ABSTRACT
The occurrence of Alsodes coppingeri is confirmed in Argentina for the first time, from Santa Cruz Province, close to the Lago del Desierto. Specimens of this species were identified according to external morphology and DNA sequences. These new records in Argentina are at the same latitude than the type locality (Puerto Río Frío, Chile) about 100 km eastwards in a straight-line, but at the opposite side of the Andes mountain range and the Southern Continental Ice Fields. Five localities from Chile (Caleta Tortel, Canal Michel, Laguna Caiquenes, Puerto Yungay, and Villa O’Higgins) are around 100 km north from our records, in a lower region of the Andes located between the Northern and Southern Continental Ice Fields. This region with discontinuous permanent ice sheet-cover may have acted as a corridor for amphibian species that are currently distributed on both sides of the Andes range.
Introduction
The temperate forests at the southern cone of South America present high levels of anuran endemisms, with moderate to low species richness, as can be seen in the Patagonia region [Citation1–4]. Recent studies discussed the diversity and phylogenetic relationships of endemic Patagonian anurans [e.g. Citation5–8]. These studies, together with additional updates on their distribution ranges, provide essential information for the implementation of conservation programs [Citation9–12]. However, there are still significant gaps regarding species distribution and taxonomy.
One of these problematic and poorly known taxa is Alsodes coppingeri (Günther 1881). This species was described into the genus Cacotus from Puerto Río Frío, Wellington Island, Chile. Soon afterwards it was named Borborocoetes coppingeri [Citation13] and later transferred to the genus Eupsophus by Codoceo [Citation14] and Capurro [Citation15] without justifications. Curiously, these last authors independently cited Puerto Montt (Chile) as the type locality of the species. Some morphological features were described for Eupsophus coppingeri, including observations on the holotype and specimens from different localities in Chile and Argentina [Citation16–20]. Since Lynch [Citation21], E. coppingeri was considered a junior synonym of Alsodes monticola Bell 1843 (the only known species of Alsodes at that moment), and a reassessment of its identity was overlooked for some decades. Later on, new information about the genus Alsodes at high latitudes was published. For instance, Díaz & Núñez [Citation22] reported some morphological larval and adult features for Alsodes verrucosus from Bahía White, Wellington Island and Formas et al. [Citation23] described Alsodes kaweshkari from Puerto Edén, in the same Island. Formas et al. [Citation24] analyzed the morphology, cytogenetics and DNA sequences of adults and larvae from the type locality of Cacotus coppingeri Günther 1881, and resurrected the species under the combination Alsodes coppingeri. In addition, they considered that the descriptions of A. coppingeri provided by Cei [Citation16–18] and Grandison [Citation19] corresponded to different species, and consequently restricted the distribution of A. coppingeri to its type locality. One year later, specimens of A. australis Formas et al. 1997 were reported from Wellington Island [Citation25], a fourth species of Alsodes cited from this place.
More recently, the validity of Alsodes coppingeri was supported in a molecular phylogenetic analysis [Citation5], and four additional populations other than that of the type locality were recognized for this species, some of them previously assigned to Alsodes australis. These new populations and additional records extended the distribution of A. coppingeri somewhat northwards, from Magallanes to the Aysén Region in Chile [Citation26–28]. In Argentina, specimens of Alsodes from the temperate forest of Santa Cruz Province were cited as Alsodes aff. coppingeri, without confirmation of their specific identity and precise location of occurrence [Citation29].
The present contribution confirm the presence of Alsodes coppingeri for the first time in Argentina at the eastern slopes of the Andes range. We also discuss about the identity and distribution of other Alsodes at these latitudes, based on external morphology and molecular characters.
Materials and methods
We carried out field work in search for Alsodes at Lago del Desierto area, in Santa Cruz Province, Argentina (49° 04ʹS; 72° 53ʹW), from 1996 to 2019. All specimens were deposited in the herpetological collection of Instituto de Diversidad y Evolución Austral (CNP.A), Chubut Province, Argentina. Collection and handling of specimens followed standard practices suggested by Heyer et al. [Citation30], under the permits (year 1997 and No 491755/16) provided by Dirección de Fauna Silvestre of Santa Cruz Province, Argentina.
To determine the specific identity, we obtained DNA sequences from two adults (CNP.A 2884 and CNP.A 4390) and observed morphological features of all specimens following Formas et al. [Citation23,Citation24] and Díaz & Nuñez [Citation22]. Genomic DNA was extracted from tissues preserved in alcohol at −20°C using the phenol/chloroform protocol [Citation31]. Partial PCR amplification of cytochrome b (Cyt b) and cytochrome oxidase I (COI) were obtained for both specimens, and 12S, tRNAVal and 16S (12S-tRNAVal-16S) only for CNP.A 2884. The primers used were as follows: MZV59/tRNAval-H, L1091/H3296, 12SM/16sa-H, and 16SC/16SD for 12S-tRNAVal-16S, MVZ15/MVZ16 for Cyt b, and LCO1490/HCO2198 and T3-AnF1/T7-AnR1 for COI [Citation32–39]. The PCR products were purified with Geneclean III (MP Biomedicals) or Millipore Montage 96-well and sequenced on an ABI 3130 capillary genetic analyzer (Applied Biosystems, Inc.). Sequencing reactions were performed following the standard protocol for Big Dye Terminators v3.1 (Applied Biosystems) in both directions, using DNABaser v. 3 (Heracle BioSoft, Pitesti, Romania) for contigs.
