine communities: an approach to statistical analysis and interpretation. 3rd ed. Plymouth UK: PRIMER-E; 2014.
- Lury DA, Fisher RA. Statistical Methods for Research Workers. The Statistician. 1972;21(3):229. The Statistician
- Hsu H, Lachenbruch PA. Paired t Test. Wiley Encycloped Clin Trial. 2008. DOI:10.1002/9780471462422.eoct969
- Fauchald K, Jumars PA. The diet of worms: a study of polychaete feeding guilds. Oceanograph Mar Biol;1979. DOI:10.12691/marine-1-1-6 An Annual Review
- Jumars PA, Dorgan KM, Lindsay SM. Diet of Worms Emended: an Update of Polychaete Feeding Guilds. Ann Rev Mar Sci. 2015;7(1):497–520.
- Law CJ, Dorgan KM, Rouse GW. Relating divergence in polychaete musculature to different burrowing behaviors: a study using opheliidae (Annelida). J Morphol. 2014;275(5):548–571.
- Department of Agriculture, Water and the Environment. Beesley , P.L., Ross, G.J.B, and Glasby , C.J. Fauna of Australia. Vol. 4A. Commonwealth of Australia: Australian Biological Resources Study/CSIRO Publishing; 2000.
- Day JH. A monograph on the Polychaeta of Southern Africa/By J.H. Day, &c. A monograph on the Polychaeta of Southern Africa/By J.H. Day, &c. London : BM(NH); 1967. DOI:10.5962/bhl.title.8596.
- http://www.annelida.net/nz/Polychaeta/Family/F-Hesionidae.htm Date accessed: 3/9/2021
- Anderson M, Greene J, Morse D, et al. Benthic Habitats. In: Greene J, Anderson M, and Odell J, et al., editors. The Northwest Atlantic Marine Ecoregional Assessment: species, Habitats and Ecosystems. Phase One. Boston M.A: The Nature Conservancy, Eastern U.S. Division. 2010;84–144.
- Martins R, Martín GS, Rodrigues AM, et al. On the diversity of the genus Pisione (Polychaeta, Pisionidae) along the Portuguese continental shelf, with a key to European species. Zootaxa. 2012;3450(1):12–22.
- Ward CH. Habitats and biota of the Gulf of Mexico: Before the Deepwater Horizon Oil Spill. New York: Springer; 2017.
- Pearson TH, Rosenberg R. Macrobenthic Succession in Relation to Organic Enrichment and Pollution of the Marine Environment. Oceanograph Mar Biol. 1987;16:229–311.
- Díaz-Castañeda V, Reish DJ. Polychaetes in Environmental Studies. Ann Mod Biol. 2009. DOI:10.1002/9780470455203.ch11
- Dean H. The use of polychaetes (Annelida) as indicator species of marine pollution: a review. Revista de Biología Tropical. 2008;56(4):11–38.
- Guzmán-Alvis AI, Carrasco FD. Influence of a tropical lagoon discharge and depth on the structure of adjacent shelf macroinfauna (Southern Caribbean). 2005;46(1):81–93.
- Guzmán Alvis AI, Lattig P, Ruiz JA. Spatial and temporal characterization of soft bottom polychaetes in a shallow tropical bay (Colombian Caribbean). Bull Mar Coast Res. 2016;35. DOI:10.25268/bimc.invemar.2006.35.0.214.
- Jackson JBC. The ecology of the molluscs of Thalassia communities, Jamaica, West Indies. II. Molluscan population variability along an environmental stress gradient. Mar Biol. 1972;14(4):304–337.
- Mccarthy SA, Laws EA, Estabrooks WA, et al. Intra-annual variability in Hawaiian shallow-water, soft-bottom macrobenthic communities adjacent to a eutrophic estuary. Estuar Coast Shelf Sci. 2000;50(2):245–258.
- Stoner A. The Role of Seagrass Biomass in the Organization of Benthic Macrofaunal Assemblages. Bulletin of Marine Science. 1980;30(3):537–551.