The sequences were included in a phylogenetic analysis, selecting terminals in accordance to relationships proposed by the extensive analysis published by Blotto et al. [Citation5]. Already available DNA sequences of Alsodes 12S-tRNAVal-16S, Cyt b, and COI were also used [Citation5,Citation24,Citation28]. The fragments of 12S-tRNAVal-16S, Cyt b, and COI were aligned with ClustalW [Citation40], executed in BioEdit [Citation41] under default parameters, and later concatenated using SequenceMatrix 1.8 [Citation42]. We performed a maximum parsimony analysis in TNT software [Citation43] choosing the “implicit enumeration” option. A preliminary analysis showed that the monophyly of Alsodes coppingeri is supported by two mutational steps in the COI fragment. For this reason, a second analysis was run including only the samples of A. coppingeri for which COI was available; the excluded samples were used only for comparisons of genetic distances (Appendix A). Support values were estimated on tree running of 1,000 replicates under parsimony jackknife [Citation44] with default TNT settings, and 0.36 of removal probability. Uncorrected p-distances were obtained employing the software MEGA 7 [Citation45].
Results
On 9 December 1996 two metamorphs (CNP.A 2763 and 2764), one male adult (CNP.A 2884), and two tadpoles (CNP.A 4086) of Alsodes sp. were found in a small stream that crosses Provincial Road N° 23 in the vicinity of the Lago del Desierto (49° 05’ 09”S; 72° 53’ 44”W; 506 m a.s.l. ± 500 m). On 27 January 1997 another male was collected (CNP.A 2885) on the forest floor and four tadpoles of Alsodes sp. (CNP.A 4911) in a small stream on the east slope of Vespignani Mountain (49° 04’ 43”S; 72° 54’ 31”W; 750 m a.s.l.). On 20 January 2018 an adult male was found (CNP.A 4390), plus another on 22 March 2019 (CNP.A 4623) and 18 tadpoles (CNP.A 4624), all of them in the same stream that crosses Provincial Road N° 23 where the so-called Salto del Anillo waterfall is formed (49° 07’ 07”S; 72° 55’ 29”W; 451 m a.s.l.). All these findings occurred in streams located within the humid forests of Nothofagus beechs, on the eastern slope of the Crestón and Vespignani range, and Campo Río Toro, between 400 and 750 m a.s.l. (; ). Around Lago del Desierto, the forest is composed by N. pumilio (lenga) and some patches of N. betuloides (coihue de Magallanes), with an underwood composed by Embothrium coccineum (notro), Chiliotrichium rosmarinifolium (romerillo), Gaultheria mucronata (chaura), Empetrum rubrum (murtilla), and Myoschilos oblongum (codocoipo) and a herbaceous layer rich in pteridophytes and bryophytes, which can spread over fallen and standing trunks. In poorly drained sites, the hydrophytic herbaceous communities were predominant and the tree Nothofagus antarctica (ñire) was present often as bushes. The frogs were found under rocks and trunks covered with bryophytic vegetation present on stream banks, while tadpoles were collected in low flow current sections of small to medium-sized streams ().
Table 1. Geographical coordinates for all populations know of Alsodes coppingeri in Chile and new records from Argentina. Type locality in bold. From Chile also was reported in Puyuhuapi, Aysén Province [14], and Península Muñoz Gamero, Última Esperanza Province [19], but both localities require new studies
Figure 1. Known geographic distribution of Alsodes coppingeri. Records from Chile: 1) Puerto Río Frío (type locality), 2) Caleta Lever, 3) Caleta Tortel, 4) Puerto Yungay, 5) Laguna Caiquenes, 6) Canal Michel, and 7) Villa O’Higgins. New records from Argentina: 8) Cerro Vespignani, 9) Lago del Desierto, and 10) Salto del Anillo. See geographical coordinates in .
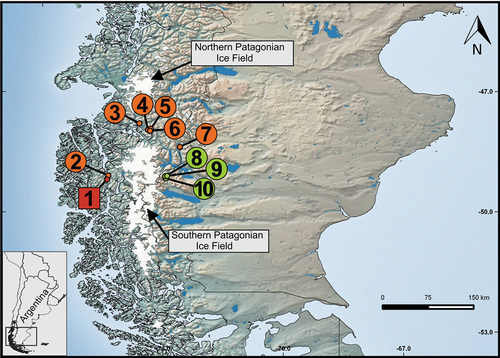
Figure 2. Habitat of Alsodes coppingeri near to Lago del Desierto (see text). (a) Overview of the temperate forests dominated by Nothofagus pumilio on mountain slopes up to about 1000 m, and (b) breeding site inside the forest.

Adult specimens presented the external characters of A. coppingeri provided by Formas et al. [Citation24]: snout profile truncate, legs with uniform coloration, almost unwebbed feet reduced to 3, 4, and 5 toes; but all presented fringes on the toes and also a tarsal fringe. Two of them had uniform brownish coloration, and the other two were uniformly grayish. The male CNP.A 4390 (SVL 58.69 mm) had well-developed fringes on toes, and marked secondary sexual characters, such as: hypertrophy of the forearms, spiny pectoral patches and nuptial pads on the fingers 1 and 2, scattered spines on the inner surface of the fingers 3 and 4, keratinous surfaces on outer bilobated metacarpal tubercle, on the dorsal and ventral surfaces of hands and feet, ventral surface of jaws, dorsal surface of the head, and flanks (). The size, the well-developed fringes on toes, and the secondary sexual characters of this specimen resembles the description of A. kaweshkari (see drawings in Formas et al. [Citation23]), but the presence of a deep notch at the anterior edge of the outer metacarpal tubercle (present in three of four specimens analyzed) resembles A. verrucosus reported from Wellington Island [Citation22]. The size of the four male specimens was larger (43.22–58.69 mm) than that given by Formas et al. [Citation24] (43.2–44.0 mm) for A. coppingeri. In addition, the two metamorphs found by us have more than mid-webbed feet, a character that shown a wide plasticity.
Figure 3. Alsodes coppingeri from Lago del Desierto, Argentina. (a) General view, (b) details of the foot, (c) hand in ventral view, and (d) hand in dorsal view of specimen CNP.A 2884; (e) General view, (f) details of the foot, and (g) ventral view of specimen CNP.A 4390.

The tadpole morphology agrees with the description of A. coppingeri provided by Formas et al. [Citation24]. They are exotrophic larvae, with dorsolateral eyes, an emarginated oral disc with a single row of marginal papillae with a wide rostral gap, one single row of mental intramarginal papillae; tooth row formula 2 (2)/3 (1), spiracle sinistral with a protruding distal end, wide and dextral vent tube, low and straight fins with sub-parallel margins, and rounded tail tip.
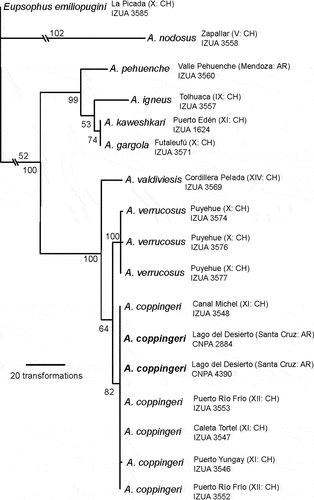
For the phylogenetic analysis, we obtained a molecular matrix of 4085 DNA base pairs (bp): 2424 bp for 12S-tRNAVal-16S, 658 bp for COI, and 1003 bp for Cyt b (most of samples have a fragment smaller than 400 bp; see Appendix A). The maximum parsimony analysis under “implicit enumeration” found a single shortest tree of 767 steps. Sequences of the two specimens of Alsodes provided herein were recovered in a clade along with other sequences of A. coppingeri, supported by two mutational transformations in COI fragments, as the sister taxon of A. verrucosus. We included a small fragment (304 bp) of Cyt b belonging to the holotype of A. kaweshkari, but this species was recovered nested in another clade together with A. gargola in a close relationship. In we show the maximum parsimony tree with jackknife supports, in which all relationships are consistent with those previously obtained by Blotto et al. [Citation5].
Figure 4. Phylogenetic analysis of Alsodes. Most parsimonious tree of 767 steps obtained with TNT. Branch lengths are proportional to the number of transformations, except when indicated by numbers. On the nodes parsimony jackknife supports are indicated. The new records from Argentina are denoted in bold.
The uncorrected p-distances between samples of A. coppingeri were extremely low, ranging between 0.0% and 0.04% for 2355 bp of 12S-tRNAVal-16S (N = 7); 0.0% when we compared only 308 bp of Cyt b (N = 10); and 0.0% in 658 bp of COI (N = 7). In the same way, the uncorrected p-distances between A. coppingeri and A. verrucosus were as follows: 0.13% to 0.21% for 12S-tRNAVal-16S; 0.65% for Cyt b; and 0.61% for COI. Appendix A shows the GenBank accession numbers of all samples used in the comparisons, some of them not included in the phylogenetic analysis.