- Lewis FG, Stoner AW. Distribution of macrofauna within seagrass beds: an explanation for patterns of abundance. Bulletin of Marine Science. 1983;33(2):296–304.
- Bell J, Pollard D (1989). Ecology of fish assemblages and fisheries associated with seagrasses. In Biology of seagrasses: a treatise on the biology of seagrasses with special reference to the Australian region.
- Hemminga MA, Duarte CM. Seagrass Ecology. Cambridge, England: Cambridge University Press.2009.
- Park JM, Kwak SN. Seasonal and habitat structures of crustacean decapod assemblages associated with Zostera marina beds in northern Jinhae Bay, Korea. J Mar Biol Assoc UK. 2019;99(2):461–471.
- Park JM, Kwak SN, Riedel R. Crustacean Decapod Assemblage Associated with Seagrass (Zostera marina) Beds in Southern Waters of Korea. Diversity. 2020;12(3):89. Diversity
- Lazzari MA. Epibenthic fishes and decapod crustaceans in northern estuaries: a comparison of vegetated and unvegetated habitats in Maine. Estuaries. 2002;25(6):1210–1218.
- Gobin JF, Warwick RM. Geographical variation in species diversity: a comparison of marine polychaetes and nematodes. J Exp Mar Biol Ecol. 2006;330(1):234–244.
- Çinar ME, Ergen Z. Faunistic analysis of Syllidae (Annelida: polychaeta) from the Aegean Sea. Cahiers de Biologie Marine. 2002;43(2):171–178.
- Giangrande A, Licciano M, Pagliara P. The diversity of diets in Syllidae (Annelida: polychaeta). Cahiers de Biologie Marine. 2000;41:55–65.
- Musco L, Lepore E, Gherardi M, et al. Sperm ultrastructure of three Syllinae (Annelida, Phyllodocida) species with considerations on syllid phylogeny and Syllis vittata reproductive biology. Zoomorphology. 2010;129(2):133–139.
- Musco L. Ecology and diversity of Mediterranean hard-bottom Syllidae (Annelida): a community-level approach. Mar Ecol Prog Ser. 2012;461:107–119.
- Abbiati M, Bianchi CN, Casteli A. Polychaete Vertical Zonation along a Littoral Cliff in the Western Méditerranean. Marine Ecol. 1987;8(1):33–48.
- Giangrande A. Polychaete zonation and its relation to algal distribution down a vertical cliff in the western Mediterranean (Italy): a structural analysis. J Exp Mar Biol Ecol. 1988;120(3):263–276.
- Ba-Akdah MA, Satheesh S, Al-Sofyani AMA, et al. Taxonomy of some species of the genus Syllis (Annelida: syllidae: syllinae) from the Red Sea found among the first colonizers of an artificial substrate. Mar Biol Res. 2018;14(8):790–805.
- Mallela J, Parkinson R, Day O. An assessment of coral reefs in Tobago. Caribbean J Sci. 2010;46(1):83–87.
- Arana IL, Díaz OD. Polychaeta (Annelida) associated with Thalassia testudinum in the northeastern coastal waters of Venezuela. Revista de Biologia Tropical. 2006;54(3). 10.15517/RBT.V54I3.14076
- Omena E, Creed JC. Polychaete fauna of seagrass beds (Halodule wrightii Ascherson) along the coast of Rio de Janeiro (Southeast Brazil). Marine Ecol. 2004;25(4):273–288.
- Bell SS, Clements LAJ, Kurdziel J. Production in natural and restored seagrasses: a case study of a macrobenthic polychaete. Ecol Appl. 1993;3(4):610–621.
- Wan Shi G, Di Min L, Ghaffar MA, et al. (2014). Macrobenthos composition, distribution and abundance within sungai pulai estuary, Johor, Malaysia. In AIP Conference Proceedings 9-11 Apr, 2014 Selangor, Malaysia.1614:591
Appendix
Table A1. Families, species and trophic categories (TC) of polychaetes discovered in Tobago at a hard bottom side and at a seagrass bed site. Carnivores (C), Deposit Feeders (DF), Filter Feeders (FF), Herbivores (H), Omnivores (O)