Discussion
DNA sequences showed that the specimens found around Lago del Desierto, including the mentioned Alsodes aff. coppingeri from Santa Cruz Province [Citation29], belong to the species A. coppingeri, confirming its presence in Argentina for the first time. All samples of this species used in the phylogenetic analysis were recovered as a well-supported clade, sister of A. verrucosus. The node A. coppingeri + A. verrucosus was weakly supported, but when other DNA markers were used for both species, a well-supported relationship was obtained [Citation5]. Alsodes verrucosus is a poorly defined species, with a non-detailed description, without assignment of type specimens, and a vaguely defined type locality that corresponds to a vast area, the Andes Range of Cautín Province in Chile [Citation46,p. 83]. The exemplars of A. verrucosus we used were sampled from Puyehue (Osorno Province), about 200 km south of Cautín Province [see Citation5]. From this last locality, karyotype and tadpoles were already described [Citation47,Citation48]. In Chile, this species was also recently recorded from Cayutué, Llanquihue Province [Citation49], and even for Wellington Island [Citation22]. In Argentina, it was cited from Río Negro Province [Citation50], a population not even detected again, and also from Neuquén Province [Citation51,Citation52] from where specimens seems to correspond to A. neuquensis [see appendix S3 in Citation5]. Caution must be taken regarding comparisons with A. verrucosus unless they include specimens from Cautín. The samples of A. coppingeri (Caleta Tortel) and A. verrucosus (Puyehue) available to us are about 800 km apart. However, their low genetic divergence suggests that future studies are needed to establish species boundaries, including intermediate populations previously assigned to either one of these two species [Citation14,Citation17], as well as specimens previously assigned to A. verrucosus from Wellington Island [Citation22,Citation53].
As early mentioned, A. kaweshkari was described in these high austral latitudes. The Cyt b sequences of A. kaweshkari we obtained did not provided differentiation from A. gargola of Futaleufú, Chile, as considered in Blotto et al. [Citation5]. This unexpected result deserves further consideration. The other Alsodes species mentioned for Wellington Island is A. australis [Citation25], but we could not study the three specimens attributed to the species that have been collected to make direct comparisons with neither with A. coppingeri nor A. verrucosus. Asencio et al. [Citation25] only considered A. kaweshkari to be present in the Island. It is worth of mention that the authors referred to Wellington Island as the type locality of A. australis, which is in fact more than 300 km away northwards from this site, at Puente Traihuanca, in the Aysén Region of Chile [Citation54]. Due to these inaccuracies, the taxonomic identity of A. australis from Wellington Island should be re-evaluated.
The adult specimens collected for this work showed some remarkable morphological variation. Three of them (SVL = 43.22–48.08 mm) slightly exceed the known size range of A. coppingeri but share other diagnostic characters of the species such as snout truncated in lateral view, uniform color on hindlimbs (without bars), reduced toe fringes and webbed feet [Citation24]. However, the characteristics of one adult male (CNP.A 4390; SVL 58.69 mm) matched with diagnostic characters of A. kaweshkari: SVL 56.5–62.2 mm, toes well fringed, webbing of feet reduced but present between all toes, granular dorsolateral surfaces, the skin around the vent and posterior thighs being granular, overall grey coloration, and notable development of secondary sexual characters [Citation23]. In spite of this, molecular data confirmed this last specimen to be A. coppingeri. The size range of adults found by us (43.22–58.69 mm) also overlaps with the only adult of A. verrucosus (43.7 mm) from Wellington Island. Double outer metacarpal tubercles were reported for this population [Citation22], a character not found again in other specimens analyzed to date from this Island [Citation23,Citation24]. Remarkably, three specimens from Lago del Desierto have outer metacarpal tubercles with deep anterior notches (bilobed), without molecular data these specimens could have been assigned to A. verrucosus. Regarding the development of webbing, the two metamorphs from Lago del Desierto have mid- or fully webbed feet, suggesting a great intraspecific variation of this character, as observed in other Alsodes [appendix S3 in 5,28]. Grandison [Citation19] described webbed feet for A. coppingeri, but according to Formas et al. [Citation24] in the Grandison’s diagnosis and the morphology provided by Cei [Citation16–18] were included specimens from a wide geographic range, many of them likely belonging to different Alsodes species.
The phenotypic plasticity found in the few specimens of A. coppingeri known from Argentina overlaps with almost all characters that were used to distinguish among A. coppingeri, A. kaweshkari, and A. verrucosus. A thorough taxonomic revision of these taxa is pending, which should include the specimens of A. australis reported from Wellington Island by Asencio et al. [Citation25]. Regarding DNA data, all available information of Alsodes at latitudes above 47° S (N = 10) appear to belong to a single species, except for a Cyt b sequence of A. kaweshkari (see discussion in [Citation5]). Nonetheless, cytogenetic characters may allow to distinguish among A. coppingeri, A. kaweshkari, and A. verrucosus. All species present 2n = 26 with bi-armed chromosomes (FN = 52), but the chromosomal configuration shows differences between the A. coppingeri – A. verrucosus and A. kaweshkari. Both Alsodes coppingeri and A. verrucosus from Wellington Island share four large, two intermediate, and seven small chromosomes, and show the nucleolus organizer regions (NORs) located within the secondary constrictions of the short arm of pair 4; but differ in the morphology of pairs 2, 7, 8, 9, 12, and 13 [Citation24,Citation55]; but see the A. verrucosus chromosome configuration from Puyehue [Citation47]. On the other hand, A. kaweshkari have five large, one intermediate, and seven small chromosomes with secondary constrictions on pairs one, four, and six [Citation23].
Like other Alsodes species, our studied larval specimens were aquatic and exotrophic tadpoles, of the lothic-benthic ecomorphological type [Citation56], which are commonly associated with streams [e.g. Citation24,Citation48,Citation57–59]. The tadpoles found in summer ranged between 37.41 and 58.96 mm of total length and between 25 and 39 developmental stages [Citation60]. The 18 tadpoles collected on March 22 (beginning of autumn) ranged between 25 and 26 Gosner’s stages, similar to the data presented from the Wellington Island by Formas et al. [Citation24]. These observations allow us to infer at least one overwintering episode during larval development in streams and permanent oligotrophic ponds of the study sites, as has been proposed for other Alsodes [e.g. Citation61–64], rather than an acceleration of metamorphosis before the arrival of winter. This is supported by data from Laguna Caiquenes, Chile, where tadpoles of A. coppingeri were found throughout the year with metamorphosing individuals in January and March [Citation26].
The area of Lago del Desierto represents the southern limits for the genus Alsodes in Argentina. On Wellington Island (Chile), A. coppingeri, A. verrucosus, and A. kaweshkari can be found in sympatry [Citation22–24]. Alsodes coppingeri [as Eupsophus coppingeri] was mentioned for Península Muñoz Gamero (Chile), found in a lowland area outside the forest by the “Royal Society Expedition to Southern Chile”, that would extends the distribution of the genus in Chile about 400 km south [Citation19]. The northernmost known locality for A. coppingeri may be Puyuhuapi, Aysén Province [Citation14], but the taxonomic identity of this population and specimens from Península Muñoz Gamero requires a revision.
All known records of A. coppingeri correspond to the temperate-cold forest altitudinal range [Citation5,Citation24,Citation26,Citation27,Citation65,this study]. At these latitudes on the western side of the Andes, the weather is cold throughout the year, with prevailing winds from the west and annual rainfall usually exceeding 4000 mm [Citation66,Citation67]. On the eastern side of the Andes, the temperate forest extends over slope of the Andes below 1000 m a.s.l., a narrow area bounded to the east by prevailing aridity [Citation68–70]. In Argentina, A. coppingeri lives in sympatry with Chaltenobatrachus grandisonae and Nannophryne variegata [Citation71], while in Chile it dwells with A. kaweshkari, A. verrucosus, Batrachyla antartandica, B. nibaldoi, B. taeniata, C. grandisonae, Eupsophus calcaratus, and N. variegata [Citation24,Citation26,Citation72].
Lago del Desierto and Wellington Island at both sides of the Andes mountain range are at almost the same latitudes, about 100 km in a straight-line, with the interposition of the Southern Continental Ice Field. However, the populations of Aysén (Chile) are located close to a gap without ice cover that separates the North and South portions of the Continental Ice Field (). It is possible that this area would act as a corridor for different amphibian species at both sides of the Andes.
The new locations of A. coppingeri in Argentina are included in the Lago del Desierto Provincial Reserve recently created in 2005, near the northern limit of Los Glaciares National Park, where the species could be present. In Chile, the species is included in the Laguna Caiquenes Natural Reserve and the Bernardo O’Higgins National Park. However, the introduction of exotic salmonids in lakes and streams from both Chile and Argentina is a matter of concern, as may pose high predation pressure on tadpoles. The known geographic range of A. coppingeri is included in the Subpolar Nothofagus Ecoregion, categorized as Vulnerable and Bioregionally Outstanding ecoregion [Citation73]. Currently, the species is classified as Data Deficient given continuing uncertainties about its actual extent of occurrence, population status, and ecological requirements [Citation74]; this categorization indicates the need for further field data because some potential extinction risk factors and the extent of geographic occurrence may have been overlooked [Citation75,Citation76].
Author contributions
DAB, CAÚ, LC, and NGB contributed equally in all stage of this manuscript.
Acknowledgments
We thank Claudio Correa and Claudio Borteiro for their valuable comments on the manuscript, and to Ricardo Ortubay, Silvia Ortubay, Daniel Wegrzyn, Sergio Rosset, Clara Volonteri, and Ximena Navoa for their help in the fieldwork. We thank Boris Blotto and José Nuñez for the information provided, and Dirección de Fauna Silvestre of Santa Cruz Province for the permits (year 1997 and No 491755 /16).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Vuilleumier F. Origin of frogs of Patagonian forests. Nature. 1968;219(5149):87–89.
- Formas JR. La herpetofauna de los bosques templados de Sudamérica. In: Duellman WE, editor. The South American herpetofauna: its origin, evolution, and dispersal. Monograph of the Museum of Natural History. Vol. 7. Lawrence: The University of Kansas; 1979. p. 341–369.
- Ortiz Z JC, Díaz-Páez H. Estado de conocimiento de los anfibios de Chile [Current state of knowledge about the amphibians of Chile]. Gayana. 2006;70(1):114–121. Spanish.
- Correa C. Nueva lista comentada de los anfibios de Chile (Amphibia, Anura) [New commented list of the amphibians of Chile (Amphibia, Anura)]. Boletín Chileno de Herpetología. 2019;6:1–14. Spanish.
- Blotto BL, Nuñez JJ, Basso NG, et al. Phylogenetic relationships of a Patagonian frog radiation, the Alsodes + Eupsophus clade (Anura: alsodidae), with comments on the supposed paraphyly of Eupsophus. Cladistics. 2013;29(2):113–131.
- Suárez-Villota EY, Quercia CA, Díaz LM, et al. Speciation in a biodiversity hotspot: phylogenetic relationships, species delimitation, and divergence times of Patagonian ground frogs from the Eupsophus roseus group (Alsodidae). Plos One. 2018;13(12):e0204968.
- Barrasso DA, Basso NG. Low genetic divergence but many names in the endemic Patagonian frogs of the genus Atelognathus (Anura, Batrachylidae): a molecular genetic and morphological perspective. J Zool Syst Evol Res. 2019;57(2):383–399.
- Correa C, Durán F. Taxonomy, systematics and geographic distribution of ground frogs (Alsodidae, Eupsophus): a comprehensive synthesis of the last six decades of research. ZooKeys. 2019;863:107–152.
- McNeely JA. The role of taxonomy in conserving biodiversity. J Nat Conserv. 2002;10(3):145–153.
- Mace GM, Godfray HCJ, Knapp S. The role of taxonomy in species conservation. Philos Trans R Soc Lond B. 2004;359(1444):711–719.
- Mota-Vargas C, Rojas-Soto OR. The importance of defining the geographic distribution of species for conservation: the case of the Bearded Wood-Partridge. J Nat Conserv. 2012;20(1):10–17.
- Azat C, Valenzuela-Sánchez A, Delgado S, et al. A flagship for Austral temperate forest conservation: an action plan for Darwin’s frogs brings key stakeholders together. Oryx. 2021;55(3):356–363.
- Boulenger GA. . Catalogue of the Batrachia Salientia s. Ecaudata in the Collection of the British Museum, 2nd. ; London: Taylor and Francis. 1882.
- Codoceo MR. Lista sistemática de Batracios de Aysén y Magallanes [Systematic list of Batracios of Aysén and Magellan]. Not Mens Mus Nac Hist Nat, Chile. 1957;2(16):2. Spanish.
- Capurro L. Lista preliminar de los anfibios de Chile, y breves apuntes sobre su distribución y biología [Preliminary list of Chilean amphibians, and brief notes on their distribution and biology]. Inv Zool Chilenas. 1958;4:289–299. Spanish.
- Cei JM. A survey of the leptodactylid frogs, genus Eupsophus, in Chile. Breviora. 1960;118:1–13.
- Cei JM. Batracios de Chile. Santiago de Chile: Editorial Universidad de Chile;1962. Spanish.
- Cei JM. El género Eupsophus en Chile [The genus Eupsophus in Chile]. Inv Zool Chilenas. 1962;8:7–42. Spanish.
- Grandison AGC. Chilean species of the genus Eupsophus (Anura, Leptodactylidae). Bull Br Mus Nat Hist Zool. 1961;8:8–149.
- Gallardo JM. Los géneros «Eupsophus» y «Batrachyla» (Anura, Leptodactylidae) en la Argentina y la verdadera identidad de Paludicola illota Barbour [The genera «Eupsophus» and «Batrachyla» (Anura, Leptodactylidae) in Argentina and the true identity of Paludicola illota Barbour]. Rev Mus Argent Cienc Nat “Bernardino Rivadavia”. 1962;8(10):113–122. Spanish.
- Lynch JD. The identity of the Chilean frog, Alsodes monticola Bell, and the status of the genus Alsodes (Amphibia: Leptodactylidae). Herpetologica. 1968;24(3):255–257.
- Díaz NF, and Nuñez H. Nuevo hallazgo de Alsodes verrucosus (Philippi, 1902) en Chile y descripción de su larva (Anura: Leptodactylidae) [New find of Alsodes verrucosus (Philippi, 1902) in Chile and description of its larva (Anura: Leptodactylidae)]. Bol Mus Nac Hist Nat. 1988;41:87–94. Spanish.
- Formas JR, Cuevas C, Nuñez J. A new species of Alsodes (Amphibia: Anura: Leptodactylidae) from southern Chile. Proc Biol Soc Wash. 1998;111(3):521–530.
- Formas JR, Núñez J, Cuevas C. Identidad de la rana austral chilena Eupsophus coppingeri (Amphibia, Anura, Neobatrachia): evidencias morfológicas, cromosómicas y moleculares [Identity of the austral Chilean frog Eupsophus coppingeri (Amphibia, Anura, Neobatrachia): morphological, chromosomic and molecular evidences]. Rev Chil Hist Nat. 2008;81(1):3–20. Spanish.
- Asencio J, Kush A, Henríquez JM, et al. Registros de anfibios en el bosque norpatagónico costero del Canal Messier, Chile [Amphibian records from the coastal Nor - Patagonian forest along the Messier Channel, Chile]. An Inst Patagon. 2009;37(1):113–116. Spanish.
- Cisternas J, Correa C, Velásquez N, et al. Reproductive features of Chaltenobatrachus grandisonae (Anura: Batrachylidae) within a protected area in Patagonia, Chile. Rev Chil Hist Nat. 2013;86(3):365–368.
- Alveal N, Díaz-Páez H, Henríquez A, et al. Aspectos dietarios de Alsodes coppingeri Günther, 1881 (Anura: Alsodidae) en Chile [Dietary aspects of Alsodes coppingeri Günther, 1881 (Anura: Alsodidae) in Chile]. Gayana. 2015;79(1):5–10. Spanish.
- Correa C, Zepeda P, Lagos N, et al. New populations of two threatened species of Alsodes (Anura, Alsodidae) reveal the scarce biogeographic knowledge of the genus in the Andes of central Chile. Zoosyst Evol. 2018;94(2):349–358.
- Úbeda C, Grigera D. El grado de protección de los anfibios patagónicos de Argentina [The degree of protection of the Patagonian amphibians of Argentina]. Ecología Austral. 2007;17(2):269–279. Spanish.
- Heyer WR, Donnelly MA, McDiarmid RW, et al. editors. Measuring and monitoring biological diversity: standard methods for amphibians. Washington and London: Smithsonian Institution Press; 1994.
- Sambrook J, and Russell D. Molecular cloning. A laboratory manual. 3rd ed. New York: Cold Spring Harbor Laboratory Press; 2001.
- Kocher TD, Thomas WK, Meyer A, et al. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. PNAS. 1989;86(16):6196–6200.
- Moritz C, Schneider CJ, Wake DB. Evolutionary relationships within the Ensatina eschscholtzii complex confirm the ring species interpretation. Syst Biol. 1992;41(3):273–291.
- Folmer O, Black M, Hoeh W, et al. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3(2):294–299.
- Richards CM, Moore WS. A phylogeny for the African treefrog family Hyperoliidae based on mitochondrial rDNA. Mol Phylogenet Evol. 1996;5(3):522–532.
- Graybeal A. Phylogenetic relationships of bufonid frogs and tests of alternate macroevolutionary hypotheses characterizing their radiation. Zool J Linnean Soc. 1997;119(3):297–338.
- Goebel AM, JM DY, Atz ME. PCR primers and amplification methods for 12S ribosomal DNA, the control region, cytochrome oxidase I, and cytochrome b in bufonids and other frogs, and an overview of PCR primers which have amplified DNA in amphibians successfully. Mol Phylogenet Evol. 1999;11(1):163–199.
- Darst CR, Cannatella DC. Novel relationships among hyloid frogs inferred from 12S and 16S mitochondrial DNA sequences. Mol Phylogenet Evol. 2004;31(2):462–475.
- Lyra ML, Haddad CFB, de Azeredo-Espin, AML. Meeting the challenge of DNA barcoding neotropical amphibians: polymerase chain reaction optimization and new COI primers. Mol Ecol Resour. 2017;17(5):966–980.
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position–specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680.
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp. 1999;41:95–98.
- Vaidya G, Lohman DJ, Meier R. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics. 2011;27(2):171–180.
- Goloboff PA, Farris JS, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008;24(5):774–786.
- Farris JS, Albert VA, Källerjo M, et al. Parsimony jackknifing outperforms neighbor-joining. Cladistics. 1996;12(2):99–124.
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874.
- Philippi RA. Suplemento a los batraquios chilenos descritos en la historia física i política de Chile de Don Claudio Gay [Supplement to the Chilean batrachians described in the physical and political history of Chile by Don Claudio Gay]. Santiago de Chile:Libreria Alemana de José Ivens;1902. Spanish.
- Formas JR, Vera MI. Karyological Relationships among frogs of the genus Alsodes, with description of the karyotypes of A. vanzolinii and A. verrucosus. Copeia. 1983;1983(4):1104–1107.
- Formas JR, Brieva L. The tadpoles of Alsodes vanzolinii and A. verrucosus (Anura: Leptodactylidae) with descriptions of their internal oral and chondrocranial morphology. Amphibia-Reptilia. 2004;25(2):151–164.
- Mella-Romero J, Lamilla-Maulén P. Alsodes verrucosus (Philippi, 1902) (Anura, Alsodidae): a new locality for a very poorly known species. Check List. 2019;15(5):811–814.
- Vellard J. Dos batracios interesantes de la región de Bariloche [Two interesting batrachians from the Bariloche región]. Acta Zool Lilloana. 1947;4:146–153. Spanish.
- Cei JM. Remarks on some Neotropical amphibians of the genus Alsodes from southern Argentina. Atti Soc Ital Sci Nat Museo Civ Stor Nat Milano. 1976;117(3–4):159–164.
- Cei JM. Additional notes to amphibians of Argentina. An update, 1980-1986. Monitore Zool Ital (N S). 1987;21(3):209–272.
- Núñez H, Gálvez O. Catálogo de la colección herpetológica del Museo Nacional de Historia Natural y nomenclátor basado en la colección [Catalog of the herpetological collection of the National Museum of Natural History and gazetteer based on the collection]. Publicación Ocasional. 2015;64:1–203. Spanish.
- Formas JR, Úbeda C, Cuevas C, et al. Alsodes australis, a new species of leptodactylid frog from the temperate Nothofagus forest of Southern Chile and Argentina. Stud Neotrop Fauna Environ. 1997;32(4):200–211.
- Cuevas CC, Formas JR. Cytogenetic analysis of four species of the genus Alsodes (Anura: Leptodactylidae) with comments about the karyological evolution of the genus. Hereditas. 2003;138(2):138–147.
- Altig R, Johnston GF. Guilds of anuran larvae: relationships among developmental modes, morphologies, and habitats. Herpetol Monogr. 1989;3:81–109.
- Lavilla EO. Lower Telmatobiinae (Anura: Leptodactylidae): generic diagnoses based on larval characters. Occas Pap Mus Nat Hist (Lawrence). 1988;124:1–19.
- Pillado MS, Alonso CA, Úbeda CA. La larva de Alsodes gargola Gallardo, 1970 (Leptodactylidae, Telmatobiinae) [The larva of Alsodes gargola Gallardo, 1970 (Leptodactylidae, Telmatobiinae)]. Alytes. 2000;18(1):62–72. Spanish.
- Barrasso DA, Alcalde L, Blotto BL, et al. Description of the tadpole of Alsodes neuquensis Cei, 1976 and comparison with the sibling species A. gargola Gallardo, 1970 (Amphibia, Anura, Alsodidae). Herpetol J. 2016;26(1):21–31.
- Gosner KL. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16(3):183–190.
- Díaz NF, Valencia J. Larval morphology and phenetic relationships of the Chilean Alsodes, Telmatobius, Caudiverbera and Insuetophrynus (Anura: Leptodactylidae). Copeia. 1985;1985(1):175–181.
- Logares RE, Úbeda CA. Alsodes gargola (Rana del Catedral). Overwintering tadpoles. Herpetol Rev. 2004;35(4):368–369.
- Logares RE, Úbeda CA. First insights into the overwintering biology of Alsodes gargola frogs and tadpoles inhabiting harsh Andean-Patagonian alpine environments. Amphibia-Reptilia. 2006;27(2):263–267.
- Corbalán V, Debandi G, Martínez F, et al. Prolonged larval development in the Critically Endangered Pehuenche’s frog Alsodes pehuenche: implications for conservation. Amphibia-Reptilia. 2014;35(3):283–292.
- Günther A. Account of the Reptiles, Batrachians, and Fishes collected during the survey of H.M.S. ‘Alert’ in the straits of Magellan and on the coast of Patagonia. Proc Zool Soc. 1881;1881:18–22. + 2 pl.
- Di Castri F. Esquisse, écologique du Chili. In: Delamare - Debouteville CL, Rapoport E, editors. Biologie de l’Amérique australe: IV [Biology of Southern America: IV]. Paris: Editions du Centre Nacional de la Recherche Scientifique; 1968. French. 7–52.
- Di Castri F, Hajek ER. Bioclimatología de Chile. Santiago de Chile [Bioclimatology of Chile]: vicerrectoría Académica de la Universidad Católica de Chile; 1976. Spanish.
- Cabrera AL, Willink A. Biogeografía de América Latina [Biogeography of Latin America]. Washington (DC): Secretaría General de la Organización de los Estados Americanos; 1973. Spanish.
- Cabrera AL. Regiones fitogeográficas argentinas [Argentine phytogeographic regions]. In: Kugler, WF editor. Enciclopedia Argentina de Agricultura y Jardinería. Tomo II. Fascículo 1 . Buenos Aires: ACME S.A.C.I.; 1976. 1–85. Spanish
- Matteucci SD. Ecorregión Bosques Patagónicos [Patagonian Forests Ecoregion]. In: Morello J, Matteucci SD, Rodriguez AF, et al. editors. Ecorregiones y complejos ecosistémicos Argentinos. Buenos Aires: Orientación Gráfica Editora. 2012. 489–547. Spanish.
- Basso NG, Úbeda CA, Bunge MM, et al. A new genus of neobatrachian frog from southern Patagonian forests, Argentina and Chile. Zootaxa. 2011;3002(1):31–44.
- Rabanal FE. Amphibia, Anura, Ceratophryidae, Batrachyla nibaldoi Formas, 1997: latitudinal extension in Patagonia, southern Chile, and distributional range actualization. Check List. 2010;6(2):287–288.
- Dinerstein E, Olson DM, Graham DJ, et al. A conservation assessment of the terrestrial ecoregions of Latin America and the Caribbean. Washington (DC): World Bank; 1995.
- IUCN SSC Amphibian Specialist Group. Alsodes coppingeri [Internet]. The IUCN red list of threatened species: e.T45477112A45477213 [ update 2018 Oct 15; cited Mar 2021]. Available from: https://dx.doi.org/10.2305/IUCN.UK.2019-2.RLTS.T45477112A45477213.en
- Morais AR, Siqueira MN, Lemes P, et al. Unraveling the conservation status of data deficient species. Biol Conserv. 2013;166:98–102.
- Howard SD, Bickford DP, Ferrier S. Amphibians over the edge: silent extinction risk of Data Deficient species. Divers Distrib. 2014;20(7):837–846.
